Abstract
Thermophilic C. jejuni/coli is reported to be the first bacterial cause of gastroenteritis worldwide and the most common zoonosis in Europe. Although non-jejuni/coli Campylobacter sp. are increasingly suspected to be responsible for diarrhoea or to be involved in inflammatory bowel disease, they remain poorly isolated due to their fastidious and non-thermophilic nature. Additionally, they are not targeted by commercial syndromic PCR assays. In this study, we present routine diagnostic results over 6 years (2017–2019 and 2021–2023) of Campylobacter sp. and related species, obtained by optimised culture from 51,065 stools by both 0.65 µm pore filtration on antibiotic-free agar, incubated in an H2-enriched atmosphere at 37 °C (also known as the Cape Town protocol), and the use of selective inhibitory Butzler medium incubated at 42 °C. This allowed the isolation of 16 Campylobacter species, 2 Aliarcobacter species, and 2 Helicobacter species, providing a completely different view of the epidemiology of Campylobacterales, in which C. jejuni/coli represents only 30.0% of all isolates, while C. concisus represents 44.4%. C. ureolyticus, representing only 5.5% of all Campylobacterales pre-COVID-19, represented 20.6% of all strains post-COVID-19 (218% increase; p < 0.05). At the same time, the proportions of C. jejuni, C. coli, and C. concisus decreased by 37, 53, and 28%, respectively (p < 0.05).
1. Introduction
Campylobacter sp. diarrhoea was first described by T. Escherich in 1886 [1] as “cholera infantum” in newborns. Then, in the first half of the 20th century, “vibrio-like” organisms were isolated in miscarrying sheep, cattle, and ewes [2,3,4,5,6,7,8,9,10,11,12], or calves and swine with diarrhoea [13,14,15,16,17]. It was not until 1947 that Vinzent et al. [18] reported a case of fatal “Vibrion” septicaemia in a pregnant woman, followed by case reports of “related Vibrio” diarrhoea, particularly in children [19,20,21,22,23]. This led to the creation of the genus Campylobacter by Sebald and Veron in 1963 [24]. Vibrio jejuni and V. coli were eventually renamed C. jejuni and C. coli, and in 1972 Dekeyser and Butzler [25] first isolated Campylobacter sp. from stool samples of diarrhoeal patients.
Subsequently, in the 1970s, two methods were proposed for the selective culture of Campylobacter sp. from stools: culture on inhibitory medium containing antibiotics [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] or culture on non-selective enriched medium after selective filtration of stools [32,38,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64], also called “The Cape Town protocol” [50]. Filtration techniques allowed to isolate non-jejuni/coli Campylobacter sp., especially C. concisus [40,51,52,55,56,57,59,60,61,63,64], C. curvus [40,51,55,56,57,59,61,64,65], C. ureolyticus [59,61,63,64], and C. upsaliensis [45,51,52,54,55,56,57,59,60,61,64] (Table S1). The best results were obtained using polycarbonate instead of cellulose filters [60,63], with pore sizes of 0.6 µm instead of 0.45 µm [58,63,64], and incubating plates in H2-enriched microaerobic atmosphere [53,54,64] at 37 °C instead of 42 °C [54], for five days instead of two days [40,50] (Table S1).
Though, regarding C. jejuni and C. coli, comparison of isolation rates obtained by filtration culture and culture on different selective media, i.e., Skirrow, Butzler, modified Charcoal-Cefoperazone-Deoxycholate agar (mCCDA), and Cefoperazone-Amphotericin B-Teicoplanin (CAT) media, showed non-significant and contradictory differences depending on the filters and selective media used [32,40,49,52,56,60,61,64,66]. Consequently, filtration culture has never been recommended as a substitute for, but rather in complement to, culture on selective media, notably for paediatric or immunosuppressed patients, or in the case of an outbreak with no identified pathogen [32,46,49,60,64,66,67]. Moreover, filtration is labour-intensive and lengthy. In addition, the pathogenicity of the most common fastidious non-jejuni/coli Campylobacter sp., i.e., C. concisus [68,69,70,71], C. curvus [65], and C. ureolyticus [72,73,74], remains to be clarified [75,76,77,78,79]. This is particularly controversial when one considers, for example, that C. concisus has been detected in the saliva of healthy carriers [80] or that C. concisus [47] and C. ureolyticus [81] are found in statistically similar proportions in the stools of diarrhoeal patients and healthy individuals. Furthermore, C. upsaliensis infections, whose clinical relevance is better established [82,83,84,85], appear to be far less common than those caused by C. jejuni and C. coli [86]. As a result, selective media were introduced into routine microbiology in the 1980s [87], eventually leading to the recognition of campylobacteriosis as the leading bacterial cause of human gastroenteritis worldwide [75,88,89] and the most reported zoonosis in Europe since 2005 [86], with over more than twice as many reported cases as salmonellosis [86,90]. In fact, in the European Union (EU) and the United States, Campylobacter sp. infections are reported to be responsible for more than 10,000 and nearly 2000 hospitalisations per year, respectively [86,90,91,92]. In both regions, this has resulted in about 30 deaths per year [86,90,91,92]. However, filtration culture remains limited to a few reference laboratories, mostly for clinical epidemiological research rather than routine diagnosis [55,61,63,64,66]. Consequently, thermophilic C. jejuni and C. coli account for over 95% of reported cases [86], while most cases of acute gastroenteritis are of unknown aetiology [93,94], which is acceptable because these infections tend to be self-limiting. Additionally, when gastroenteritis is documented, viral causes are more common than bacterial causes [95].
In contrast, the genus Campylobacter currently comprises 45 species and 13 subspecies [96], of which more than 10 are associated with human infections [68,75,79]. Recently, non-jejuni/coli Campylobacter species have been associated with inflammatory bowel disease (IBD). In 2009, Zhang et al. [97] noted higher PCR detection of C. concisus in intestinal biopsies from children with Crohn’s disease than in controls (51% vs. 2%; p < 0.0001). Again, in 2010, Man et al. [98] reported a higher prevalence of PCR-detected C. concisus in stools of newly diagnosed Crohn’s disease patients than in healthy and non-inflammatory bowel disease controls (65% vs. 33% vs. 37%, respectively; p < 0.05). Then, in 2011, Mukhopadhya and colleagues [99] showed significantly higher prevalence rates of C. concisus and C. ureolyticus in colon biopsy specimens from adults with ulcerative colitis than in healthy controls (p < 0.01). Finally, in 2012, the C. concisus incidence rate among 8302 patients presenting gastroenteritis in North Jutland, Denmark, was measured at 35/100,000 inhabitants, almost as high as the C. jejuni plus C. coli incidence rate [100].
However, they are still seriously underdiagnosed [51,54,61,66,75,77], though with the development of genomics over the last two decades, the diversity of Campylobacter sp. has re-emerged [101]. In addition, rapid supplanting of culture-based methods in routine diagnostics by commercial PCR assays, also called culture-independent diagnostic tests, for the detection of mainly C. jejuni, C. coli, and, to a lesser extent, C. upsaliensis [102,103], is ongoing. As a result, funding for the culture of fastidious Campylobacter species is more threatened than ever.
Finally, a 28.1% global decrease in the annual notification rate of campylobacteriosis was observed among the 27 EU Member States when comparing the 2017–2019 period to 2021 [90]. Notably, the number of travel-associated cases was significantly lowered [90]. Indeed, in 6 out of 27 countries, including Belgium, a statistically significant decrease in the number of confirmed cases was observed when comparing the pre-COVID-19 period to 2021. This contrasts with the statistically significant increasing trend observed over the preceding seven-year period of 2008–2014 [104]. This decline was particularly evident in 2020, which was severely affected by the pandemic, resulting in a reduction in international travel [86,90,105]. In fact, this decreasing tendency between 2021 and the pre-COVID-19 period was observed for all zoonosis in Europe, with the exception of tularaemia [90]. In 2022, the notification rate in most EU Member States did not match that of recent pre-pandemic years [86].
In contrast, in 2020, Kuhn et al. [106] predicted a 25 and 196% increase in campylobacteriosis incidences by the end of the 2040s and the 2080s, respectively, in the four Nordic countries (i.e., Finland, Sweden, Norway, and Denmark), secondary to global warming and increases in precipitation, particularly heavy rainfall.
Considering all the upheavals in the clinical approach to Campylobacterales, this longitudinal clinical study aimed to evaluate their diversity among clinical samples, as well as the ongoing epidemiological dynamics in Brussels, Belgium, using an optimised filtration culture in parallel with selective inhibitory medium culture of stools collected for routine diagnosis.
