Sex Differences during Influenza A Virus Infection and Vaccination and Comparison of Cytokine and Antibody Responses between Plasma and Serum Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viruses
2.3. Influenza Virus Infection and Pathogenesis Study
2.4. Influenza Virus Vaccination and Challenge Study
2.5. Virus Titration in Lung Homogenates
2.6. Collection of Plasma and Serum Samples
2.7. Measurement of Cytokines
2.8. Antibody Measurement by Enzyme-Linked Immunosorbent Assays (ELISAs)
2.9. Antibody Avidity Assay
2.10. Microneutralization Assay
2.11. Hemagglutination Inhibition (HI) Assay
2.12. Antibody-Dependent Cellular Cytotoxicity (ADCC) Reporter Assay
2.13. Statistical Analysis
3. Results
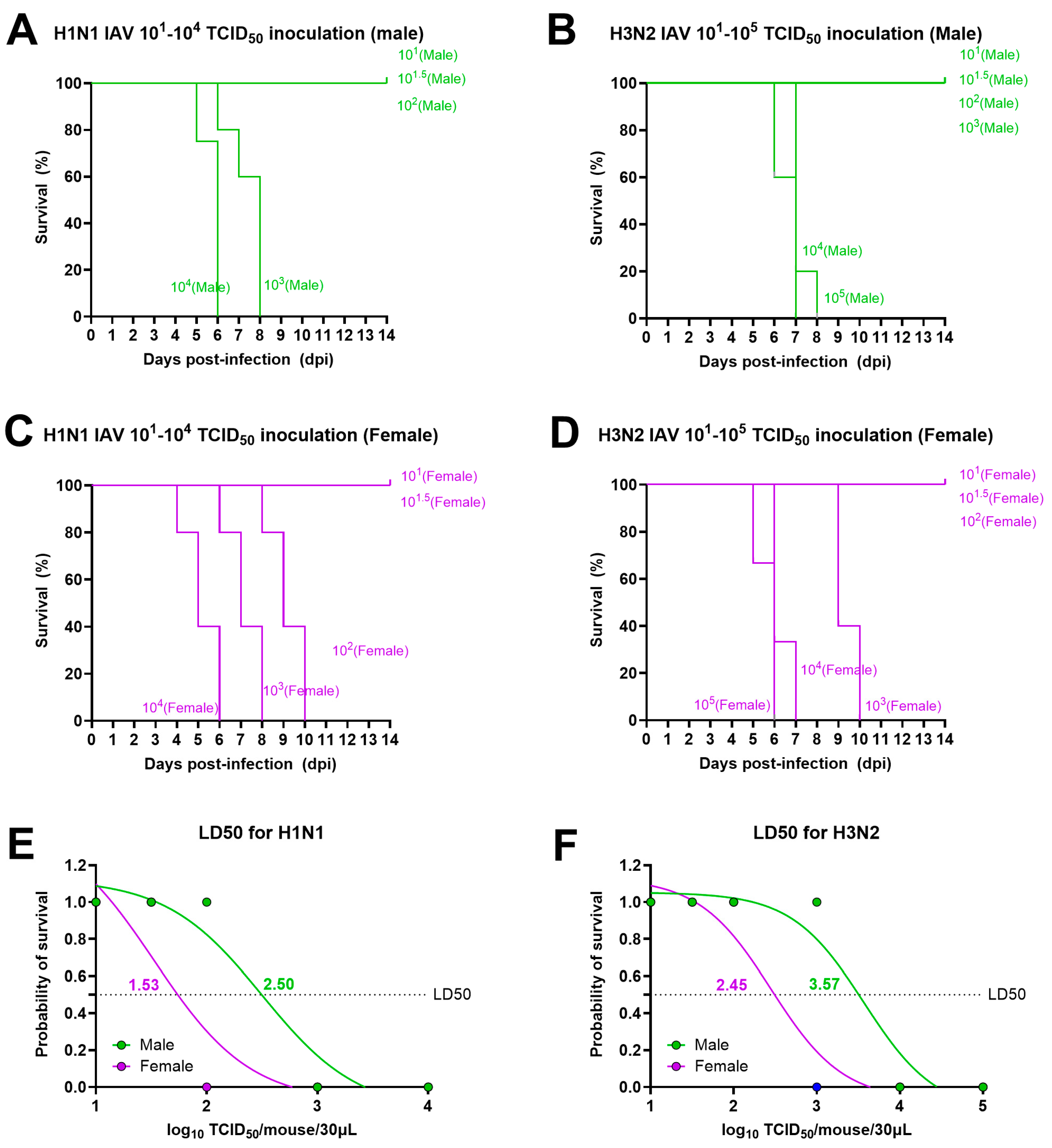
3.1. Lethal Doses for Both H1N1 and H3N2 IAVs Are Lower for Adult Females Than for Male Mice
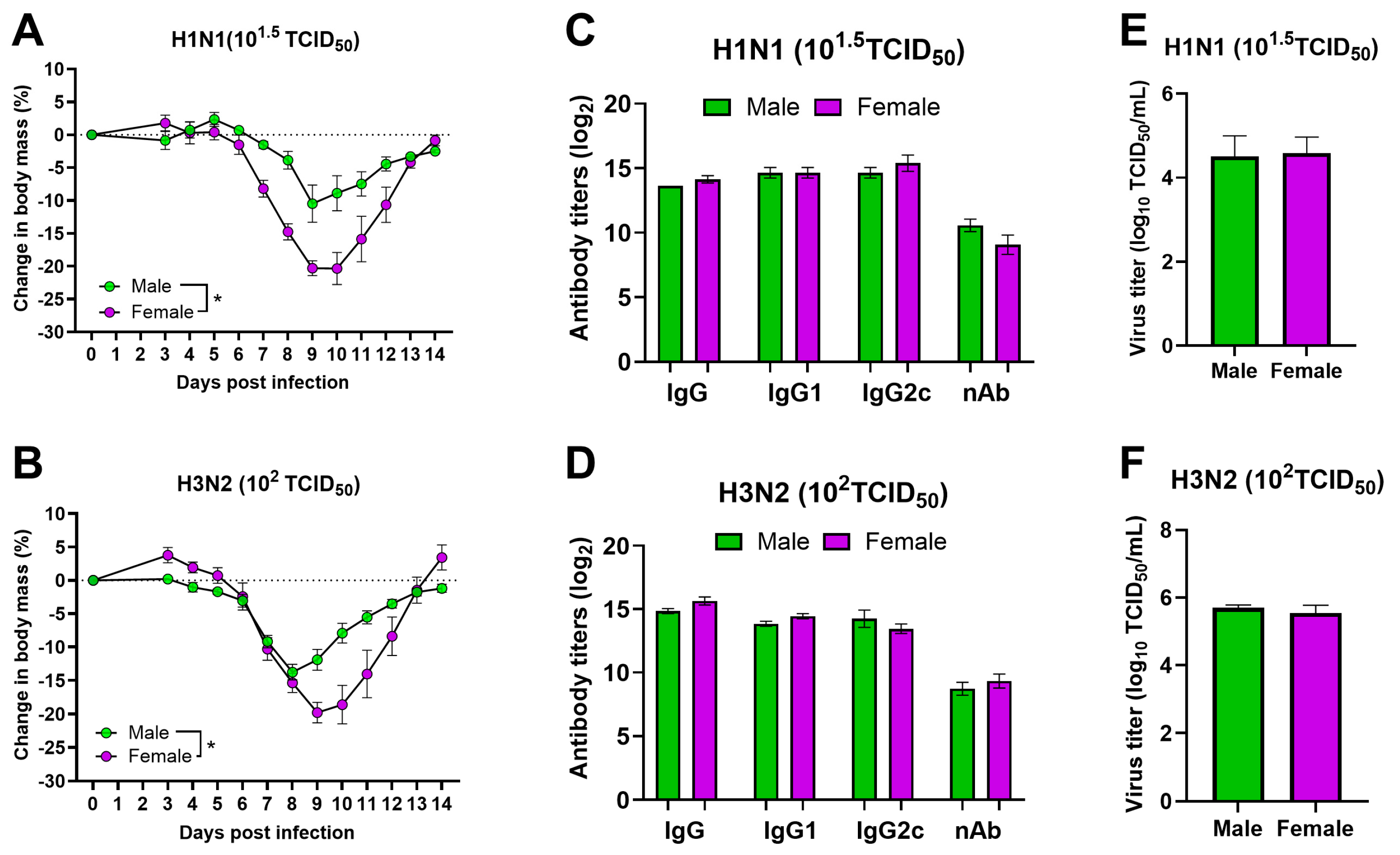
3.2. After Infection with Sublethal Doses of Both H1N1 and H3N2 IAVs, Adult Females Suffer from More Severe Disease Than Male Mice
3.3. Cytokine Responses between Plasma and Serum Samples Differ during Infection with Sublethal Doses of H1N1 and H3N2 IAVs
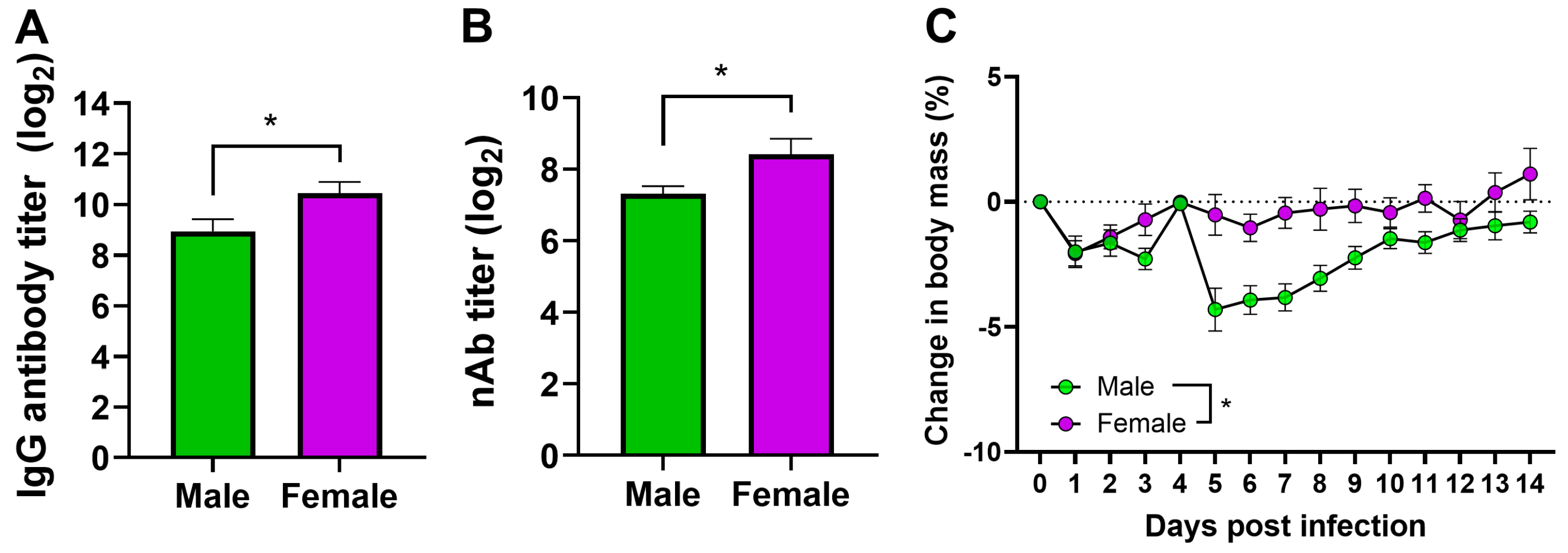
3.4. Vaccinated Adult Female Mice Have a Higher Antibody Response and Are Better Protected Than Male Mice
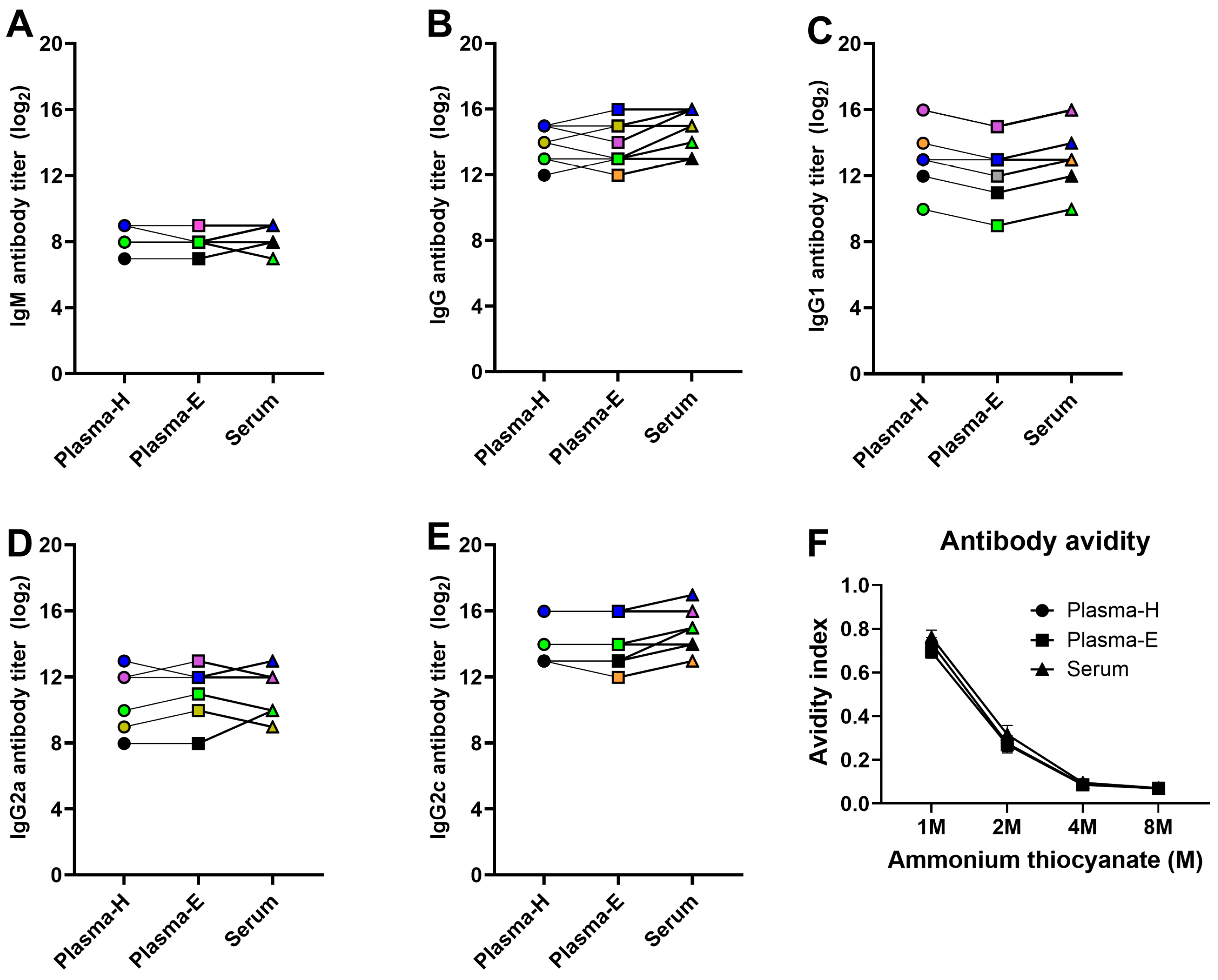
3.5. ELISA-Based Antibody Responses Are Comparable between Plasma and Serum Samples in Vaccinated and Challenged Mice
