The Impact of Acute EBV Infection on Changes in the Serum Proteome in Children—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Sample Collection
2.1.1. The Tested Material Was Divided into Three Research Groups
2.1.2. Clinical Evaluation
2.2. Biochemical Research
2.3. Serological Tests
2.4. Proteomics
2.5. Statistics
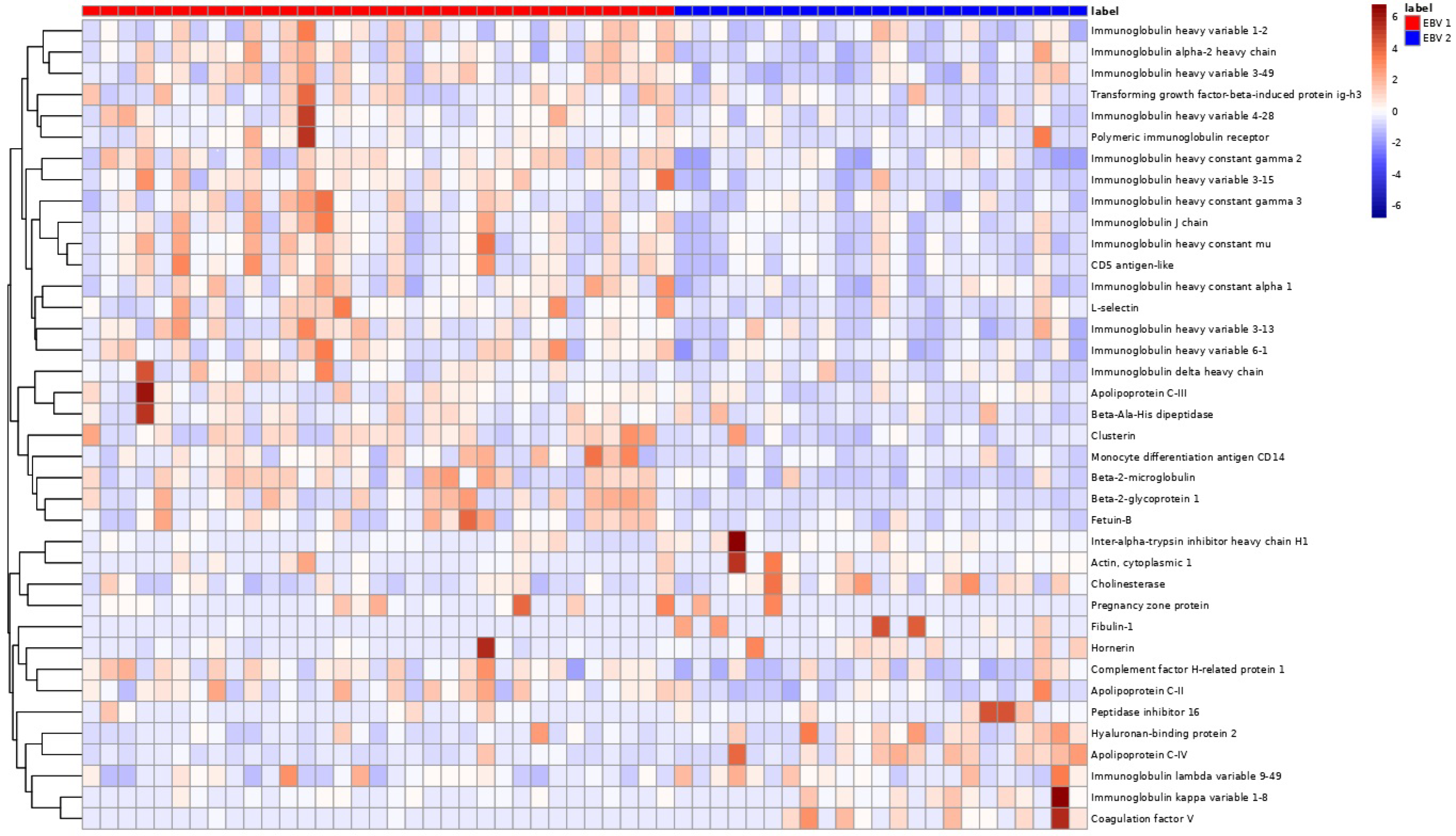
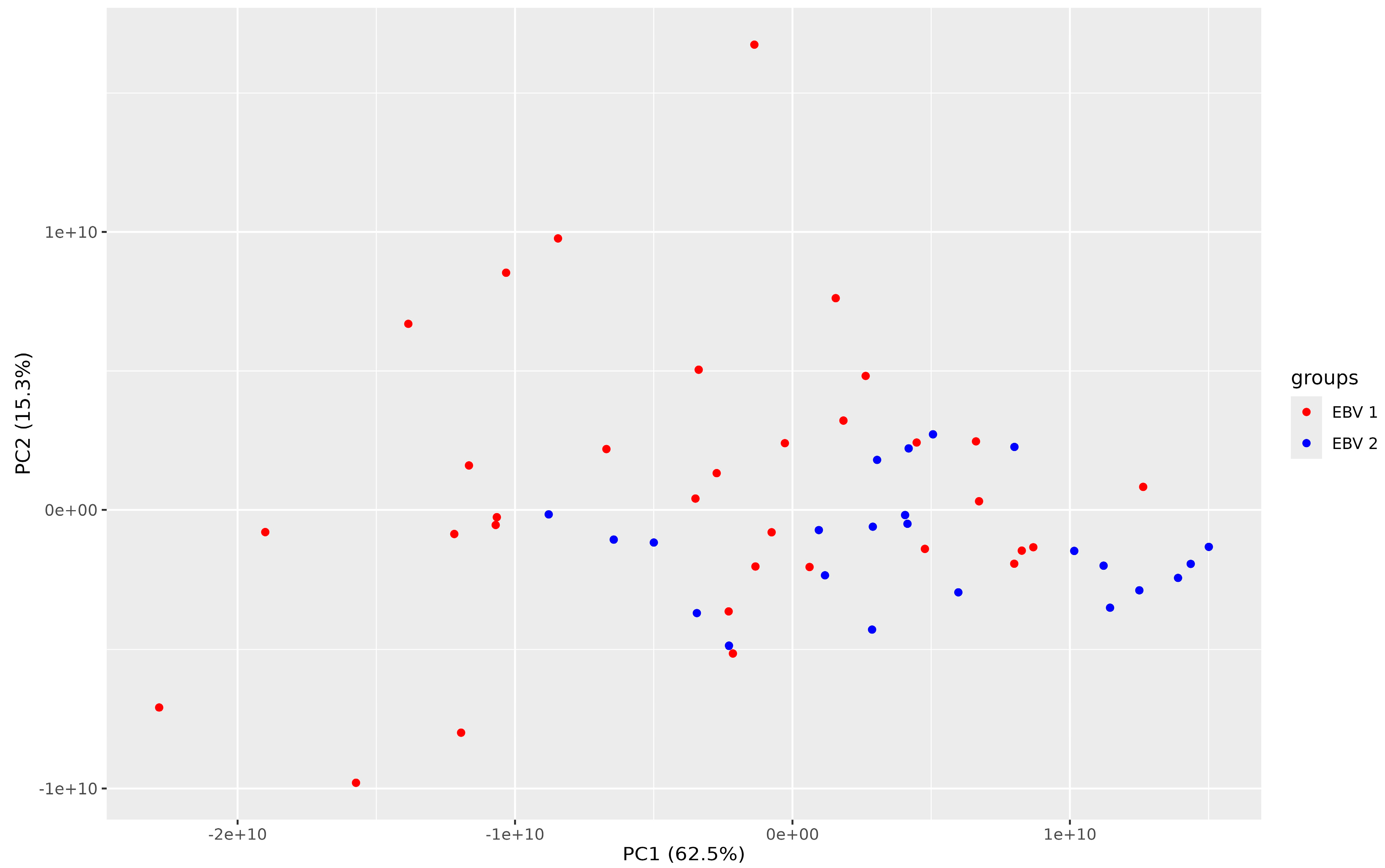
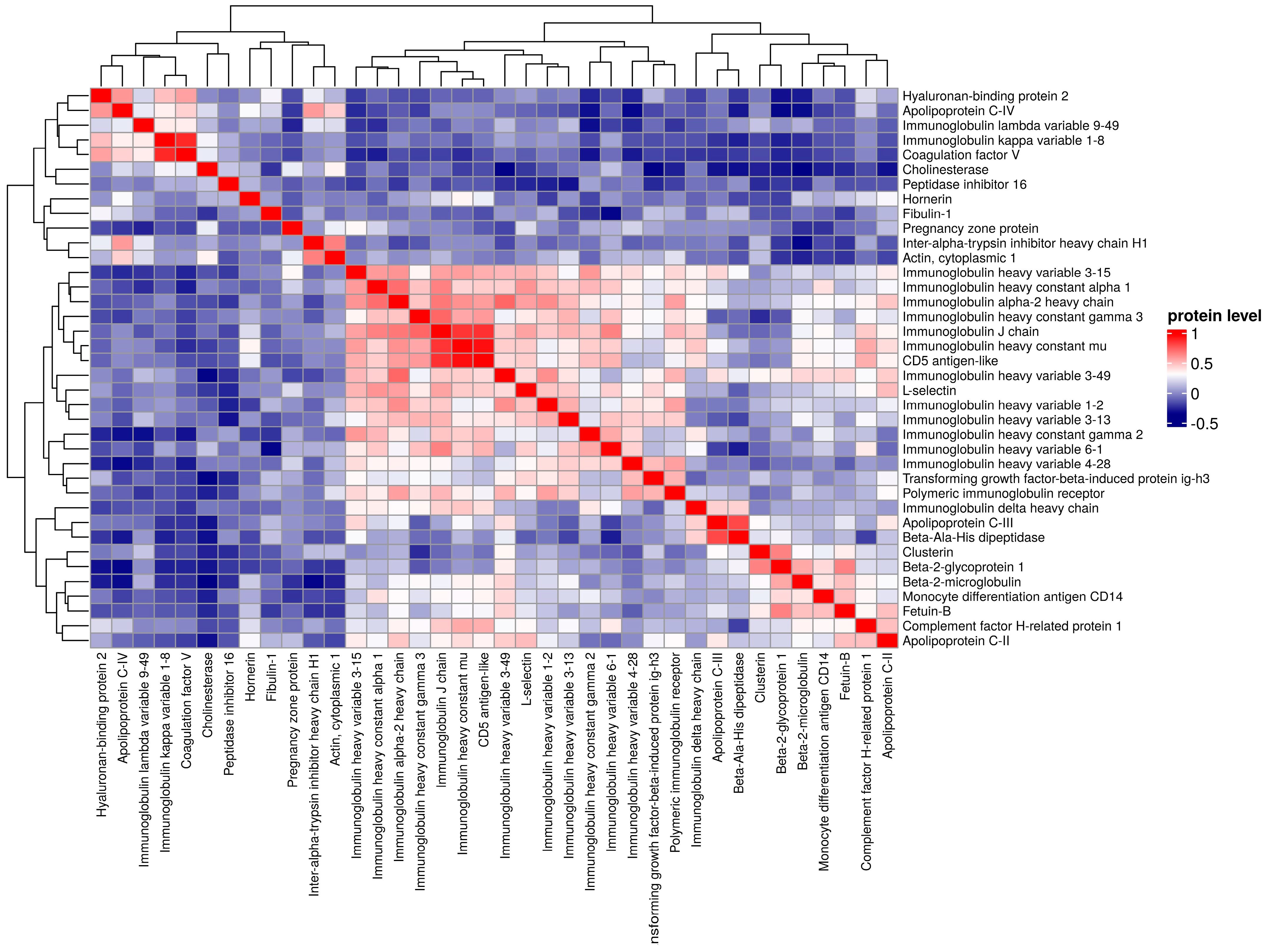
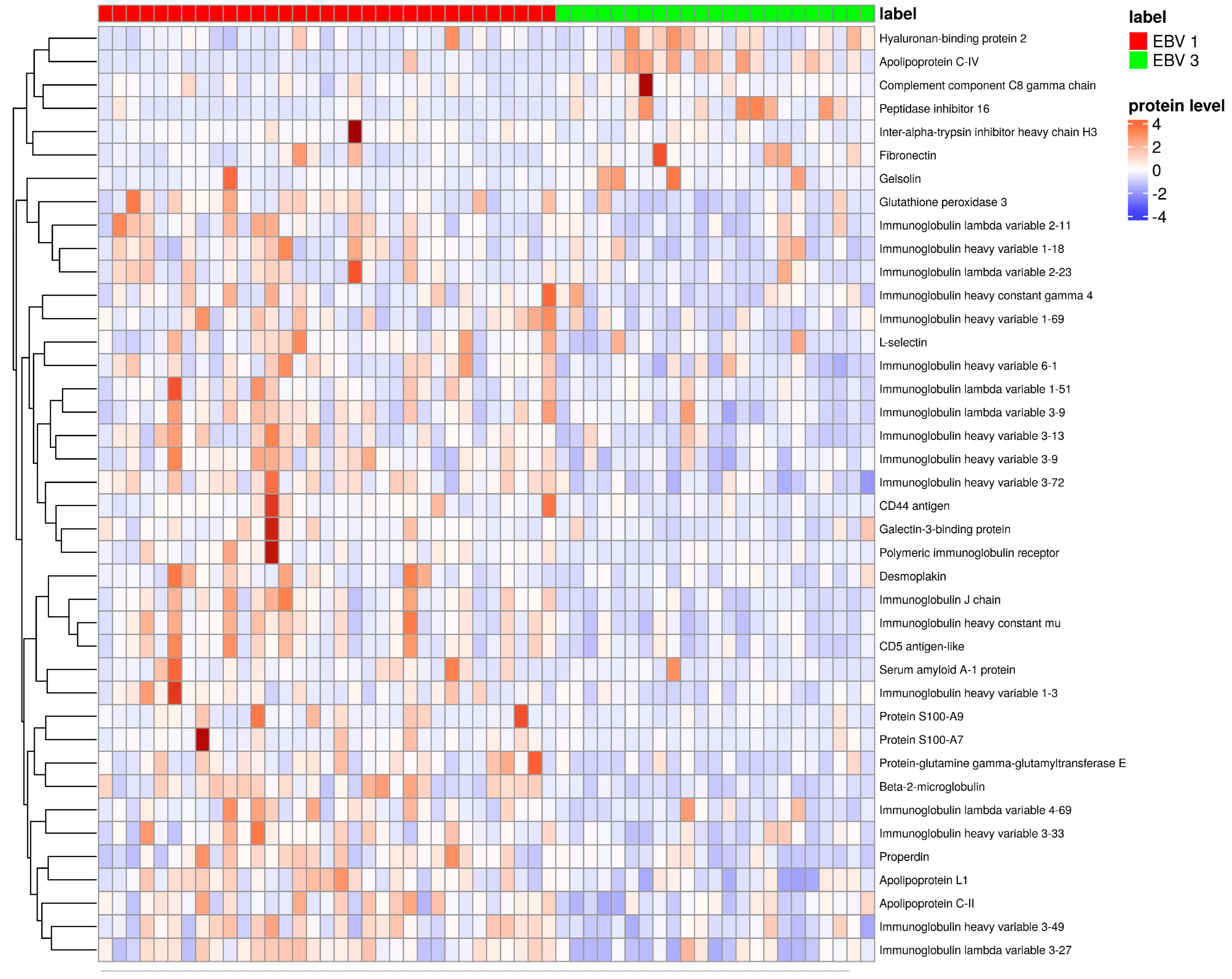
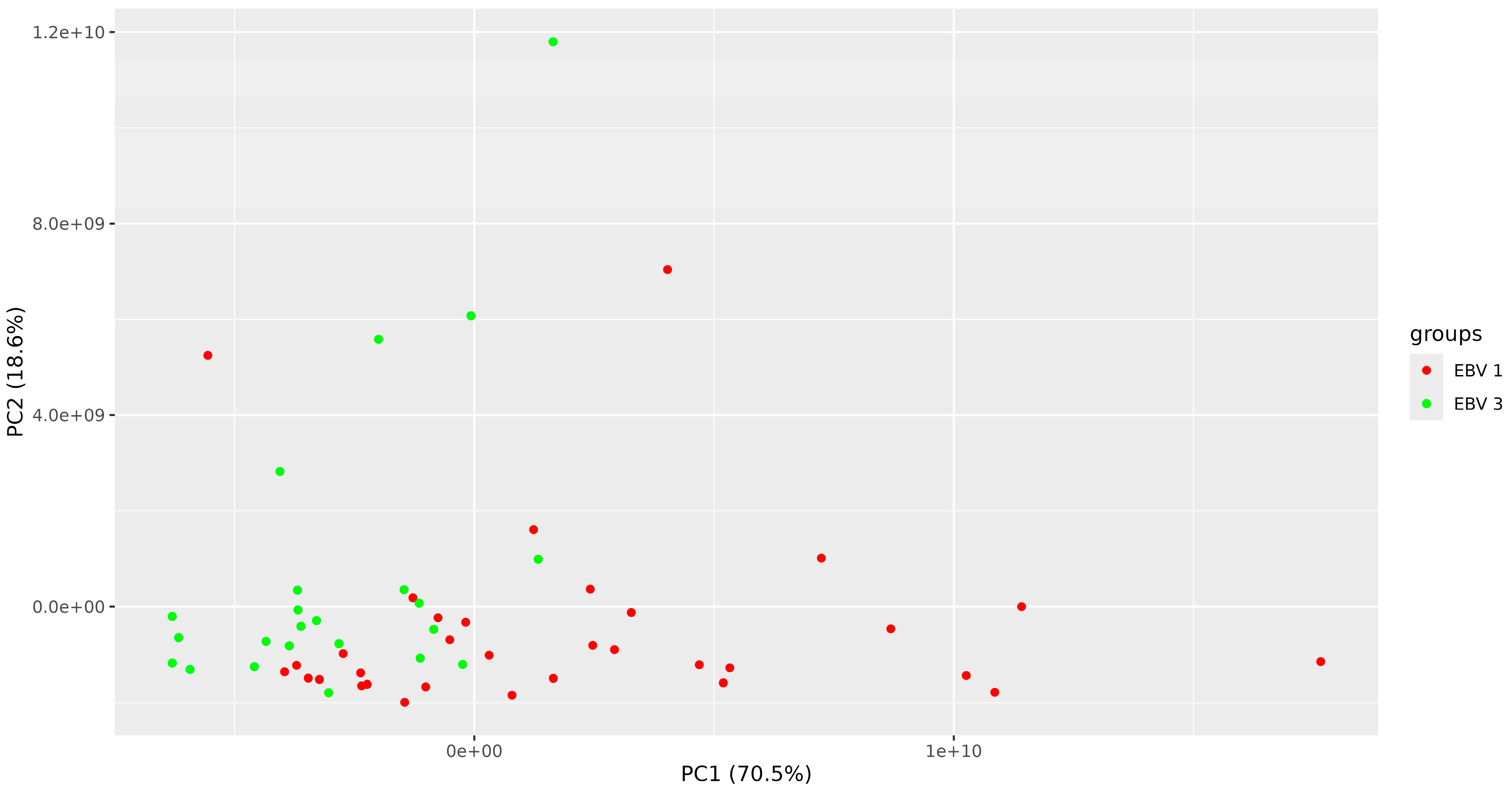
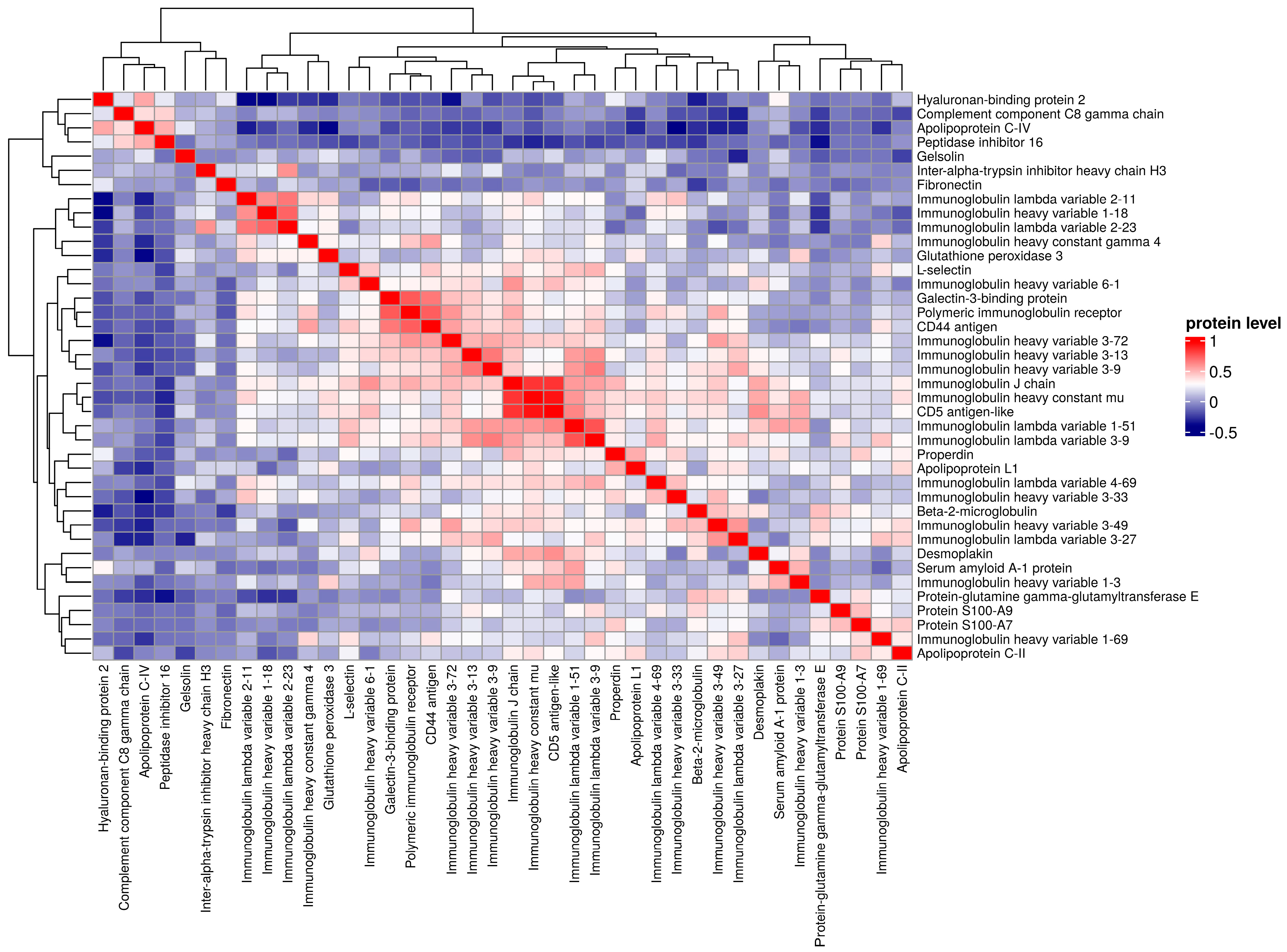
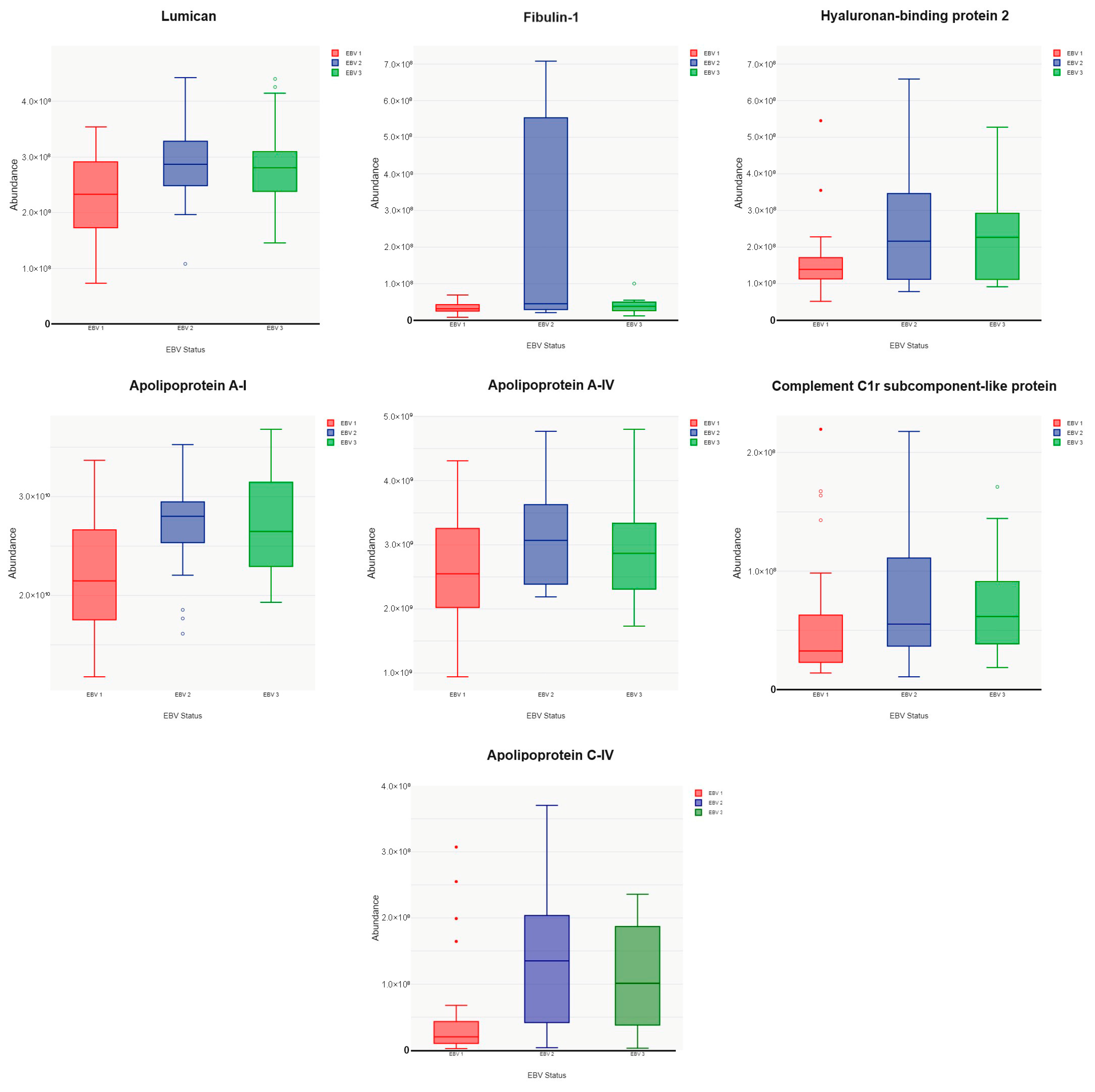
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazur-Melewska, K.; Breńska, I.; Jończyk-Potoczna, K.; Kemnitz, P.; Pieczonka-Ruszkowska, I.; Mania, A.; Służewski, W.; Figlerowicz, M. Neurologic Complications Caused by Epstein-Barr Virus in Pediatric Patients. J. Child Neurol. 2016, 31, 700–708. [Google Scholar] [CrossRef]
- Mazur-Melewska, K.; Derwich, A.; Mania, A.; Kemnitz, P.; Służewski, W.; Figlerowicz, M. Epstein-Barr Virus Infection with Acute Acalculous Cholecystitis in Previously Healthy Children. Int. J. Clin. Pract. 2019, 73, 1–6. [Google Scholar] [CrossRef]