2. Materials and Methods
2.1. Stool Samples
Over a 6-year period, from 2017 to 2019 (pre-COVID-19 period) and 2021 to 2023 (post-COVID-19 period), a total of 51,065 stool samples sent from 4 university hospitals in Brussels, Belgium, to the Laboratoire Hospitalier Universitaire de Bruxelles-Brussel Universitair Laboratorium (LHUB-ULB) for testing for bacterial gastrointestinal pathogens, were considered for analysis. Samples collected in 2020 were excluded because some culture reagents, in particular filters, were not available in the LHUB-ULB for more than six months due to the COVID-19 pandemic. Samples obtained from a fifth partner university hospital were excluded, as a distinct culture methodology was employed in the years 2017 and 2018.
Strains belonging to the same species exhibiting the same antibiotic susceptibility profile, isolated from stools collected within less than a one-month interval from the same patient, were considered as related to the same clinical episode. Only the first collected stool from each episode was included in the study, with the following stools being considered as duplicates and excluded from positive stool statistics.
2.2. Culture
Upon arrival at the LHUB-ULB, the samples were stored at 4 °C. They were inoculated into media within 24 h of their arrival, with the exception of samples received on Friday after 3 pm and on Saturday, which were inoculated on the following Monday (within 72 h of their arrival). For Campylobacterales isolation, samples were plated on a selective inhibitory agar medium (Campylobacter Selective Agar (Butzler)™, ThermoFisher Scientific, Waltham, MA, USA) and incubated for 48 h at 40–43 °C in a microaerobic atmosphere (85% N2—10% CO2—5% O2—0% H2). In parallel, 2 mL of liquid stool (or liquefied soft stool by the addition of isotonic saline) was diluted 1:3 in a Brucella broth (EO Labs, Cumbernauld, UK) and incubated for 30 min at 35–38 °C in the same atmosphere as used in the same atmosphere as used for Butzler plates Butzler plates (85% N2—10% CO2—5% O2—0% H2). Six drops of this broth were then transferred to the surface of a 0.6 µm polycarbonate filter (Whatman™ Nuclepore™ Hydrophilic Membrane, Cytiva, Marlborough, MA, USA), placed on an antibiotic-free Columbia agar containing 5% sheep’s blood (BD™), and incubated at 35–38 °C in the same atmosphere again (85% N2—10% CO2—5% O2—0% H2). The Brucella broth was re-incubated for a further 30 min. Then, a further six drops of the re-incubated broth were transferred to the surface of the same filter and the Columbia agar was re-incubated for a further 30 min. Finally, the filters were removed, and the Columbia agars were incubated for five days at 35–38 °C in a microaerobic atmosphere enriched with H2 (80% N2—7% CO2—6% O2—7% H2).
2.3. Identification
Butzler plates were examined after 24 and 48 h of incubation, while Columbia plates were examined after 48 h and 5 days of incubation. All growing colonies were identified using matrix-assisted laser desorption ionisation–time of flight mass spectrometry (MALDI-TOF MS; Biotyper database; Bruker Daltonics, Bremen, Germany). The databases utilized were those that have been employed over time. Each new iteration was validated using a diverse panel of Campylobacter species, in accordance with our quality assurance protocols.
2.4. Statistical Analyses
The proportions of Campylobacter isolates from the pre-COVID-19 (2017–2019) and post-COVID-19 (2021–2023) pandemic periods were compared using the Chi-squared test, and continuous data were compared using the non-parametric Mann–Whitney test. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Prevalence and Species Repartition
The mean positivity rate of stool cultures for Campylobacterales over the entire study period was 7.1%, with annual positivity rates ranging from minimum 5.9% (2018) to maximum 8.0% (2021). Sixteen species of the genus Campylobacter and four species of related organisms were identified. Throughout the study period, C. concisus and C. curvus represented 36.4–51.2% and 4.3–9.8% (minimum–maximum), respectively, of annual isolates, totalling 1600 and 272 strains, whereas C. jejuni and C. coli represented only 20.9–32.2% and 1.9–5.0% (minimum–maximum), respectively, of annual isolates, totalling 970 and 112 strains (Table 1). Interestingly, 447 C. ureolyticus strains were isolated, representing 3.7–23.0% of the annual isolates (minimum in 2018—maximum in 2023, respectively). It was the fourth most common species after C. coli in the pre-COVID-19 period, but the third most common in the post-COVID-19 period, with almost the same incidence as C. jejuni infections. C. upsaliensis and Aliarcobacter butzleri were the next most common species isolated, averaging 1.5 and 1.4% annually (n = 53 and 50 strains, respectively; Table 1).

Table 1.
Species prevalence of Campylobacterales in 51,065 stools at the Department of Microbiology, LHUB-ULB, Belgium, from 1 January 2017 to 31 December 2023, using Butzler selective medium plus filtration culture with a 0.6 µm pore size polycarbonate filter and Columbia agar. Percentages indicate the proportion of each species in the total number of strains of Campylobacterales in a year.
Another thirteen species combined accounted for 2.8% of the total. Among them, 45 strains belonging to 10 additional Campylobacter species were identified, i.e., C. showae (n = 25), C. hyointestinalis (n = 7), C. fetus (n = 5), C. gracilis (n = 2), C. lari (n = 2), C. hominis (n = 1), C. rectus (n = 1), C. sputorum (n = 1), C. lanienae (n = 1), and C. peloridis (n = 1), accounting in total for only 1.3% of all isolates. In addition to Campylobacter sp. isolates, 44 strains from 2 Helicobacter species were identified, namely, H. pullorum (n = 33) and H. cinaedi (n = 11), representing 1.2% of all isolates. Finally, in addition to A. butzleri, 10 Aliarcobacter cryoaerophilus strains were isolated (Table 1).
3.2. Dynamics from 2017 to 2023
There were 28,911 and 22,154 stool samples collected during the pre- and post-COVID-19 periods, respectively, with mean positivity rates of 6.7 and 7.5%, respectively.
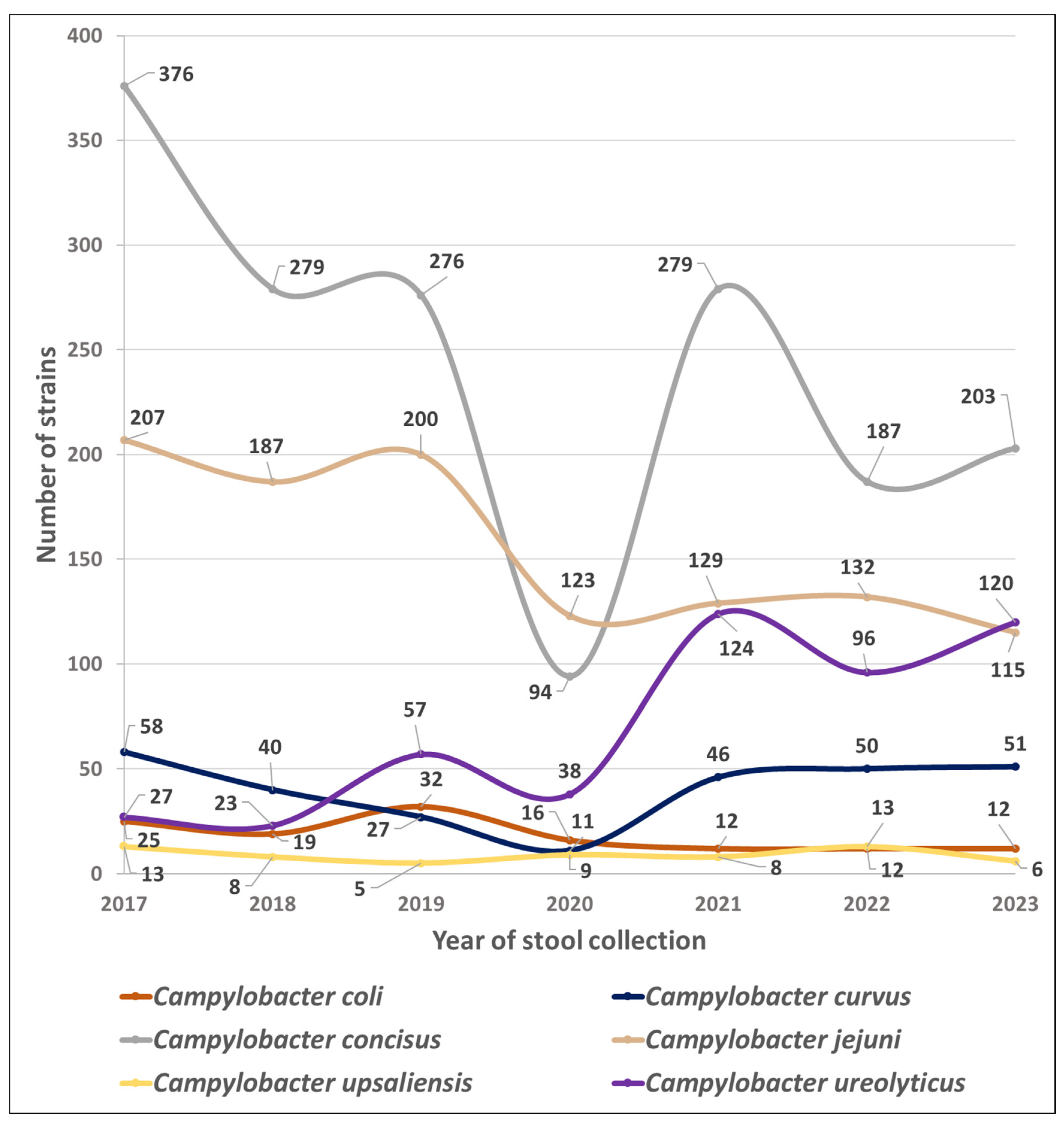
Figure 1 shows the trends in the number of isolates of the six most common Campyloacter sp. collected throughout the study period.
