Assessing the In Vitro Individual and Combined Effect of Arthrobotrys oligospora and A. musiformis (Orbiliales) Liquid Culture Filtrates against Infective Larvae of the Sheep Blood-Feeding Nematode Haemonchus contortus (Trichostrongylidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nematodes
2.1.1. Obtaining the Free-Living Nematode Panagrellus redivivus for Use as Bait to Isolate Nematophagous Fungi
2.1.2. Procurement of Haemonchus contortus Infective Larvae (L3)
2.2. Isolation of Nematophagous Fungi
2.3. Procurement of Fungal Liquid Culture Filtrates
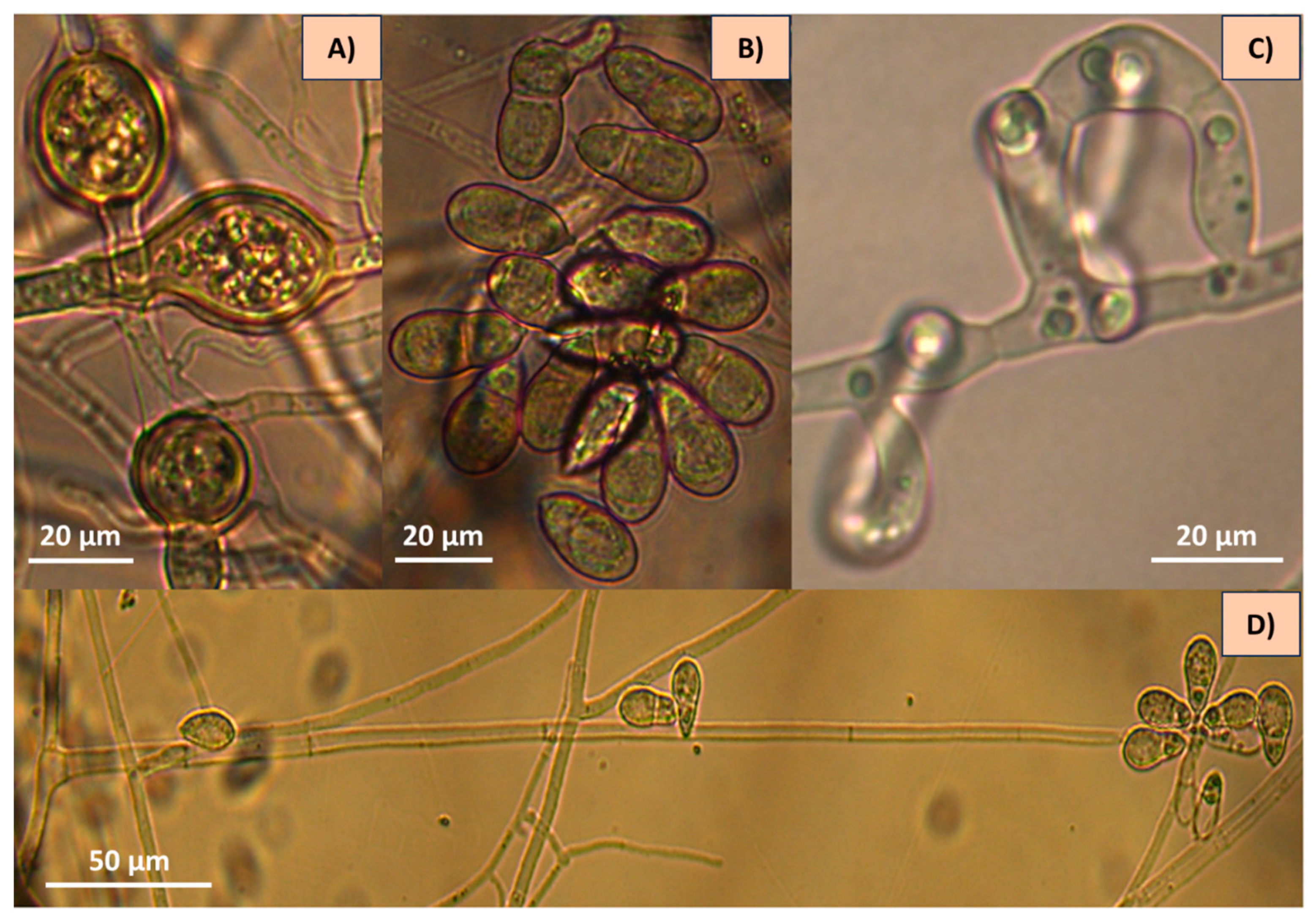
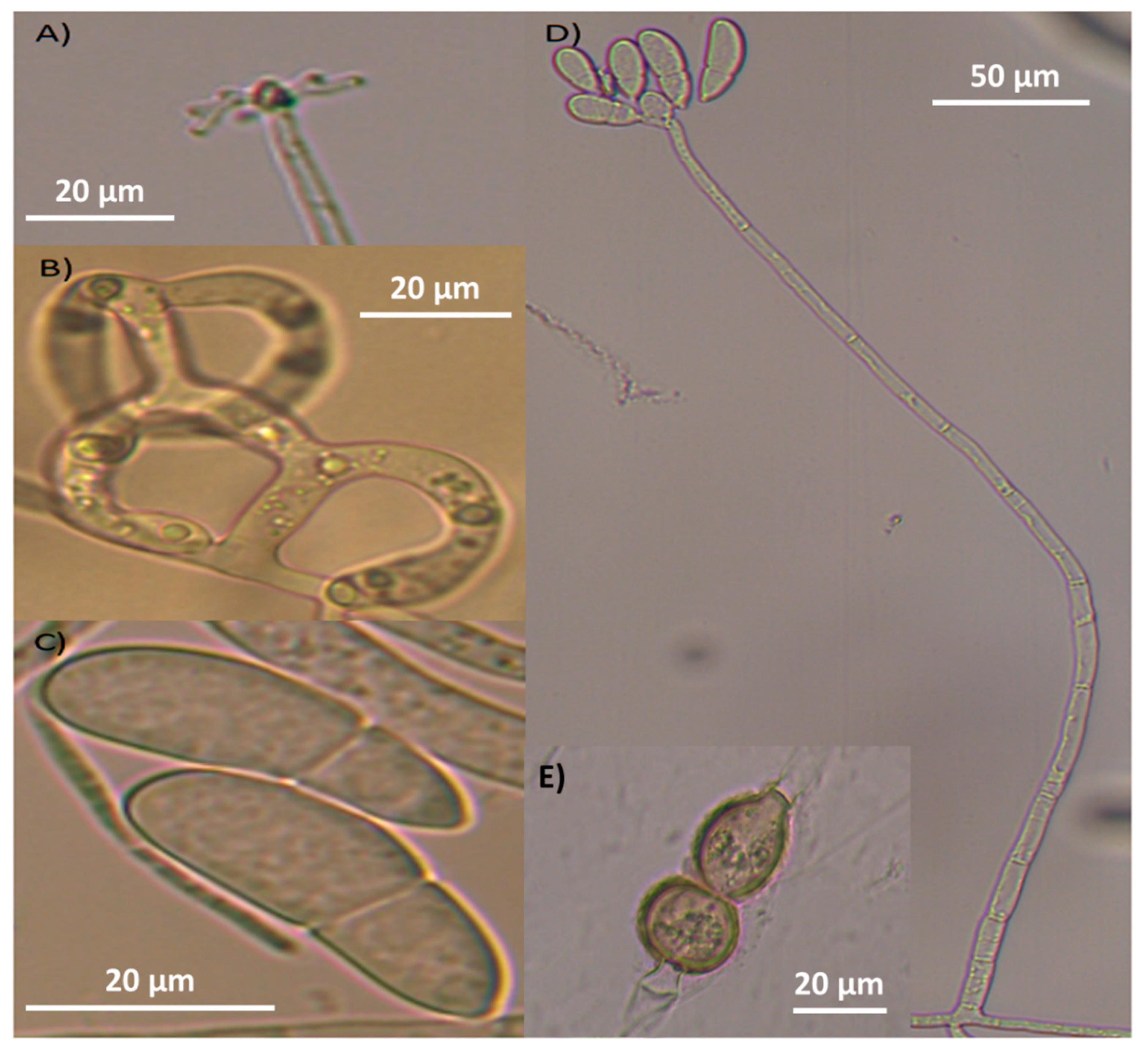
2.4. Traditional Taxonomic Identification of Fungal Isolates by Morphometry
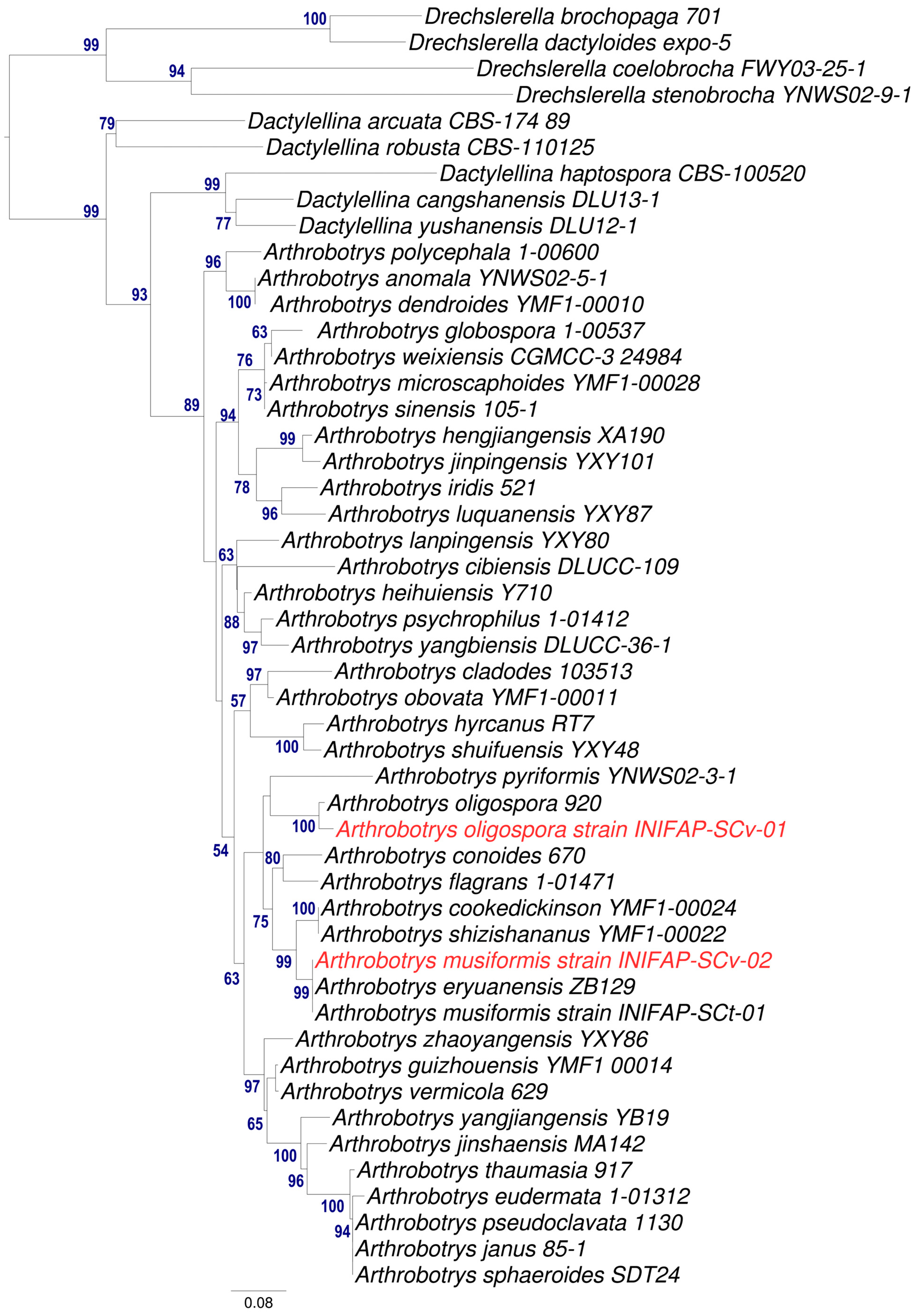
2.5. Molecular Identification
2.6. Assessing the Predatory Activity of Fungi against Haemonchus contortus Infective Larvae