- Watanabe, M.; Panetta, G.L.; Piccirillo, F.; Spoto, S.; Myers, J.; Serino, F.M.; Costantino, S.; Di Sciascio, G. Acute Epstein-Barr Related Myocarditis: An Unusual but Life-Threatening Disease in an Immunocompetent Patient. J. Cardiol. Cases 2020, 21, 137–140. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S.; Ahmed, R. CD8 T-Cell Memory Differentiation during Acute and Chronic Viral Infections; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Cohen, J.I.; Fauci, A.S.; Varmus, H.; Nabel, G.J. Epstein-Barr Virus: An Important Vaccine Target for Cancer Prevention. Sci. Transl. Med. 2011, 3, 107fs7. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein–Barr Virus-Associated Lymphomas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef]
- Chijioke, O.; Azzi, T.; Nadal, D.; Münz, C. Innate Immune Responses against Epstein Barr Virus Infection. J. Leukoc. Biol. 2013, 94, 1185–1190. [Google Scholar] [CrossRef]
- Chappell, D.L.; White, M.C.; Damania, B. Proteomic Approaches to Investigate Gammaherpesvirus Biology and Associated Tumorigenesis. Adv. Virus Res. 2021, 109, 201–254. [Google Scholar] [CrossRef]
- Ersing, I.; Nobre, L.; Wang, L.W.; Soday, L.; Ma, Y.; Paulo, J.A.; Narita, Y.; Ashbaugh, C.W.; Jiang, C.; Grayson, N.E.; et al. A Temporal Proteomic Map of Epstein-Barr Virus Lytic Replication in B Cells. Cell Rep. 2017, 19, 1479–1493. [Google Scholar] [CrossRef]
- Li, X.; Luo, T.; Yan, H.; Xie, L.; Yang, Y.; Gong, L.; Tang, Z.; Tang, M.; Zhang, X.; Huang, J.; et al. Proteomic Analysis of Pediatric Hemophagocytic Lymphohistiocytosis: A Comparative Study with Healthy Controls, Sepsis, Critical Ill, and Active Epstein-Barr virus Infection to Identify Altered Pathways and Candidate Biomarkers. J. Clin. Immunol. 2023, 43, 1997–2010. [Google Scholar] [CrossRef]
- Dreyfus, D.H.; Farina, A.; Farina, G.A. Molecular mimicry, genetic homology, and gene sharing proteomic “molecular fingerprints” using an EBV (Epstein-Barr virus)-derived microarray as a potential diagnostic method in autoimmune disease. Immunol. Res. 2018, 66, 686–695, Erratum in Immunol. Res. 2019, 67, 460. [Google Scholar] [CrossRef]