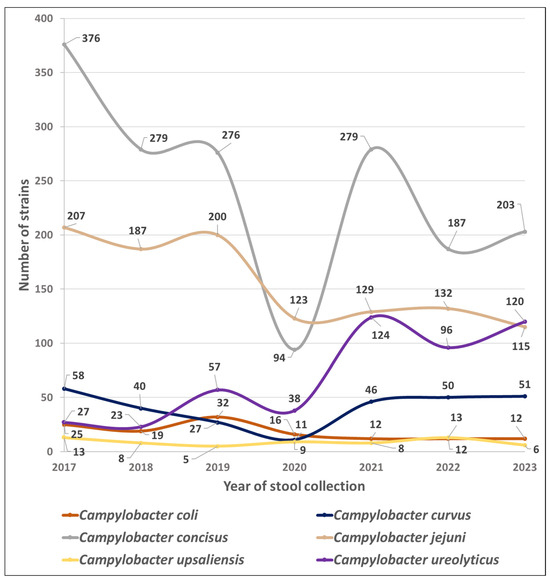
Figure 1.
Species prevalence dynamics of Campylobacterales in 51,065 stools at the Department of Microbiology, LHUB-ULB, Belgium, from 1 January 2017 to 31 December 2023, using Butzler selective medium plus filtration culture with a 0.6 µm pore size polycarbonate filter and Columbia agar. Percentages indicate the proportion of each species in the total number of strains of Campylobacterales in a year.
In contrast between these two time periods, the mean numbers of C. jejuni, C. coli, and C. concisus isolates decreased by 37, 53, and 28%, respectively (p < 0.05), while the mean number of C. curvus isolates increased by 18%, and those of C. upsaliensis isolates remained stable. Mean numbers of Aliarcobacter sp. and Helicobacter sp. also decreased after the COVID-19 pandemic by 24 and 57%, respectively.
Finally, C. ureolyticus, which accounted for only 3.5, 4.0, and 9.0% of the yearly isolates in 2017, 2018, and 2019, respectively, accounted for 20.1, 18.7, and 23.0% of the yearly isolates in 2021, 2022, and 2023 (p < 0.05; Table 1).
3.3. Seasonal Prevalence
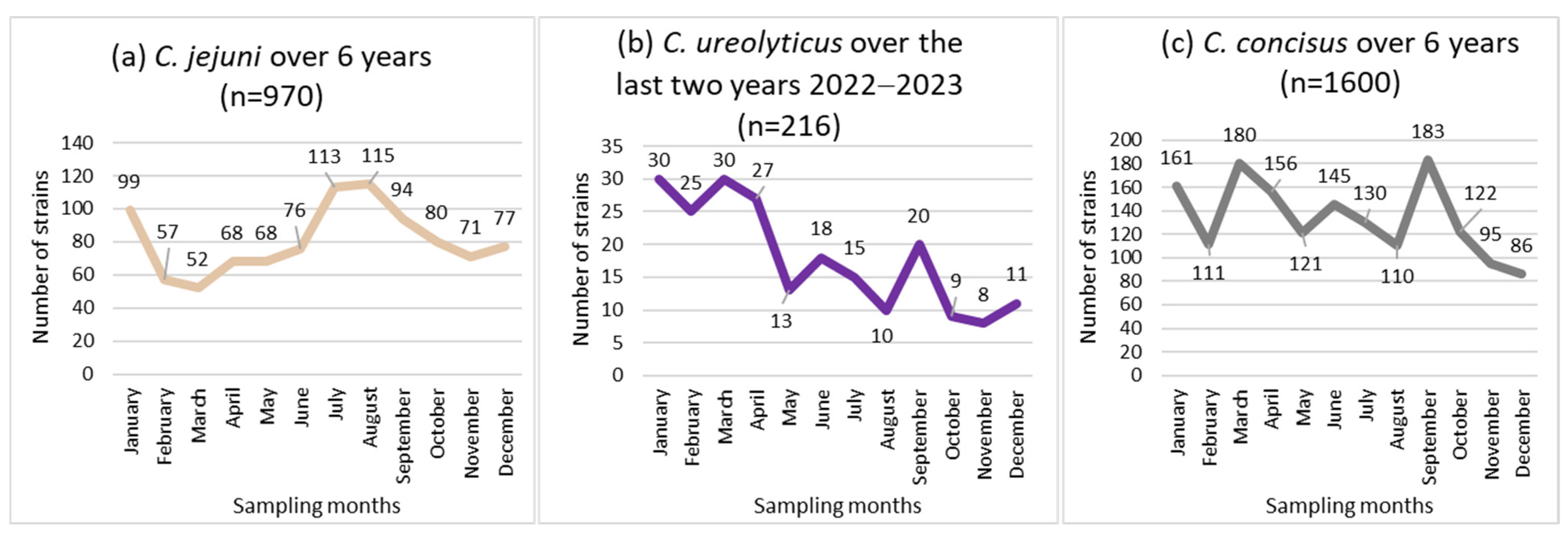
Non-uniform distributions of the incidence of C. jejuni and C. ureolyticus were found across the 12 months of the year (p < 0.05; Figure 2a,b), while the incidence of C. concisus and C. curvus showed no seasonal trend (Figure 2c).
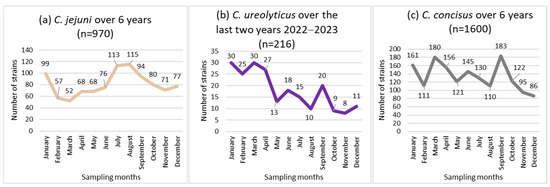
Figure 2.
Seasonal distribution of the most prevalent Campylobacter species, i.e., C. jejuni (a), C. concicus (b), and C. ureolyticus (c) in 51,065 stools at the Department of Microbiology, LHUB-ULB, Belgium, from 1 January 2017 to 31 December 2023, excluding year 2020 using Butzler selective medium plus filtration culture, with a 0.6 µm pore size polycarbonate filter and Columbia agar.
C. jejuni isolates peaked twice each year, in January (99 isolates) and in July (113 isolates)/August (115 isolates), representing 33.7% of the total (970 isolates; Figure 2a).
C. ureolyticus incidence increased from January to April (51.9% of the total number of isolates), and decreased from May to December, with the fewest C. ureolyticus detections in November (Figure 2b).