2.7. Assessing the Nematocidal Activity of Fungal Liquid Culture Filtrates against Haemonchus contortus Infective Larvae
2.8. Microscopic Analysis of Haemonchus contortus Infective Larvae Exposed to Fungal Liquid Culture Filtrates
2.9. Myco-Chemical Profile
2.10. Statistical Analysis
3. Results and Discussion
3.1. Traditional Taxonomy (Morphometrics)
3.2. Molecular Identification
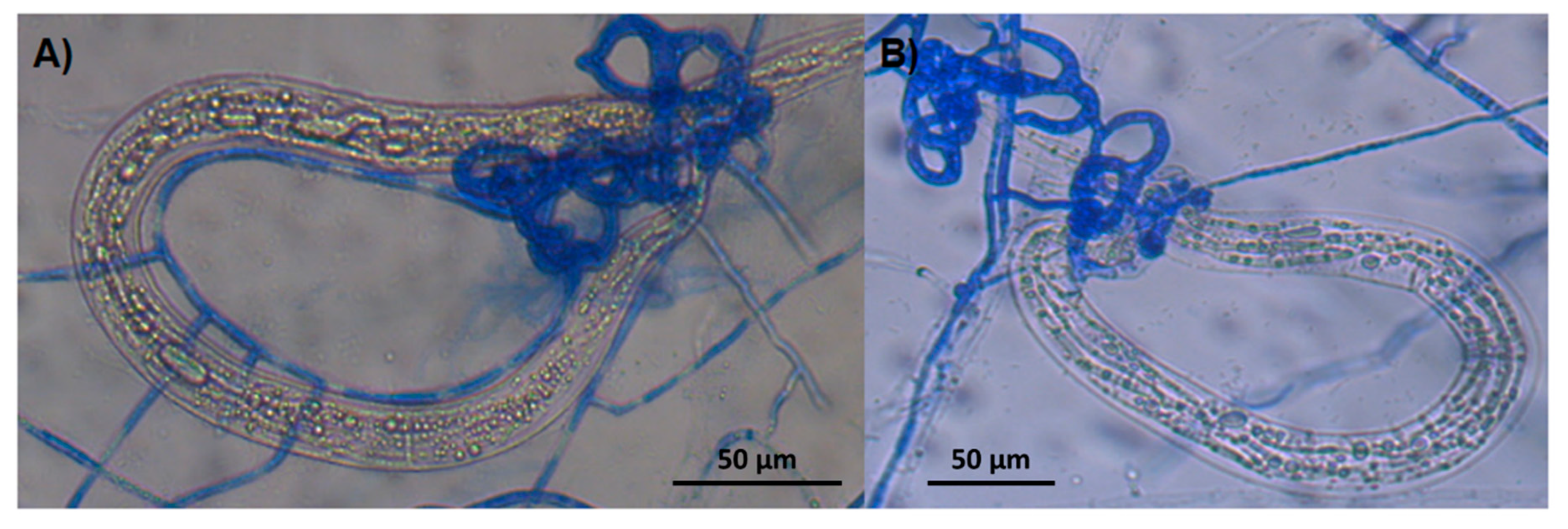
3.3. Predatory Activity Assessment of the Two Nematophagous Fungal Isolates against Haemonchus contortus Infective Larvae
3.4. Microscopic Findings about Predatory Activity of Fungi
3.5. Assessment of the In Vitro Nematocidal Activity of Liquid Culture Filtrates of Arthrobotrys oligospora and Arthrobotrys musiformis Produced in SPDB and CzDB against Haemonchus contortus Infective Larvae
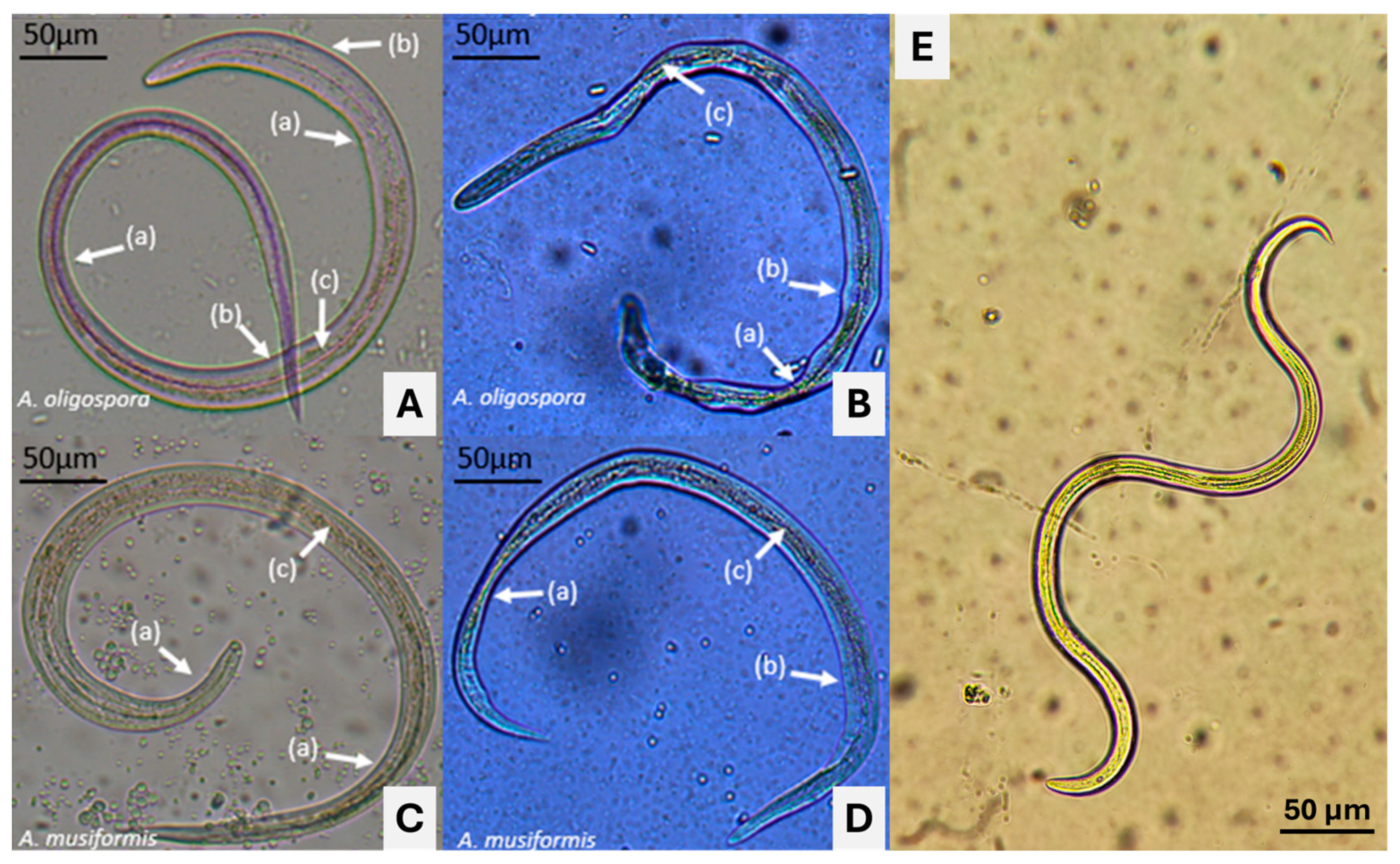