- Luczak, M.; Suszynska-Zajczyk, J.; Marczak, L.; Formanowicz, D.; Pawliczak, E.; Wanic-Kossowska, M.; Stobiecki, M. Label-Free Quantitative Proteomics Reveals Differences in Molecular Mechanism of Atherosclerosis Related and Non-Related to Chronic Kidney Disease. Int. J. Mol. Sci. 2016, 17, 631. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- McBride, A.A. The Promise of Proteomics in the Study of Oncogenic Viruses. Mol. Cell. Proteom. 2017, 16, S65–S74. [Google Scholar] [CrossRef]
- Maiti, G.; Frikeche, J.; Lam, C.Y.-M.; Biswas, A.; Shinde, V.; Samanovic, M.; Kagan, J.C.; Mulligan, M.J.; Chakravarti, S. Matrix Lumican Endocytosed by Immune Cells Controls Receptor Ligand Trafficking to Promote TLR4 and Restrict TLR9 in Sepsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2100999118. [Google Scholar] [CrossRef]
- Dalli, J.; Norling, L.V.; Montero-Melendez, T.; Federici Canova, D.; Lashin, H.; Pavlov, A.M.; Sukhorukov, G.B.; Hinds, C.J.; Perretti, M. Microparticle Alpha-2-Macroglobulin Enhances pro-Resolving Responses and Promotes Survival in Sepsis. EMBO Mol. Med. 2014, 6, 27–42. [Google Scholar] [CrossRef]
- Valencia, S.M.; Hutt-Fletcher, L.M. Important but Differential Roles for Actin in Trafficking of Epstein-Barr Virus in B Cells and Epithelial Cells. J. Virol. 2012, 86, 2–10. [Google Scholar] [CrossRef]