3.6. Functional Antibody Assays Are Affected by Plasma or Serum Sample Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Gomez Lorenzo, M.M.; Fenton, M.J. Immunobiology of influenza vaccines. Chest 2013, 143, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Chaulagain, S.; Klein, S.L. Sex biases in infectious diseases research. J. Exp. Med. 2022, 219, e20211486. [Google Scholar] [CrossRef]
- Dhakal, S.; Klein, S.L. Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. J. Virol. 2019, 93, e00797-19. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, A.N.; Foong, R.E.; Bozanich, E.M.; Berry, L.J.; Garratt, L.W.; Gualano, R.C.; Jones, J.E.; Dousha, L.F.; Zosky, G.R.; Sly, P.D. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir. Viruses 2011, 5, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.E.; Hodgson, A.; Robinson, D.P.; Kaplan, J.B.; Pekosz, A.; Klein, S.L. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 2011, 29, 9246–9255. [Google Scholar] [CrossRef]
- Vermillion, M.S.; Ursin, R.L.; Kuok, D.I.T.; Vom Steeg, L.G.; Wohlgemuth, N.; Hall, O.J.; Fink, A.L.; Sasse, E.; Nelson, A.; Ndeh, R.; et al. Production of amphiregulin and recovery from influenza is greater in males than females. Biol. Sex. Differ. 2018, 9, 24. [Google Scholar] [CrossRef]
- Giurgea, L.T.; Cervantes-Medina, A.; Walters, K.A.; Scherler, K.; Han, A.; Czajkowski, L.M.; Baus, H.A.; Hunsberger, S.; Klein, S.L.; Kash, J.C.; et al. Sex Differences in Influenza: The Challenge Study Experience. J. Infect. Dis. 2022, 225, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Eshima, N.; Tokumaru, O.; Hara, S.; Bacal, K.; Korematsu, S.; Tabata, M.; Karukaya, S.; Yasui, Y.; Okabe, N.; Matsuishi, T. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS ONE 2011, 6, e19409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zarychanski, R.; Pinto, R.; Cook, D.J.; Marshall, J.; Lacroix, J.; Stelfox, T.; Bagshaw, S.; Choong, K.; Lamontagne, F.; et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009, 302, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.J.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): Age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; Vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L.; Engle, K.; Ursin, R.L.; Tang, W.Y.; Klein, S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12477–12482. [Google Scholar] [CrossRef] [PubMed]
- Živković, I.; Petrović, R.; Arsenović-Ranin, N.; Petrušić, V.; Minić, R.; Bufan, B.; Popović, O.; Leposavić, G. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals 2018, 52, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Živković, I.; Bufan, B.; Petrušić, V.; Minić, R.; Arsenović-Ranin, N.; Petrović, R.; Leposavić, G. Sexual diergism in antibody response to whole virus trivalent inactivated influenza vaccine in outbred mice. Vaccine 2015, 33, 5546–5552. [Google Scholar] [CrossRef]
- Ursin, R.L.; Dhakal, S.; Liu, H.; Jayaraman, S.; Park, H.S.; Powell, H.R.; Sherer, M.L.; Littlefield, K.E.; Fink, A.L.; Ma, Z.; et al. Greater Breadth of Vaccine-Induced Immunity in Females than Males Is Mediated by Increased Antibody Diversity in Germinal Center B Cells. mBio 2022, 13, e0183922. [Google Scholar] [CrossRef]
- Dhakal, S.; Park, H.-S.; Seddu, K.; Lee, J.S.; Creisher, P.S.; Seibert, B.; Davis, K.M.; Hernandez, I.R.; Maul, R.W.; Klein, S.L. Estradiol mediates greater germinal center responses to influenza vaccination in female than male mice. mBio 2024, 15, e00326-24. [Google Scholar] [CrossRef] [PubMed]
- Creisher, P.S.; Campbell, A.D.; Perry, J.L.; Roznik, K.; Burd, I.; Klein, S.L. Influenza subtype-specific maternal antibodies protect offspring against infection but inhibit vaccine-induced immunity and protection in mice. Vaccine 2022, 40, 6818–6829. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Deshpande, S.; McMahon, M.; Strohmeier, S.; Krammer, F.; Klein, S.L. Female-biased effects of aging on a chimeric hemagglutinin stalk-based universal influenza virus vaccine in mice. Vaccine 2022, 40, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Vom Steeg, L.G.; Dhakal, S.; Woldetsadik, Y.A.; Park, H.S.; Mulka, K.R.; Reilly, E.C.; Topham, D.J.; Klein, S.L. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PLoS Pathog. 2020, 16, e1008506. [Google Scholar] [CrossRef] [PubMed]