3.4. Age Distribution
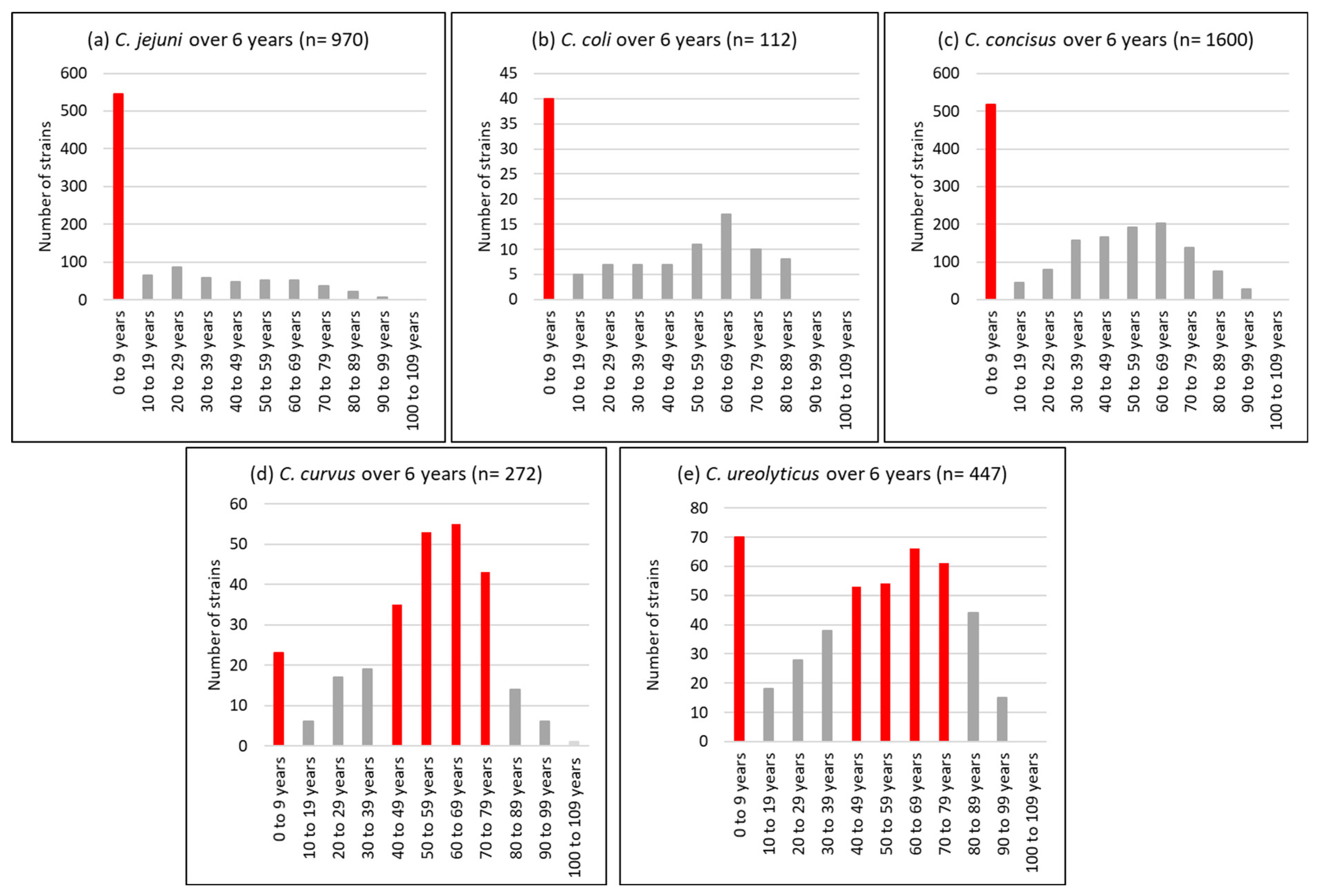
With regard to the age profile, a unimodal distribution was observed for C. jejuni isolates, 56% of which were detected in children under the age of 10 years. Conversely, C. curvus and C. ureolyticus were bimodally distributed, with 13 and 23% detected in children under 10 years and 42 and 25% isolated from patients aged 50 to 69 years (Figure 3).
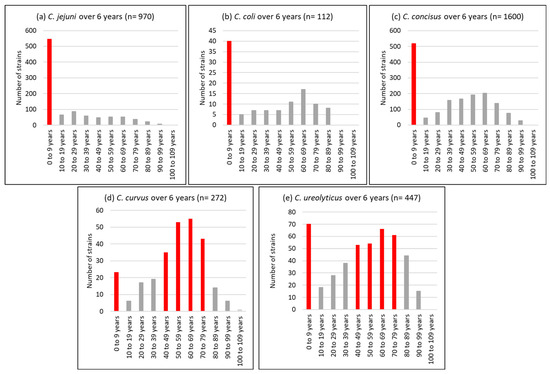
Figure 3.
Age profile of the most prevalent Campylobacter species, i.e., C. jejuni (a), C. coli (b), C. concisus (c), C. curvus (d), and C. ureolyticus (e) in 51,065 stools at the Department of Microbiology, LHUB-ULB, Belgium, from 1 January 2017 to 31 December 2023, excluding year 2020 using Butzler selective medium plus filtration culture, with a 0.6 µm pore size polycarbonate filter and Columbia agar.
4. Discussion
4.1. Prevalence and Species Repartition
In line with the literature on stool filtration culture, the species distribution among Campylobacter sp.-positive samples reflected a much greater diversity than that commonly described by the vast majority of routine diagnostic laboratories [86,87,105] using either standard culture on selective inhibitory medium or commercial multiplex PCR assays (e.g., BD Max, Seegene, Qiastat, or FilmArray assays), which only allow the detection of C. jejuni, C. coli, and rarely, C. upsaliensis and C. fetus.
Firstly, C. concisus was the most common isolated species in our laboratory (Table 1). As it was twice as frequent as C. jejuni, it seems to be the most common Campylobacter species encountered in the gastrointestinal tract.
These findings agree with the literature. Indeed, when the Cape Town protocol [50] (0.6 μm pore cellulose acetate filters and tryptose agar plates containing 10% un-lysed sheep or horse blood) was employed as an alternative to yeast-enriched blood agar plates incubated in a H₂-enriched microaerobic atmosphere, Lastovica et al. [55] discovered that 24.63% of the 5443 Campylobacterales strains isolated from a paediatric population were C. concisus (Table S1). Subsequently, Nielsen et al. [60] demonstrated that polycarbonate is a more efficient stool filtration material than cellulose acetate using 0.6 μm pore size filters: 26% more C. concisus strains was observed in 1791 diarrhoeal stools collected in Denmark in 2012 after replacing cellulose acetate with polycarbonate (114/134 vs. 79/134; p < 0.0001) (Table S1). The authors suggested that the smooth, glassy surface of the polycarbonate filter would be more suitable for the penetration of motile Campylobacter sp. than the rough surface of the cellulose acetate filter, which could catch random particles and block Campylobacter sp. from passing through the filter [60].
In contrast, only 1.9% of 1394 stool isolates were C. concisus (n = 27) in a study by Vandenberg et al. [56] in Belgium, who adapted the Cape Town protocol, replacing tryptose agar plates containing 10% un-lysed blood with Mueller–Hinton agar plates with 5% sheep blood agar, and 0.6 μm with 0.45 μm pore size cellulose filters (Table S1). This could be explained by the fact that the 0.45 µm pore size is too small for motile Campylobacter sp., especially C. concisus, to efficiently penetrate the filter.
The superior efficiency of the polycarbonate filter with a 0.6 μm pore size over the cellulose filter with 0.45 μm pore size for the isolation of non-jejuni/coli Campylobacter sp. was again confirmed by Nachamkin et al. in 2017 [63] and by our team in 2019 [64] (Table S1). In addition, we showed that C. concisus isolation was also facilitated by the use of Columbia agar instead of blood-enriched Mueller–Hinton agar incubated in an H2-enriched atmosphere. With regard to C. curvus and C. ureolyticus, although studies [40,51,52,55,56,61,63,64] include small numbers of isolates, the use of 0.45 μm pore size filters also appears to be a dramatic limiting factor on their growth (Table S1). Indeed, in our laboratory, when we switched from 0.6 μm pore size polycarbonate filters, which were out of stock due to the COVID-19 pandemic, to 0.45 μm pore size cellulose acetate filters (Porafil CA, Macherey-Nagel) from March to September 2020, we observed a more than 90% decrease in C. concisus, C. ureolyticus, and C. curvus isolation rates.
Only 53 C. upsaliensis strains were isolated from 51,065 stool samples, resulting in a prevalence of 0.1% (Table 1). These results are consistent with those reported in Denmark in 2000 by Engberg et al. [40]. Using three selective media and a filtration technique, they were unable to isolate C. upsaliensis from 1376 clinical stool samples from patients with diarrhoea (Table S1). Also in Denmark, Nielsen et al. [60] isolated only 1 C. upsaliensis strain from 1791 diarrhoeal stools (prevalence: 0.06%) collected in 2012 using the same technique, and 2 years later, cultures yielded only 5 C. upsaliensis strains from 5963 diarrhoeal stools (prevalence: 0.08%) [61] (Table S1). The same observation was made by Nachamkin et al. [63] in Pennsylvania, US, who isolated 7 distinct Campylobacter species, but not C. upsaliensis, from 225 faecal samples collected in 2016. This was despite performing filtration culture on three types of filters: cellulose acetate filters of 0.45 and 0.65 μm pore sizes, and polycarbonate filters of 0.6 μm pore size, as well as Brucella agar plates containing 5% sheep blood, hemin, and vitamin K (Table S1). More recently, our team [64] attempted to optimize the filtration technique by evaluating the influence of the agar (Mueller–Hinton versus Columbia agar, both containing 5% sheep blood), the filter (0.6 μm polycarbonate versus 0.45 μm cellulose acetate), and the atmosphere (7% H2-enriched versus non-H2-enriched microaerobic atmosphere) on more than 2000 stool samples collected in Brussels, Belgium, in 2014, 2016, and 2018. Irrespective of the parameters, less than one in five hundred stool cultures yielded C. upsaliensis (prevalence <0.2%) (Table S1). This is again in line with a previous study carried out in our laboratory in 2004, where only 85 C. upsaliensis were isolated from 67,599 stools (prevalence: 0.13%) collected between 1995 and 2002, performing filtration culture with Mueller–Hinton agar containing 5% sheep blood and a 0.45 μm pore size cellulose acetate filter [56] (Table S1).