3.6. Microscopic Findings Regarding Haemonchus contortus Infective Larvae Exposed to Liquid Culture Filtrates of Two Nematophagous Fungi Grown in Sweet Potato Dextrose Broth and Czapek-Dox Broth
3.7. Myco-Chemical Compound Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. Diagnosis, treatment and management of Haemonchus contortus in small ruminants. Adv. Parasitol. 2016, 93, 181–238. [Google Scholar] [CrossRef] [PubMed]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus Infection in Pasture-Based Sheep Production Systems, with a Focus on the Pathogenesis of Anaemia and Changes in Haematological Parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Gustafsson, K.; Ljungström, B.L.; Skarin, M.; Varady, M.; Engström, F. Failure of ivermectin treatment in Haemonchus contortus infected-Swedish sheep flocks. Veter.-Parasitol. Reg. Stud. Rep. 2015, 1–2, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Martindah, E.; Sawitri, D.H.; Wardhana, A.H.; Ekawasti, F.; Dewi, D.A. Anthelmintic Resistance Status in Gastrointestinal Nematodes of Seven Different Breeds of Sheep in intensive management. IOP Conf. Ser. Earth Environ. 2023, 1174, 012030. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-trapping fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Guerrero, D.E.; Olmedo-Juárez, A.; Mendoza-de Gives, P. Control and prevention of nematodiasis in small ruminants: Background, challenges and outlook in Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 186–204. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B. Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora - an extensive plasticity of infection structures. Mycologist 1999, 18, 125–133. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: https://www.indexfungorum.org/names/namesrecord.asp?RecordID=149365 (accessed on 10 March 2024).