- Kumarasinghe, N.; Moss, W.N. Analysis of a Structured Intronic Region of the LMP2 Pre-mRNA from EBV Reveals Associations with Human Regulatory Proteins and Nuclear Actin. BMC Res. Notes 2019, 12, 33. [Google Scholar] [CrossRef]
- Timpl, R.; Sasaki, T.; Kostka, G.; Chu, M.-L. Fibulins: A Versatile Family of Extracellular Matrix Proteins. Nat. Rev. Mol. Cell Biol. 2003, 4, 479–489. [Google Scholar] [CrossRef]
- Obaya, A.J.; Rua, S.; Moncada-Pazos, A.; Cal, S. The Dual Role of Fibulins in Tumorigenesis. Cancer Lett. 2012, 325, 132–138. [Google Scholar] [CrossRef]
- Du, M.; Fan, X.; Hong, E.; Chen, J.J. Interaction of Oncogenic Papillomavirus E6 Proteins with Fibulin-1. Biochem. Biophys. Res. Commun. 2002, 296, 962–969. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X.; et al. Targeting the Signaling in Epstein–Barr Virus-Associated Diseases: Mechanism, Regulation, and Clinical Study. Signal Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef]
- Karpenko, I.L.; Valuev-Elliston, V.T.; Ivanova, O.N.; Smirnova, O.A.; Ivanov, A.V. Peroxiredoxins-The Underrated Actors during Virus-Induced Oxidative Stress. Antioxidants 2021, 10, 977. [Google Scholar] [CrossRef]