However, significantly higher isolation rates of C. upsaliensis from stool samples were reported by Goossens et al. in Belgium in 1989 (prevalence: 0.73%) [46] and Lindblom et al. in Sweden in 1993 (prevalence: 0.82%) [84]. Furthermore, in 2000, the Cape Town protocol allowed 4122 strains belonging to Campylobacter and related species to be isolated from 19,535 stool samples collected from children with diarrhoea in Cape Town, South Africa, between 1990 and 2000, of which 23% were identified as C. upsaliensis (prevalence: 4.9%) [51].
It can be hypothesized that the choice of an appropriate growth medium, e.g., tryptose agar containing 10% un-lysed sheep or horse blood, as recommended by the Cape Town protocol, is an overriding constraint for the culture of C. upsaliensis. Therefore, the prevalence of C. upsaliensis in Belgium may be higher than that reported here. Further studies comparing agar media among themselves or with results obtained by molecular techniques are needed to clarify this point. However, local animal-to-human and human-to-human transmission, especially among young children, travellers, and immigrants, may contribute to the higher prevalence observed in some cohorts in some world regions compared to Belgium [107].
As far as Aliarcobacter sp. are concerned, A. butzleri and A. cryoaerophilus have persistently represented less than 1.5% of isolates per year (Table 1). Conversely, Vandenberg et al. [56], in the same laboratory, reported that 4.0% of the 1906 isolates obtained from 67,599 stools collected between 1995 and 2002 belonged to the genus Aliarcobacter. Nevertheless, 71 of the 77 strains were isolated only by the method of De Boer et al. [108], which consists of 24 h selective enrichment of 0.5 g of stool in Brucella broth supplemented with antibiotics, followed by culture on Aliarcobacter selective plates incubated for 3 days at 25 °C in a microaerobic atmosphere. Specific conditions for culturing Aliarcobacter sp. were also recommended by Lastovica and Le Roux [50], according to the Cape Town protocol. By not following these recommendations, Lastovica et al. failed to isolate A. cryoaerophilus strains from 19,535 (2000) and more than 20,000 (2006) diarrhoeal stools, and A. butzleri isolates represented only 0.39 and 0.36% of all strains grown in these studies [51,55]. As a result, the prevalence of Aliarcobacter sp. in clinical stools reported here may be underestimated. It also confirms the need for using a culture method suitable for Aliarcobacter sp. that includes incubation below 35 °C.
4.2. Dynamics from 2017 to 2023
The mean number of C. ureolyticus annual isolates increased by 218% (from 36 to 113; p < 0.05; Table 1) between the pre- and post-COVID-19 periods, even exceeding the C. jejuni rate in 2023 (Figure 1). At the same time, the mean annual number of C. jejuni, C. coli, and C. concisus isolates decreased by 37, 53, and 28% (from 198 to 125, 25 to 12, and 310 to 223, respectively; p < 0.05; Table 1). Implementing hygienic measures in 2020 may also have accelerated an existing trend. However, it is interesting to note that the annual isolation rate of C. ureolyticus had already doubled between 2018 and 2019 (from 23 to 57, respectively; Table 1), before the COVID-19 pandemic. As the variations are in opposite directions, they cannot be explained by a global decrease or increase in laboratory activity, even though 28,911 stools were cultured in the pre-COVID-19 period, while only 22,154 were analysed in the post-COVID-19 years.
Such a high proportion of C. ureolyticus among stool-detected Campylobacter sp. was previously reported by Bullman et al. in 2011 [109], before the COVID-19 pandemic, conducting in-house genus- and species-specific PCR testing of 7194 samples. In this study, 27.3% of the 373 Campylobacter sp. detected in the stools of 349 diarrhoeal patients in Southern Ireland were non-jejuni/coli Campylobacter species, of which 81.7% were actually C. ureolyticus (n = 83 stools). In addition, Hatanaka et al. [110] reported that 51.9% of Campylobacter sp. detected by PCR in the stools of children with diarrhoea in Japan were C. ureolyticus. Conversely, using PCR, Collado et al. [78] detected C. ureolyticus in low and statistically similar proportions in stools from both diarrhoeal and healthy groups.
To confirm the increase in the presence of C. ureolyticus in human clinical samples and explain the possible partial replacement of C. jejuni, C. coli, and C. concisus with C. ureolyticus, further studies are needed, especially on stools collected in other regions of the world. Indeed, several authors have predicted an increase in the incidence of campylobacteriosis in Europe and Asia as a consequence of climate change, together with an increase in temperature, humidity, and especially, heavy precipitation [111,112,113]. However, these predictions may not apply to C. ureolyticus, as very little is known about its reservoir and transmission route to humans [114,115]. In addition, studies over a longer period of time, together with climate observations, are needed in order to make further assumptions.
4.3. Seasonality
In line with the literature, C. jejuni incidence peaked in July/August [86,90,109], whereas C. ureolyticus incidence increased from January to April [109] (coinciding with increased cattle shedding) [72,73,74] (Figure 2). Notably, C. ureolyticus has been detected by PCR in unpasteurised milk in Ireland [114] and in cat, cow, and pig faeces [115]. This is consistent with studies suggesting that C. ureolyticus is not human commensal [72,74]. Furthermore, regarding Campylobacter sp. bacteraemia, Tinévez et al. [116] reported that in a nationwide study conducted in France from 2015 to 2019, 22 out of 592 episodes (3.7%) were attributed to C. ureolyticus, which was the fourth most frequently isolated species after C. jejuni, C. fetus, and C. coli (42.9, 42.6, and 6.8%, respectively). This also indicates the potential invasiveness of this species, for which an increasing number of putative virulence factors have been described [72,73,74]. Moreover, when C. ureolyticus was isolated, it was observed that in over 95% of cases, no additional Campylobacterales were present, thus supporting its involvement in the observed symptoms.
Besides, the incidence of C. concisus showed no seasonal trend (Figure 2), as observed in Denmark by Nielsen et al. [100]. This tends to indicate that humans commensally carry this species, in agreement with Zhang et al. [80] and Macuch et al. [117], who suggested that the human oral cavity may serve as a reservoir for this species. Consistent with this hypothesis, neither Tinévez et al. [116], as the French Campylobacter NRC from 2015 to 2019, Lastovica et al. [55] in South Africa from 1990 to 2005, nor our laboratory, as the Belgium Campylobacter NRC from 2014 to 2023 [118], reported C. concisus bacteraemia. In addition, only two C. curvus blood isolates were obtained in France. This does not support the invasiveness of these two species, especially in view of the important detection rate of C. concisus in human stools. The possibility exists that cases of C. concisus and C. curvus bacteraemia remain undiagnosed owing to the inability of the bacteria to grow in a commercial blood culture bottle, in particular due to a lack of H2 enrichment of the atmosphere. However, the susceptibility of C. concisus to the bactericidal effects of human serum has been clearly demonstrated. Consequently, this bacterium is likely to be less able to cause bacteraemia [119].
4.4. Age Distribution
Our results (Figure 3) agree with those of Bullman et al. [109], with C. ureolyticus and C. curvus being more prevalent at the two extremes of life when immunity is compromised, in contrast to C. jejuni, whose cases were mostly reported in children. This suggests that C. ureolyticus is more of an opportunistic pathogen. However, three putative virulence and colonisation factors, the surface antigen CjaA, an outer membrane fibronectin-binding protein, and an S-layer RTX toxin, have been detected in its secretome [72].
5. Conclusions
In conclusion, 3604 strains belonging to 20 species of Campylobacter and related organisms were isolated from 51,065 stool samples collected over 6 years (2017–2019 and 2021–2023, pre- and post-COVID-19 periods, respectively) using filtration culture. The most frequently detected species was C. concisus, which accounted for almost half of all isolates. Meanwhile, the annual isolation rate of C. ureolyticus increased by 218% between pre- and post-COVID-19 periods. This raises concerns about the pathogenic potential of these species, particularly in vulnerable patients at risk of non-self-limiting infections. Currently, a growing number of laboratories are diagnosing campylobacteriosis using commercial multiplex PCR assays, the efficacy of which remains to be evaluated in the context of such emerging species. Furthermore, these assays provide no information about antimicrobial resistance. It is, therefore, recommended that a routine reflex culture be carried out in the event of a positive multiplex PCR result. In the event of a negative result in a patient exhibiting persistent gastrointestinal symptoms with an unidentified aetiology, a control stool sample may also be collected and submitted to an NRC that performs filtration culture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13060475/s1, Table S1: Filtration culture review.