- Hennø, L.T.; Storjord, E.; Christiansen, D.; Bergseth, G.; Ludviksen, J.K.; Fure, H.; Barene, S.; Nielsen, E.W.; Mollnes, T.E.; Brekke, O.-L. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines—Consequences for defining reference values in healthy humans. Cytokine 2017, 97, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Biancotto, A.; Feng, X.; Langweiler, M.; Young, N.S.; Philip McCoy, J. Effect of anticoagulants on multiplexed measurement of cytokine/chemokines in healthy subjects. Cytokine 2012, 60, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann, M.; Bosshart, H. Heparin Binds to Lipopolysaccharide (LPS)-Binding Protein, Facilitates the Transfer of LPS to CD14, and Enhances LPS-Induced Activation of Peripheral Blood Monocytes1. J. Immunol. 2005, 174, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.H.; Resto-Ruiz, S.; Washington, G.C.; Ethridge, S.; Palini, A.; Vogt, R.; Waxdal, M.; Fleisher, T.; Noguchi, P.D.; Marti, G.E. Flow cytometric analysis of whole blood lysis, three anticoagulants, and five cell preparations. Cytometry 1992, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Elghetany, M.T.; Davis, B.H. Impact of preanalytical variables on granulocytic surface antigen expression: A review. Cytom. B Clin. Cytom. 2005, 65, 1–5. [Google Scholar] [CrossRef]
- Hodge, G.L.; Flower, R.; Han, P. Optimal storage conditions for preserving granulocyte viability as monitored by Annexin V binding in whole blood. J. Immunol. Methods 1999, 225, 27–38. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Rubin, S.J.S.; Bai, L.; Aluko, S.; Yang, Y.; Park, W.; Habtezion, A. Effects of processing conditions on stability of immune analytes in human blood. Sci. Rep. 2020, 10, 17328. [Google Scholar] [CrossRef] [PubMed]