- Gargouri, B.; Nasr, R.; Mseddi, M.; Benmansour, R.; Lassoued, S. Induction of Epstein-Barr Virus (EBV) Lytic Cycle in Vitro Causes Lipid Peroxidation, Protein Oxidation and DNA Damage in Lymphoblastoid B Cell Lines. Lipids Health Dis. 2011, 10, 111. [Google Scholar] [CrossRef]
- Apostolou, F.; Gazi, I.F.; Lagos, K.; Tellis, C.C.; Tselepis, A.D.; Liberopoulos, E.N.; Elisaf, M. Acute Infection with Epstein–Barr Virus Is Associated with Atherogenic Lipid Changes. Atherosclerosis 2010, 212, 607–613. [Google Scholar] [CrossRef]
- Sayyahfar, S.; Lavasani, A.; Nateghian, A.; Karimi, A. Evaluation of Lipid Profile Changes in Pediatric Patients with Acute Mononucleosis. Infect. Chemother. 2017, 49, 44–50. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.-S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of Infection and Inflammation on Lipid and Lipoprotein Metabolism: Mechanisms and Consequences to the Host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef]
- Cristescu, C.V.; Alain, S.; Ruță, S.M. The Role of CMV Infection in Primary Lesions, Development and Clinical Expression of Atherosclerosis. J. Clin. Med. 2022, 11, 3832. [Google Scholar] [CrossRef]
- Mold, C.; Bradt, B.M.; Nemerow, G.R.; Cooper, N.R. Epstein-Barr Virus Regulates Activation and Processing of the Third Component of Complement. J. Exp. Med. 1988, 168, 949–969. [Google Scholar] [CrossRef]
- Almitairi, J.O.M.; Venkatraman Girija, U.; Furze, C.M.; Simpson-Gray, X.; Badakshi, F.; Marshall, J.E.; Schwaeble, W.J.; Mitchell, D.A.; Moody, P.C.E.; Wallis, R. Structure of the C1r–C1s Interaction of the C1 Complex of Complement Activation. Proc. Natl. Acad. Sci. USA 2018, 115, 768–773. [Google Scholar] [CrossRef]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement Membrane Attack Complex. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Bubeck, D.; Roversi, P.; Donev, R.; Morgan, B.P.; Llorca, O.; Lea, S.M. Structure of Human Complement C8, a Precursor to Membrane Attack. J. Mol. Biol. 2011, 405, 325–330. [Google Scholar] [CrossRef] [PubMed]
| EBV-1 | EBV-2 | EBV-3 | |
|---|---|---|---|
| n | 33 | 23 | 23 |
| Mean age (years) | 8.57 ± 3.56 | 9.09 ± 2.53 | 10.13 ± 2.44 |