- Hyde, K.; Swe, A.; Zhang, K.Q. Nematode-Trapping Fungi; Fungal Diversity Research Series; Zhang, K.Q., Hyde, K., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 23, pp. 1–12. [Google Scholar]
- Rahman, M.U.; Chen, P.; Zhang, X.; Fan, B. Predacious Strategies of Nematophagous Fungi as Bio-Control Agents. Agronomy 2023, 13, 2685. [Google Scholar] [CrossRef]
- de Lara, R.; Castro, T.; Castro, J.; Castro, G. Cultivo del nematodo Panagrellus redivivus (Goodey, 1945) en un medio de avena enriquecida con Spirulina sp. Rev. Biol. Mar. Oceanogr. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- Valcárcel-Sancho, F.; Rojo-Vázquez, F.A.; Olmeda-García, A.S.; Arribas-Novillo, B.; Márquez-Sopeña, L.; Fernando-Pat, N. Atlas de Parasitología Ovina; Editorial Servet: Zaragoza, Spain, 2009; 137p. [Google Scholar]
- Iliev, P.; Ivanov, A.; Prelezov, P. Effects of temperature and desiccation on survival rate of Haemonchus contortus infective larval stage. Trakia J. Sci. 2018, 16, 17–21. [Google Scholar] [CrossRef]
- Cedillo-Borda, M.; López-Arellano, M.; Reyes-Guerrero, D.E. In vitro assessment of ivermectin resistance and gene expression profiles of P-glycoprotein genes from Haemonchus contortus (L3). Bio-Protocol 2020, 10, e3851. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Cedillo-Borda, M.; Alonso-Morales, R.A.; Alonso-Díaz, M.A.; Olmedo-Juárez, A.; Mendoza-de-Gives, P.; López-Arellano, M.E. Comparative study of transcription profiles of the P-glycoprotein transporters of two Haemonchus contortus isolates: Susceptible and resistant to ivermectin. Mol. Biochem. Parasitol. 2020, 238, 111281. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, E.; Aguilar-Marcelino, L.; Hernández-Romano, J.; Castañeda-Ramírez, G.S.; Mendoza-de-Gives, P. Taxonomic and biological characterization and predatory activity of four nematophagous fungi isolates of Arthrobotrys species from Tapachula, Chiapas, Mexico. Arch. Agron. Soil Sci. 2021, 69, 327–343. [Google Scholar] [CrossRef]
- Drechsler, C. Some hyphomycetes that prey on free-living terricolous nematodes. Mycologia 1937, 2, 447–552. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Van Oorschot, C.A.N. Taxonomy of the Dactylaria complex, V. A review of Arthrobotrys and allied genera. Stud. Mycol. 1985, 26, 61–96. [Google Scholar]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.-Y. New Arthrobotrys Nematode-Trapping Species (Orbiliaceae) from Terrestrial Soils and Freshwater Sediments in China. J. Fungi 2022, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Tigano-Milani, M.S.; Honeycutt, R.J.; Lacey, L.A.; Assis, R.; McClelland, M.; Sobral, B.W. Genetic Variability of Paecilomyces fumosoroseus Isolates Revealed by Molecular Markers. J. Invertebr. Pathol. 1995, 65, 274–282. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Pérez-Anzúrez, G.; Olmedo-Juárez, A.; de Fernex, E.V.-S.; Alonso-Díaz, M.; Delgado-Núñez, E.J.; López-Arellano, M.E.; González-Cortázar, M.; Zamilpa, A.; Ocampo-Gutierrez, A.Y.; Paz-Silva, A.; et al. Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens 2022, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Zamilpa, A.; Mendoza-de Gives, P.; Arece-García, J.; López-Arellano, M.E.; Fernex, E.v.S.d. In vitro larvicidal effect of a hydroalcoholic extract from Acacia cochliacantha leaf against ruminant parasitic nematodes. Veter Res. Commun. 2017, 41, 227–232. [Google Scholar] [CrossRef]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A Nematocidal Flavonoid from Prosopis laevigata Leaves Against Haemonchus contortus Eggs and Larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef]
- Baral, H.O.; Weber, E.; Marson, G. Monograph of Orbiliomycetes (Ascomycota) Based on Vital Taxonomy. Part II, 1st ed.; National Museum of Natural History: Luxembourg, 2020; 1752p. [Google Scholar]