Author Contributions
Conceptualisation, E.G. and D.M.; methodology, E.G. and D.M.; formal analysis, E.G.; data curation, E.G.; writing—original draft preparation, E.G.; writing—review and editing, D.M.; supervision, D.M. and V.Y.M.D.; project administration, D.M. and V.Y.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In accordance with the Belgian law on human experimentation, and considering the retrospective and non-interventional nature of the present study, in which no medical records were consulted and no personal health data were collected, the study did not require the approval of an ethics committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Escherich, T. Beitrage zur Kenntniss der Darmbacterien. II. Ueber das Vorkommen von Vibrionen im Darmcanal und den Stuhlgangen der Sauglinge. (Articles adding to the knowledge of intestinal bacteria. III. On the existence of vibrios in the intestines and feces of babies.). Münchener. Med. Wochenschr. 1886, 33, 815–817. [Google Scholar]
- McFadyean, J.; Stockman, S. Report of the Departmental Committee Appointed by the Board of Agriculture and Fisheries to Inquire into Epizootic Abortion. Part III. Abortion in Sheep; HMSO: London, UK, 1913. [Google Scholar]
- Smith, T. Spirilla associated with disease of fetal membrane in cattle. J. Exper. Med. 1918, 28, 701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, T. Etiologic relation of spirilla (Vibrio fetus) to bovine abortion. J. Exper. Med. 1919, 30, 313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, T.; Taylor, M.S. Some morphological and biological characters of Spirilla (Vibrio foetus n.sp.) associated with disease of fetal membranes in cattle. J. Exp. Med. 1919, 30, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. Etiologic significance of Vibrio fetus. J. Exper. Med. 1920, 32, 683. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Little, R.B.; Taylor, M.S. Further studies on the etiological r6le of Vibrio fetus. J. Exp. Med. 1923, 37, 352. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.; Marsh, H. Vibrionic abortion in sheep. J. Am. Vet. Med. Asso. 1924, 65, 203. [Google Scholar] [CrossRef]
- Welch, H.; Marsh, H. Vibrionic abortion in sheep. J. Am. Vet. Med. Asso. 1930, 76, 568. [Google Scholar]
- Graham, R.; Thorp, F. Vibrionic abortion in sheep. J. Am. Vet. Med. Asso. 1930, 76, 568. [Google Scholar]
- Gilman, H.L. Vibrionic abortion in sheep. Rep. N. Y. State Cornell Vet. Coll. 1932, 33, 81. [Google Scholar]
- Stegenga, T.; Terpstra, J. Over Vibrio fetus infecties bij het rund en enzootishe steriliteit. Tijdschr. Diergeneeskd. 1949, 74, 293–296. [Google Scholar]
- Jones, F.S.; Orcutt, M.; Little, R.B. Vibrio associated with intestinal disorder of cows and calves. J. Exper. Med. 1931, 53, 853. [Google Scholar] [CrossRef] [PubMed]
- Politzer, R. Further observations on a cholera-like vibrio. Rep. Nat. Quar. Serv. 1935, 36, 70. [Google Scholar]
- Doyle, L.P. A vibrio associated with swine dysentery. Am. J. Vet. Res. 1944, 5, 3–5. [Google Scholar]
- Smith, T.; Orcutt, M.L. Vibrios from calves. Their serologic relationship to Vibrio fetus. J. Exper. Med. 1927, 45, 491. [Google Scholar] [CrossRef] [PubMed]
- Florent, A. Les deux vibrioses génitales de la bête bovine: La vibriose vénérienne, due à Vibrio fœtus venerialis, et la vibriose d’origine intestinale due à V. fœtus intestinalis. In Proceedings of the 10th International Veterinary Congress Madrid 1959, Madrid, Spain, 21–27 May 1959; Volume 2, pp. 953–957. [Google Scholar]
- Vinzent, R.; Dumas, J.; Picard, N. Septicémie grave au cours de la grossesse due à un Vibrion. Bull. Acad. Nat. Med. Paris 1947, 131, 90–92. [Google Scholar]
- King, E.O. Human infections with Vibrio fetus and a closely related vibrio. J. Infect. Dis. 1957, 101, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, W.E.; Borchers, J. Vibrionic enteritis in infants. Am. J. Dis. Child. 1961, 101, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp, J.M.; Wolf, H.A. Infection due to a ‘related’ vibrio. J. Pediatr. 1961, 59, 318–321. [Google Scholar] [CrossRef]
- White, W.D. Human vibriosis: Indigenous case in England. BMJ 1967, 2, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Darrell, J.H.; Farrell, B.C.; Mulligan, R.A. Case of human vibriosis. BMJ 1967, 2, 287–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebald, M.; Veron, M. Teneur en bases de l’ADN et classification des vibrions. Ann. Inst. Pasteur 1963, 105, 897–910. [Google Scholar]
- Dekeyser, P.; Gossuin-Detrain, M.; Butzler, J.P.; Sternon, J. Acute enteritis due to a related vibrio: First positive stool cultures. J. Infect. Dis. 1972, 125, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Butzler, J.P.; De Boeck, M.; Goossens, H. New selective medium for isolation of Campylobacter jejuni from faecal specimens. Lancet 1983, 321, 818. [Google Scholar] [CrossRef] [PubMed]
- Skirrow, M.B. Campylobacter enteritis: A ‘new’ disease. BMJ 1977, 2, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Bolton, F.J.; Robertson, L. A selective medium for isolating Campylobacter jejuni/coli. J. Clin. Pathol. 1982, 35, 462–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolton, F.J.; Coates, D.; Hinchliffe, P.M.; Robertson, L. Comparison of selective media for isolation of Campylobacter jejuni/coli. J. Clin. Pathol. 1983, 36, 78–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolton, F.J.; Hutchinson, D.N.; Coates, D. Blood-free selective medium for isolation of Campylobacter jejuni from feces. J. Clin. Microbiol. 1984, 19, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; De Boeck, M.; Butzler, J.P. A new selective medium for the isolation of Campylobacter jejuni from human faeces. Eur. J. Clin. Microbiol. 1983, 2, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; De Boeck, M.; Coignau, H.; Vlaes, L.; Van den Borre, C.; Butzler, J.P. Modified selective medium for isolation of Campylobacter spp. from feces: Comparison with Preston medium, a blood-free medium, and a filtration system. J. Clin. Microbiol. 1986, 24, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Simor, A.E.; Roscoe, M.; Fleming, P.C.; Smith, S.S.; Lane, J. Evaluation of a blood-free, charcoal based, selective medium for the isolation of Campylobacter organisms from feces. J. Clin. Microb. 1986, 23, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Eley, A.; Clarry, T.; Bennett, K.W. Selective and differential medium for isolation of Bacteroides ureolyticus from clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.L.; Karmali, M.A. Direct isolation of atypical Campylobacter species from human feces on selective agar medium. J. Clin. Microbiol. 1989, 27, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Endtz, H.P.; Ruijs, G.J.; Zwinderman, A.H.; van der Reijden, T.; Biever, M.; Mouton, R.P. Comparison of six media, including a semisolid agar, for the isolation of various Campylobacter species from stool specimens. J. Clin. Microbiol. 1991, 29, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aspinall, S.T.; Wareing, D.R.; Hayward, P.G.; Hutchinson, D.N. Selective medium for thermophilic campylobacters including Campylobacter upsaliensis. J. Clin. Pathol. 1993, 46, 829–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Piersimoni, C.; Bornigia, S.; Curzi, L.; De Sio, G. Comparison of two selective media and a membrane filter technique for isolation of Campylobacter species from diarrhoeal stools. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Corry, J.E.; Post, D.E.; Colin, P.; Laisney, M.J. Culture media for the isolation of campylobacters. Int. J. Food Microbiol. 1995, 26, 43–76. [Google Scholar] [CrossRef] [PubMed]