- Defang, G.N.; Martin, N.J.; Burgess, T.H.; Millar, E.V.; Pecenka, L.A.; Danko, J.R.; Arnold, J.C.; Kochel, T.J.; Luke, T.C. Comparative analysis of hemagglutination inhibition titers generated using temporally matched serum and plasma samples. PLoS ONE 2012, 7, e48229. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-Y.; Cao, J.; Ou-Yang, P.; Tsai, C.-Y.; Lin, C.-W.; Chen, C.-C.; Tsai, M.-K.; Lee, C.-Y. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci. Rep. 2022, 12, 5713. [Google Scholar] [CrossRef]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, S.; Wolfe, B.W.; Pantha, S.; Vijayakumar, S. Sex Differences during Influenza A Virus Infection and Vaccination and Comparison of Cytokine and Antibody Responses between Plasma and Serum Samples. Pathogens 2024, 13, 468. https://doi.org/10.3390/pathogens13060468
Dhakal S, Wolfe BW, Pantha S, Vijayakumar S. Sex Differences during Influenza A Virus Infection and Vaccination and Comparison of Cytokine and Antibody Responses between Plasma and Serum Samples. Pathogens. 2024; 13(6):468. https://doi.org/10.3390/pathogens13060468
Chicago/Turabian StyleDhakal, Santosh, Brian W. Wolfe, Saurav Pantha, and Saranya Vijayakumar. 2024. "Sex Differences during Influenza A Virus Infection and Vaccination and Comparison of Cytokine and Antibody Responses between Plasma and Serum Samples" Pathogens 13, no. 6: 468. https://doi.org/10.3390/pathogens13060468