- Bahena-Nuñez, D.S.; Ocampo-Gutiérrez, A.Y.; Mendoza-de Gives, P.; González-Cortázar, M.; Zamilpa, A.; Higuera-Piedrahita, R.I.; Pérez-Anzúrez, G.; Olmedo-Juárez, A.; López-Arellano, M.E.; Delgado-Núñez, E.J.; et al. Arthrobotrys oligospora (Fungi: Orbiliales) and its liquid culture filtrate myco-constituents kill Haemonchus contortus infective larvae (Nematoda: Trichostrongylidae). Biocont. Sci. Technol. 2024; in press. [Google Scholar]
- Cao, X.; Xu, Q.; Wan, X.M.; Yang, X.C.; Jia, X.Y.; Xue, X.J.; Du, J.L.; Cai, K.Z. Isolation, identification, and in vitro predatory activity of nematophagous fungus Arthrobotrys musiformis and its interaction with nematodes using scanning electron microscopy. Biocontrol Sci. Techn. 2018, 28, 94–104. [Google Scholar] [CrossRef]
- Dhawan, S.C.; Narayana, R.; and Babn, N.P. Influence of abiotic and biotic factors on growth of Paecilomyces lilacinus, Arthrobotrys oligospora and Pochonia chlamydosporia and parasitization of eggs/trapping of Meloidogyne incognita juveniles. Ann. Plant Prot. Sci. 2004, 12, 369–372. [Google Scholar]
- Alfaro-Gutiérrez, I.C.; Mendoza-de Gives, P.; Liébano-Hernández, E.; López-Arellano, M.E.; Valero-Coss, R.O.; Hernández-Velázquez, V.M. Nematophagous fungi (Orbiliales) capturing, destroying and feeding on the histotrophic larvae of Haemonchus contortus (Nematoda: Trichostrongylidae). Rev. Mex. Micol. 2011, 33, 29–35. [Google Scholar]
- Ojeda-Robertos, N.F.; Aguilar-Marcelino, L.; Olmedo-Juárez, A.; Luna-Palomera, C.; Peralta-Torres, J.A.; López-Arellano, M.E.; Mendoza-de-Gives, P. In vitro predatory activity of nematophagous fungi isolated from water buffalo feces and from soil in the Mexican southeastern. Rev. Bras. Parasitol. Veter 2019, 28, 314–319. [Google Scholar] [CrossRef]
- Jaramillo-Tlalapango, J.; Mendoza-de Gives, P.; Higuera-Piedrahita, R.I.; Ocampo-Gutiérrez, A.Y.; López-Arellano, M.E.; Pérez-Anzúrez, G.; Olmedo-Juárez, A.; Hernández-Romano, J.; Maza-López, J.; Delgado-Núñez, E.J.; et al. Study of a Mexican isolate of Arthrobotrys musiformis (Orbiliales): Predatory behavior and nematocidal activity of liquid culture filtrates against Haemonchus contortus (Trichostrongylidae), protein profile and myco-constituent groups. Fungal Biol. 2023, 127, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Jiang, Z.; Nizamani, M.M.; Zhang, H.; Liu, M.; Shan, W.; Wang, Y.; Li, K. Isolation, Identification, and Evaluation of the Predatory Activity of Chinese Arthrobotrys Species towards Economically Important Plant-Parasitic Nematodes. J. Fungi 2023, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.C.; Li, X.M.; Zhao, N.; Yang, L.; Zhang, K.Q.; Yang, J.K. Regulatory Mechanism of Trap Formation in the Nematode-Trapping Fungi. J. Fungi 2022, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Tzean, Y.; Chou, T.-H.; Hsiao, C.-C.; Shu, P.-Y.; Walton, J.D.; Tzean, S.-S. Cloning and characterization of cuticle-degrading serine protease from nematode-trapping fungus Arthrobotrys musiformis. Mycoscience 2016, 57, 136–143. [Google Scholar] [CrossRef]
- Lafta, A.A.; Kasim, A.A. Effect of nematode-trapping fungi, Trichoderma harzianum and Pseudomonas fluorescens in controlling Meloidogyne spp. Plant Arch. 2019, 19, 1163–1168. [Google Scholar]
- Jahanbazian, L.; Abdollahi, M.; Rezaie, R. Combined effect of Metarhizium anisopliae and Pseudomonas fluorescens CHA0 on root-knot nematode, Meloidogyne incognita in tomato. Iran. J. Plant Pathol. 2015, 51, 339–355. [Google Scholar]