- Engberg, J.; On, S.L.; Harrington, C.S.; Gerner-Smidt, P. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. J. Clin. Microbiol. 2000, 38, 286–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, K.W.; Mattick, K.L.; Harrison, M.; Humphrey, T.J. Evaluation of selective media for Campylobacter isolation when cycloheximide is replaced with amphotericin B. Lett. Appl. Microbiol. 2002, 34, 124–129. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, A.; Koziel, M.; De Barra, L.; Corcoran, D.; Bullman, S.; Lucey, B.; Sleator, R.D. Development of nalidixic acid amphotericin B vancomycin (NAV) medium for the isolation of Campylobacter ureolyticus from the stools of patients presenting with acute gastroenteritis. Br. J. Biomed. Sci. 2014, 71, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Doherty, D.; Mooney, A.; Byrne, M.; Woodward, D.; Johnson, W.; Rodgers, F.; Bourke, B. Basis of the superiority of cefoperazone amphotericin teicoplanin for isolating Campylobacter upsaliensis from stools. J. Clin. Microbiol. 2001, 39, 2713–2716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Steele, T.W.; McDermott, S.N. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 1984, 16, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Elharrif, Z. Isolation of Campylobacter species by filtration. Eur. J. Clin. Microbiol. 1985, 4, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Butzler, J.P. Isolation of Campylobacter upsaliensis from stool specimens. J. Clin. Microbiol. 1989, 27, 2143–2144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Etterijck, R.; Breynaert, J.; Revets, H.; Devreker, T.; Vandenplas, Y.; Vandamme, P.; Lauwers, S. Isolation of Campylobacter concisus from feces of children with and without diarrhea. J. Clin. Microbiol. 1996, 34, 2304–2306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aspinall, S.T.; Wareing, D.R.; Hayward, P.G.; Hutchinson, D.N. A comparison of a new campylobacter selective medium (CAT) with membrane filtration for the isolation of thermophilic campylobacters including Campylobacter upsaliensis. J. Appl. Bacteriol. 1996, 80, 645–650. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Castillo, F.J.; Clavel, A.; Rubio, M.C. Use of a selective medium and a membrane filter method for isolation of Campylobacter species from Spanish paediatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, E.; Lastovica, A.J. The Protocol: How to Isolate the Most Campylobacters for Your Dollar, Pound, Franc, Yen, etc. In International Workshop on Campylobacter, Helicobacter and Related Organisms; Lastovica, A.J., Newell, D.G., Lastovica, E.E., Eds.; Institute of Child Heath: Cape Town, South Africa, 1998; pp. 30–33. [Google Scholar]
- Lastovica, A.J.; le Roux, E. Efficient isolation of campylobacteria from stools. J. Clin. Microbiol. 2000, 38, 2798–2799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lastovica, A.J.; Le Roux, E. Efficient isolation of Campylobacter upsaliensis from stools. J. Clin. Microbiol. 2001, 39, 4222–4223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Lastovica, A.J.; Le Roux, E. Optimal detection of Campylobacter spp. in stools. J. Clin. Pathol. 2003, 56, 480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lastovica, A.J.; Le Roux, E. Prevalence and optimal detection of C. upsaliensis in stool specimens. Clin. Infect. Dis. 2003, 36, 1624–1625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lastovica, A.J. Emerging Campylobacter spp.: The tip of the iceberg. Clin. Microbiol. Newsl. 2006, 28, 49–56. [Google Scholar] [CrossRef]
- Vandenberg, O.; Dediste, A.; Houf, K.; Ibekwem, S.; Souayah, H.; Cadranel, S.; Douat, N.; Zissis, G.; Butzler, J.P.; Vandamme, P. Arcobacter species in humans. Emerg. Infect. Dis. 2004, 10, 1863–1867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandenberg, O.; Houf, K.; Douat, N.; Vlaes, L.; Retore, P.; Butzler, J.P.; Dediste, A. Antimicrobial susceptibility of clinical isolates of non-jejuni/coli campylobacters and arcobacters from Belgium. J. Antimicrob. Chemother. 2006, 57, 908–913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Speegle, L.; Miller, M.E.; Backert, S.; Oyarzabal, O.A. Use of cellulose filters to isolate Campylobacter spp. from naturally contaminated retail broiler meat. J. Food Prot. 2009, 72, 2592–2596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lynch, O.A.; Cagney, C.; McDowell, D.A.; Duffy, G. A method for the growth and recovery of 17 species of Campylobacter and its subsequent application to inoculated beef. J. Microbiol. Methods 2010, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.L.; Engberg, J.; Ejlertsen, T.; Nielsen, H. Comparison of polycarbonate and cellulose acetate membrane filters for isolation of Campylobacter concisus from stool samples. Diagn. Microbiol. Infect. Dis. 2013, 76, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.L.; Ejlertsen, T.; Nielsen, H. Polycarbonate filtration technique is noninferior to mCCDA for isolation of Campylobacter species from stool samples. Diagn. Microbiol. Infect. Dis. 2015, 83, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Schweiger, A.; von Steiger, N.; Droz, S.; Marschall, J. Campylobacter concisus pseudo-outbreak caused by improved culture conditions. J. Clin. Microbiol. 2015, 53, 660–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nachamkin, I.; Nguyen, P. Isolation of Campylobacter Species from Stool Samples by Use of a Filtration Method: Assessment from a United States-Based Population. J. Clin. Microbiol. 2017, 55, 2204–2207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Tilmanne, A.; Kandet Yattara, H.M.; Herpol, M.; Vlaes, L.; Retore, P.; Quach, C.; Vandenberg, O.; Hallin, M.; Martiny, D. Multi-step optimization of the filtration method for the isolation of Campylobacter species from stool samples. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Waddington, M.; Lindquist, D.; Ware, J.; Cheung, W.; Ely, J.; Janda, J.M. Description of Campylobacter curvus and C. curvus-like strains associated with sporadic episodes of bloody gastroenteritis and Brainerd’s diarrhea. J. Clin. Microbiol. 2005, 43, 585–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClurg, K.R.; McClurg, R.B.; Moore, J.E.; Dooley, J.S. Efficient isolation of campylobacters from stools: What are we missing? J. Clin. Pathol. 2002, 55, 239–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engberg, I.; Gerner-Smidt, P. Author’s reply to: Lastovica, A.J.; le Roux, E. Efficient isolation of campylobacteria from stools. J. Clin. Microbiol. 2000, 38, 2798–2799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.; Ma, R.; Wang, Y.; Zhang, L. The Clinical Importance of Campylobacter concisus and Other Human Hosted Campylobacter Species. Front Cell Infect. Microbiol. 2018, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.L.; Engberg, J.; Ejlertsen, T.; Bücker, R.; Nielsen, H. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin. Microbiol. Infect. 2012, 18, E459–E465. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.L.; Engberg, J.; Ejlertsen, T.; Nielsen, H. Clinical manifestations of Campylobacter concisus infection in children. Pediatr. Infect. Dis. J. 2013, 32, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, V.; Riordan, S.M.; Grimm, M.C.; Tran, T.A.; Major, J.; Kaakoush, N.O.; Mitchell, H.; Zhang, L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE 2011, 6, e25417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgos-Portugal, J.A.; Kaakoush, N.O.; Raftery, M.J.; Mitchell, H.M. Pathogenic potential of Campylobacter ureolyticus. Infect. Immun. 2012, 80, 883–890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donovan, D.; Corcoran, G.D.; Lucey, B.; Sleator, R.D. Campylobacter ureolyticus: A portrait of the pathogen. Virulence 2014, 5, 498–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maki, J.J.; Howard, M.; Connelly, S.; Pettengill, M.A.; Hardy, D.J.; Cameron, A. Species Delineation and Comparative Genomics within the Campylobacter ureolyticus Complex. J. Clin. Microbiol. 2023, 61, e0004623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Man, S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Boras, V.F.; Houde, A. Enteric campylobacteria and RNA viruses associated with healthy and diarrheic humans in the Chinook health region of southwestern Alberta, Canada. J. Clin. Microbiol. 2011, 49, 209–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Global View of Campylobacteriosis: Report of an Expert Consultation; World Health Organization, Food and Agriculture: Utrecht, The Netherlands, 2012; ISBN 9789241564601.