| Female/male | 12/21 | 11/12 | 11/12 |
| p | 0.533 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Melewska, K.; Luczak, M.; Watral, J.; Małecki, P.; Mania, A.; Figlerowicz, M. The Impact of Acute EBV Infection on Changes in the Serum Proteome in Children—A Pilot Study. Pathogens 2024, 13, 471. https://doi.org/10.3390/pathogens13060471
Mazur-Melewska K, Luczak M, Watral J, Małecki P, Mania A, Figlerowicz M. The Impact of Acute EBV Infection on Changes in the Serum Proteome in Children—A Pilot Study. Pathogens. 2024; 13(6):471. https://doi.org/10.3390/pathogens13060471
Chicago/Turabian StyleMazur-Melewska, Katarzyna, Magdalena Luczak, Joanna Watral, Paweł Małecki, Anna Mania, and Magdalena Figlerowicz. 2024. "The Impact of Acute EBV Infection on Changes in the Serum Proteome in Children—A Pilot Study" Pathogens 13, no. 6: 471. https://doi.org/10.3390/pathogens13060471
APA StyleMazur-Melewska, K., Luczak, M., Watral, J., Małecki, P., Mania, A., & Figlerowicz, M. (2024). The Impact of Acute EBV Infection on Changes in the Serum Proteome in Children—A Pilot Study. Pathogens, 13(6), 471. https://doi.org/10.3390/pathogens13060471