- Cortes-Morales, J.A.; Zamilpa, A.; Salinas-Sánchez, D.O.; González-Cortazar, M.; Tapia-Maruri, D.; Mendoza-de Gives, P.; Rivas-González, J.M.; Olmedo-Juárez, A. In vitro ovicidal effect of p-coumaric acid from Acacia bilimekii aerial parts against Haemonchus contortus. Veter. Parasitol. 2023, 320, 109971. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Saha, S. Coumarins: An Important Phytochemical with Therapeutic Potential. In Plant-Derived Bioactives, 2nd ed.; Swamy, M., Ed.; Springer: Singapore, 2020; Volume 1, pp. 205–222. [Google Scholar]
- Jasso-Díaz, G.; Torres-Hernández, G.; Zamilpa, A.; Becerril-Pérez, C.; Ramírez-Bribiesca, J.E.; Hernández-Mendo, O.; Sánchez-Arroyo, H.; Olmedo-Juárez, A.; González-Cortazar, M.; Mendoza-de Gives, P. Ruta chalepensis full extract and organic phases exhibit nematocidal activity against Haemonchus contortus eggs and infective larvae (L3). Helminthologia 2022, 59, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Qin, C.; Liu, X.; Li, R.; Wang, C.; Li, C.; Du, G.; Guo, Q. Nematicidal Coumarins from Cnidium monnieri Fruits and Angelica dahurica Roots and Their Physiological Effect on Pine Wood Nematode (Bursaphelenchus xylophilus). Molecules 2023, 28, 4109. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.D.; de Lima, H.G.; de Sousa, N.B.; Genipapeiro, I.L.d.J.; Uzêda, R.S.; Branco, A.; Costa, S.L.; Batatinha, M.J.M.; Botura, M.B. In vitro anthelmintic evaluation of three alkaloids against gastrointestinal nematodes of goats. Veter. Parasitol. 2021, 296, 109505. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Koshino, H.; Uzawa, J.; Fujiok, S.; Kawano, T.; Kimura, Y. Nematicidal alkaloids and related compounds produced by the fungus Penicillium cf. simplicissimum. Biosci. Biotechnol. Biochem. 2000, 64, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- D’addabbo, T.; Argentieri, M.P.; Żuchowski, J.; Biazzi, E.; Tava, A.; Oleszek, W.; Avato, P. Activity of Saponins from Medicago Species against Phytoparasitic Nematodes. Plants 2020, 9, 443. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Cruz-Santiago, H.; del Prado-Vera, I.C.; Ramirez-Mares, M.V.; Gutiérrez-Ortiz, M.d.R.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Lira-de León, K.I.; Hernández-Carlos, B. Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential. Molecules 2021, 26, 2216. [Google Scholar] [CrossRef]
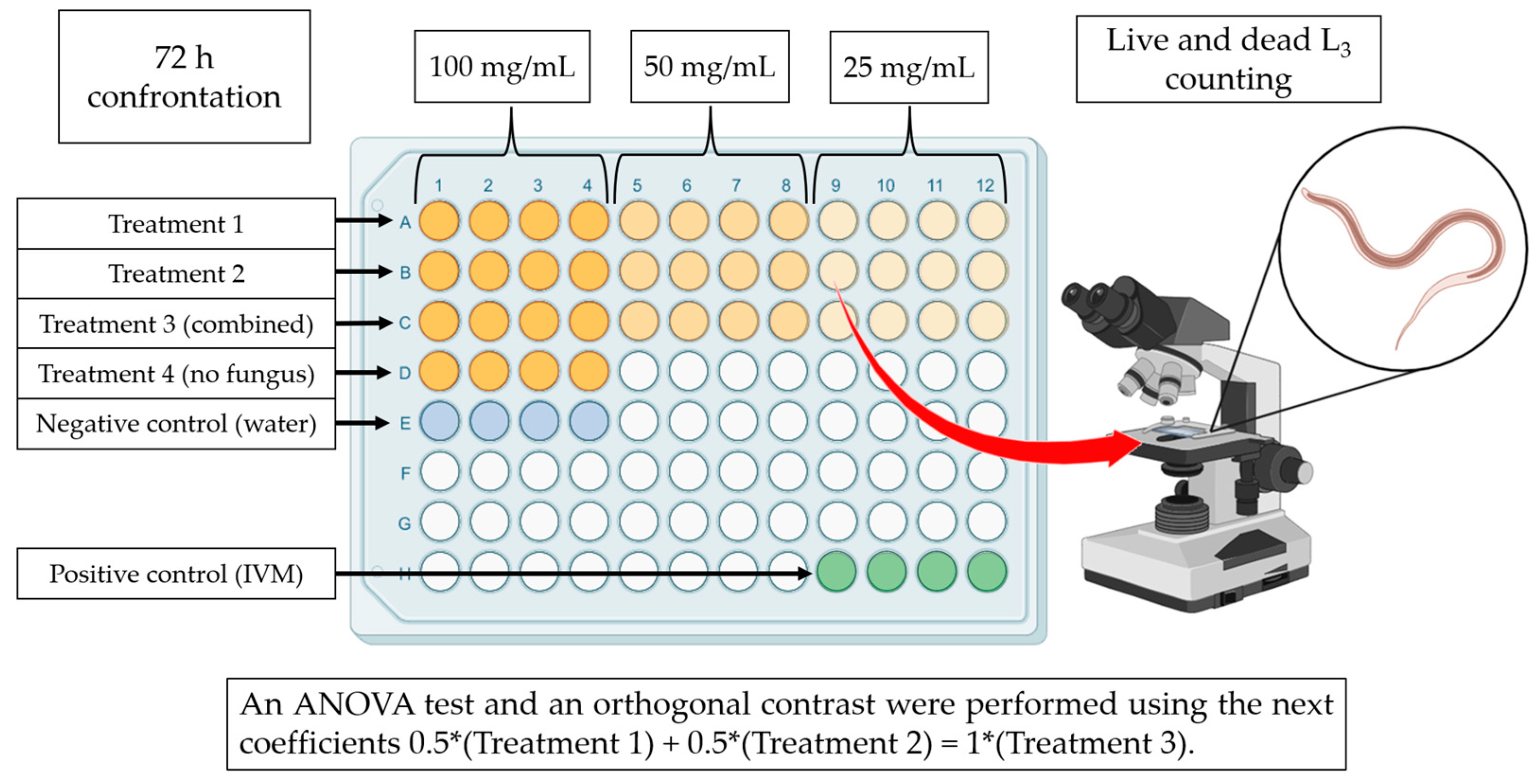

| Treatment | Medium | Fungal Filtrate |
|---|---|---|
| 1 | Sweet Potato Dextrose Broth | Arthrobotrys oligospora (*) |
| 2 | A. musiformis (*) | |
| 3 | A. oligospora + A. musiformis (*) | |
| 4 | Control (without fungus) | |
| 5 | Czapek-Dox Broth | Arthrobotrys oligospora (*) |
| 6 | A. musiformis (*) | |
| 7 | A. oligospora + A. musiformis (*) | |
| 8 | Control (without fungus) | |
| Controls | ||
| 9 | Phosphate Buffered Saline | Negative control |
| 10 | Ivermectin (0.5%) | Positive control |
| Characteristic | Strain 1 (AspT) | Strain 2 (AspG) |
|---|---|---|
| Conidium shape | Globose, slightly constricted in the septum (on occasion). | Ellipsoidal to obovoidal, slightly curved |
| Septum | One septum situated slightly below the half conidium | One septum situated slightly below the half conidium |
| Number of conidia per conidiophore | 6 (4–8) | 7 (3–11) |
| Conidia length (µm) | 21.1 (19–23) | 32.7 (27–40) |
| Conidia width (µm) | 11.4 (10–12) | 13 (11–17) |
| Conidiophores length (µm) | 344 (193–429) | 291 (141–403) |
| Identified species | Arthrobotrys oligospora | Arthrobotrys musiformis |
| Isolate (Species) | Recovered Larvae Control Group (Mean ± SE) | Recovered Larvae Treated Group (Mean ± SE) | Larval Reduction % |
|---|---|---|---|
| Arthrobotrys oligospora | 192 ± 38.21 | 105 ± 26.42 | 45.14 a |
| Arthrobotrys musiformis | 197 ± 60.53 | 57 ± 30.35 | 70.95 b |
| Isolate | Source of Isolation | Nematode Target | Predatory Activity % | Author (s) |