- Collado, L.; Gutiérrez, M.; González, M.; Fernández, H. Assessment of the prevalence and diversity of emergent campylobacteria in human stool samples using a combination of traditional and molecular methods. Diagn. Microbiol. Infect. Dis. 2013, 75, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Iraola, G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019, 32, e00072-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Budiman, V.; Day, A.S.; Mitchell, H.; Lemberg, D.A.; Riordan, S.M.; Grimm, M.; Leach, S.T.; Ismail, Y. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J. Clin. Microbiol. 2010, 48, 2965–2967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serichantalergs, O.; Ruekit, S.; Pandey, P.; Anuras, S.; Mason, C.; Bodhidatta, L.; Swierczewski, B. Incidence of Campylobacter concisus and C. ureolyticus in traveler’s diarrhea cases and asymptomatic controls in Nepal and Thailand. Gut Pathog. 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goossens, H.; Vlaes, L.; De Boeck, M.; Pot, B.; Kersters, K.; Levy, J.; De Mol, P.; Butzler, J.P.; Vandamme, P. Is “Campylobacter upsaliensis” an unrecognised cause of human diarrhoea? Lancet 1990, 335, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Giesendorf, B.A.; Vandamme, P.; Vlaes, L.; Van den Borre, C.; Koeken, A.; Quint, W.G.; Blomme, W.; Hanicq, P.; Koster, D.S.; et al. Investigation of an outbreak of Campylobacter upsaliensis in day care centers in Brussels: Analysis of relationships among isolates by phenotypic and genotypic typing methods. J. Infect. Dis. 1995, 172, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, G.B.; Sjögren, E.; Hansson-Westerberg, J.; Kaijser, B. Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhoea in Swedish children. Scand. J. Infect. Dis. 1995, 27, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Labarca, J.A.; Sturgeon, J.; Borenstein, L.; Salem, N.; Harvey, S.M.; Lehnkering, E.; Reporter, R.; Mascola, L. Campylobacter upsaliensis: Another pathogen for consideration in the United States. Clin. Infect. Dis. 2002, 34, E59–E60. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 21, e8442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butzler, J.P. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 2004, 10, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Swerdlow, D.L.; Stern, N.J. Microbial food borne pathogens. Campylobacter jejuni. Vet. Clin. N. Am. Food Anim. Pract. 1998, 14, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2022 Zoonoses Report. EFSA J. 2021, 20, e07666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, J.P.; Shah, H.J.; Weller, D.L.; Ray, L.C.; Smith, K.; McGuire, S.; Trevejo, R.T.; Jervis, R.H.; Vugia, D.J.; Rissman, T.; et al. Preliminary Incidence and Trends of Infections Caused by Pathogens Transmitted Commonly through Food-Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1260–1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delahoy, M.J.; Shah, H.J.; Weller, D.L.; Ray, L.C.; Smith, K.; McGuire, S.; Trevejo, R.T.; Scallan Walter, E.; Wymore, K.; Rissman, T.; et al. Preliminary Incidence and Trends of Infections Caused by Pathogens Transmitted Commonly Through Food-Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 701–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flint, J.A.; Van Duynhoven, Y.T.; Angulo, F.J.; DeLong, S.M.; Braun, P.; Kirk, M.; Scallan, E.; Fitzgerald, M.; Adak, G.K.; Sockett, P.; et al. Estimating the burden of acute gastroenteritis, foodborne disease, and pathogens commonly transmitted by food: An international review. Clin. Infect. Dis. 2005, 41, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Rosenthal, M.; Gregoricus, N.; Greene, S.A.; Ferguson, J.; Henao, O.L.; Vinjé, J.; Lopman, B.A.; Parashar, U.D.; Widdowson, M.A. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg. Infect. Dis. 2011, 17, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, R.M.; Mattison, C.P.; Marsh, Z.; Shioda, K.; Donald, J.; Salas, S.B.; Naleway, A.L.; Biggs, C.; Schmidt, M.A.; Hall, A.J. Norovirus and Other Viral Causes of Medically Attended Acute Gastroenteritis Across the Age Spectrum: Results from the Medically Attended Acute Gastroenteritis Study in the United States. Clin. Infect. Dis. 2021, 73, e913–e920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the, D.S.M.Z. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.M.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Dutt, S.; Stormon, M.; Otley, A.; O’Loughlin, E.V.; Magoffin, A.; et al. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn’s disease. J. Clin. Microbiol. 2009, 47, 453–455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Man, S.M.; Zhang, L.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Mitchell, H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Thomson, J.M.; Hansen, R.; Berry, S.H.; El-Omar, E.M.; Hold, G.L. Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS ONE 2011, 6, e21490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, H.L.; Ejlertsen, T.; Engberg, J.; Nielsen, H. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: A population-based study. Clin. Microbiol. Infect. 2013, 19, 445–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newell, D.G. Campylobacter concisus: An emerging pathogen? Eur. J. Gastroenterol. Hepatol. 2005, 17, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.C.; Griffin, P.M.; Wymore, K.; Wilson, E.; Hurd, S.; LaClair, B.; Wozny, S.; Eikmeier, D.; Nicholson, C.; Burzlaff, K.; et al. Changing Diagnostic Testing Practices for Foodborne Pathogens, Foodborne Diseases Active Surveillance Network, 2012–2019. Open Forum. Infect. Dis. 2022, 9, ofac344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benedetti, G.; Holm Hansen, C.; Tølbøll Svendsen, A.; Grimstrup Joensen, K.; Sørensen, G.; Engsbro, A.L.; Torpdahl, M.; Møller Nielsen, E.; Ethelberg, S. The effect of changing diagnostic method from culture to PCR on the number of episodes of human campylobacteriosis in Denmark: A retrospective study (2015–2022). Microbiol. Spectr. 2024, 12, e0341823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar] [CrossRef]
- Liu, F.; Lee, S.A.; Xue, J.; Riordan, S.M.; Zhang, L. Global epidemiology of campylobacteriosis and the impact of COVID-19. Front. Cell Infect. Microbiol. 2022, 12, 979055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhn, K.G.; Nygård, K.M.; Guzman-Herrador, B.; Sunde, L.S.; Rimhanen-Finne, R.; Trönnberg, L.; Jepsen, M.R.; Ruuhela, R.; Wong, W.K.; Ethelberg, S. Campylobacter infections expected to increase due to climate change in Northern Europe. Sci. Rep. 2020, 10, 13874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bourke, B.; Chan, V.L.; Sherman, P. Campylobacter upsaliensis: Waiting in the wings. Clin. Microbiol. Rev. 1998, 11, 440–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Boer, E.; Tilburg, J.J.; Woodward, D.L.; Lior, H.; Johnson, W.M. A selective medium for the isolation of Arcobacter from meats. Lett. Appl. Microbiol. 1996, 23, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Corcoran, D.; O’Leary, J.; O’Hare, D.; Lucey, B.; Sleator, R.D. Emerging dynamics of human campylobacteriosis in Southern Ireland. FEMS Immunol. Med. Microbiol. 2011, 63, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, N.; Shimizu, A.; Somroop, S.; Li, Y.; Asakura, M.; Nagita, A.; Prasad Awasthi, S.; Hinenoya, A.; Yamasaki, S. High Prevalence of Campylobacter ureolyticus in Stool Specimens of Children with Diarrhea in Japan. Jpn J. Infect. Dis. 2017, 70, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Christiansen, L.E.; Wainø, M.; Ethelberg, S.; Madsen, H.; Wegener, H.C. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol. 2004, 70, 7474–7480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prachantasena, S.; Charununtakorn, P.; Muangnoicharoen, S.; Hankla, L.; Techawal, N.; Chaveerach, P.; Tuitemwong, P.; Chokesajjawatee, N.; Williams, N.; Humphrey, T.; et al. Climatic factors and prevalence of Campylobacter in commercial broiler flocks in Thailand. Poult. Sci. 2017, 96, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Chuma, T.; Andoh, M.; Yamashita, M.; Asakura, H.; Yamamoto, S. Effect of climatic elements on Campylobacter colonization in broiler flocks reared in southern Japan from 2008 to 2012. Poult. Sci. 2017, 96, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Koziel, M.; Lucey, B.; Bullman, S.; Corcoran, G.D.; Sleator, R.D. Molecular-based detection of the gastrointestinal pathogen Campylobacter ureolyticus in unpasteurized milk samples from two cattle farms in Ireland. Gut Pathog. 2012, 4, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koziel, M.; Corcoran, G.D.; Sleator, R.D.; Lucey, B. Detection and molecular analysis of Campylobacter ureolyticus in domestic animals. Gut Pathog. 2014, 6, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tinévez, C.; Velardo, F.; Ranc, A.G.; Dubois, D.; Pailhoriès, H.; Codde, C.; Join-Lambert, O.; Gras, E.; Corvec, S.; Neuwirth, C.; et al. Retrospective Multicentric Study on Campylobacter spp. Bacteremia in France: The Campylobacteremia Study. Clin. Infect. Dis. 2022, 75, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Macuch, P.J.; Tanner, A.C. Campylobacter species in health, gingivitis, and periodontitis. J. Dent. Res. 2000, 79, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Giraudon, E.; Prevost, B.; Martiny, D. Retrospective study on Campylobacter spp. bacteremia in Belgium: 2014–2023. In Sciensano Scientific Seminar on Infectious Diseases 2024, Brussels, Belgium, 16 May 2024; p. 76. Available online: https://www.sciensano.be/sites/default/files/book2024.pdf (accessed on 2 May 2024).
- Kirk, K.F.; Nielsen, H.L.; Nielsen, H. The susceptibility of Campylobacter concisus to the bactericidal effects of normal human serum. APMIS 2015, 123, 269–274. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).