|---|---|---|---|---|
| A. oligospora | Not available | Meloidogyne incognita (**) | 79.6–87.5 | [28] |
| A. musiformis | Mossy soil, decaying plant material (a rotten trunk) and soil containing Brahea palm roots | H. contortus (L3) | >97 | [29] |
| A. oligospora | Faeces of water buffalo | H. contortus (L3) | >89 | [30] |
| A. oligospora | Soil and animal faeces | Panagrellus redivivus (*) | 57.2 | [16] |
| A. musiformis | Soil sample | H. contortus (L3) | >74 | [31] |
| A. oligospora | Soil samples | Aphelenchoides besseyi, Bursaphelenchus xylophilus and Ditylenchus destructor (**) | 54.6–97.3 | [32] |
| Concentration (mg/mL) | Fungal Filtrate | DL/TL | Mortality % | SE | Significance * |
|---|---|---|---|---|---|
| Sweet potato dextrose broth | |||||
| 0 | A. oligospora | 6/119 | 5.03 | 0.87 | 1 |
| A. musiformis | 6/119 | 5.03 | 0.87 | ||
| Combination | 6/119 | 5.03 | 0.87 | ||
| 25 | A. oligospora | 12/96 | 12.14 | 2.74 | 0.072 |
| A. musiformis | 15/107 | 13.70 | 3.24 | ||
| Combination | 21/101 | 20.54 | 3.95 | ||
| 50 | A. oligospora | 31/104 | 29.83 | 6.03 | 0.456 |
| A. musiformis | 25/104 | 24.50 | 5.08 | ||
| Combination | 19/86 | 22.18 | 5.06 | ||
| 100 | A. oligospora | 82/104 | 78.29 | 2.65 | 0.543 |
| A. musiformis | 83/110 | 75.55 | 5.37 | ||
| Combination | 79/99 | 79.84 | 3.04 | ||
| Czapek-Dox Broth | |||||
| 0 | A. oligospora | 2/80 | 2.12 | 0.38 | 0.588 |
| A. musiformis | 2/86 | 2.65 | 0.40 | ||
| Combination | 2/80 | 2.12 | 0.38 | ||
| 25 | A. oligospora | 23/86 | 27.34 | 5.75 | 0.011 |
| A. musiformis | 22/81 | 27.08 | 3.42 | ||
| Combination | 37/84 | 44.07 | 5.75 | ||
| 50 | A. oligospora | 38/89 | 43.03 | 8.84 | 0.916 |
| A. musiformis | 44/86 | 51.59 | 5.54 | ||
| Combination | 36/75 | 48.29 | 7.96 | ||
| 100 | A. oligospora | 75/87 | 86.17 | 3.39 | 0.308 |
| A. musiformis | 83/91 | 91.35 | 3.05 | ||
| Combination | 81/87 | 92.55 | 2.47 | ||
| Metabolites and Reagents | Arthrobotrys musiformis | Arthrobotrys oligospora | ||
|---|---|---|---|---|
| CzDB | SPDB | CCzDox | SPDB | |
| Alkaloids | + | + | + | + |
| Coumarins | - | + | + | + |
| Flavonoids | - | - | - | - |
| Tannins | - | - | - | - |
| Triterpenes | - | - | - | - |
| Sterols | - | + | + | + |
| Saponins | - | + | +++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colinas-Picazo, A.; Mendoza-de Gives, P.; Pérez-Anzúrez, G.; Gutiérrez-Medina, E.; Bautista-García, G.A.; Delgado-Núñez, E.J.; Olmedo-Juárez, A. Assessing the In Vitro Individual and Combined Effect of Arthrobotrys oligospora and A. musiformis (Orbiliales) Liquid Culture Filtrates against Infective Larvae of the Sheep Blood-Feeding Nematode Haemonchus contortus (Trichostrongylidae). Pathogens 2024, 13, 498. https://doi.org/10.3390/pathogens13060498
Colinas-Picazo A, Mendoza-de Gives P, Pérez-Anzúrez G, Gutiérrez-Medina E, Bautista-García GA, Delgado-Núñez EJ, Olmedo-Juárez A. Assessing the In Vitro Individual and Combined Effect of Arthrobotrys oligospora and A. musiformis (Orbiliales) Liquid Culture Filtrates against Infective Larvae of the Sheep Blood-Feeding Nematode Haemonchus contortus (Trichostrongylidae). Pathogens. 2024; 13(6):498. https://doi.org/10.3390/pathogens13060498
Chicago/Turabian StyleColinas-Picazo, Antonio, Pedro Mendoza-de Gives, Gustavo Pérez-Anzúrez, Enrique Gutiérrez-Medina, Génesis Andrea Bautista-García, Edgar Jesús Delgado-Núñez, and Agustín Olmedo-Juárez. 2024. "Assessing the In Vitro Individual and Combined Effect of Arthrobotrys oligospora and A. musiformis (Orbiliales) Liquid Culture Filtrates against Infective Larvae of the Sheep Blood-Feeding Nematode Haemonchus contortus (Trichostrongylidae)" Pathogens 13, no. 6: 498. https://doi.org/10.3390/pathogens13060498






