Abstract
Our study focused exclusively on analyzing Escherichia coli (E. coli) contamination in fresh raw mussels and ready-to-eat (RTE) stuffed mussels obtained from authorized and regulated facilities. However, it is critical to recognize that such contamination represents a significant public health threat in regions where unauthorized harvesting and sales practices are prevalent. This study aimed to comprehensively assess the prevalence, molecular characteristics, and antibacterial resistance profiles of E. coli in fresh raw mussels and RTE stuffed mussels. E. coli counts in fresh raw mussel samples ranged from 1 to 2.89 log CFU/g before cooking, with a significant reduction observed post-cooking. RTE stuffed mussel samples predominantly exhibited negligible E. coli presence (<1 log CFU/g). A phylogenetic analysis revealed a dominance of phylogroup A, with variations in the distribution observed across different sampling months. Antibacterial resistance was prevalent among the E. coli isolates, notably showing resistance to ampicillin, streptomycin, and cefotaxime. Extended-spectrum β-lactamase (ESβL) production was rare, with only one positive isolate detected. A variety of antibacterial resistance genes, including tetB and sul1, were identified among the isolates. Notably, virulence factor genes associated with pathogenicity were absent. In light of these findings, it is imperative to maintain rigorous compliance with quality and safety standards at all stages of the mussel production process, encompassing harvesting, processing, cooking, and consumption. Continuous monitoring, implementation of rigorous hygiene protocols, and responsible antibacterial drug use are crucial measures in mitigating food safety risks and combating antibacterial resistance. Stakeholders, including seafood industry players, regulatory agencies, and healthcare professionals, are essential to ensure effective risk mitigation and safeguard public health in the context of seafood consumption.
1. Introduction
Mussels are a nutritious shellfish that are rich in protein, minerals, vitamins, and omega-3 fatty acids. They can be consumed both cooked and fresh. They are also low in fat (less saturated fat) and calories, making them a healthy choice for a balanced diet. The consumption of mussels can provide various health benefits, such as improving immune function, reducing inflammation, enhancing brain function, and preventing anemia [1,2]. Mussels are also considered a sustainable and eco-friendly seafood option, as they do not require feed or fertilizers and can filter and improve water quality [1].
Stuffed mussels are a popular traditional food sold by vendors in the Mediterranean Sea countries. The mussel variety used for stuffed mussels is Mytilus galloprovincialis, which is commonly known as the “black mussel”. Ready-to-eat stuffed (RTE) mussels are typically prepared by first cleaning the cockleshells thoroughly, removing all feather-like structures, and then stuffing them with a mixture of rice, oil, salt, and spices. The cockleshells are then closed firmly and steamed [3].
E. coli is widely recognized as a key indicator of fecal contamination and is used to assess the microbiological quality of seafood, particularly mussels. Its presence in food, especially those that are RTE, can signal potential contamination with pathogenic microorganisms. E. coli can cause severe illnesses in humans, including gastroenteritis, urinary tract infections, and neonatal meningitis. Some strains, like EHEC, produce Shiga toxins that can lead to severe conditions such as hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS), which is a leading cause of acute kidney failure in children. Additionally, some of other serotypes of E. coli possess higher risks, such as carrying the stx and eae virulence genes, named STEC E. coli. These infections, often associated with E. coli, can result from consuming contaminated and undercooked foods and may also be transmitted through the fecal–oral route [4]. It can contaminate marine environments and mussels through various sources like sewage, agricultural runoff, and animal manure and consequently enter the food chain [5,6]. The prevalence of E. coli serotypes in both the marine environment and mussels is influenced by factors such as environmental conditions, seasonal changes, mussel species, and pollution levels in harvesting areas [7,8,9]. These serotypes may possess different surface antigens affecting their pathogenicity and host range. The impact of these factors on public health is significant, especially for vulnerable populations [10,11]. E. coli species may also harbor antibacterial resistance genes, posing a global concern [12].
Despite the nutritional benefits of mussels, harvesting areas that are polluted, with improper preparation, storage, and sale conditions, can lead to E. coli contamination. Contaminated water, exposure to temperature fluctuations, and processing with contaminated equipment can result in cross-contamination and rapid bacterial growth. The consumption of raw or undercooked mussel products may lead to serious illnesses. Consequently, the presence of E. coli in seafood products, particularly mussels, requires a thorough risk assessment to address potential health risks to consumers [13,14]. Numerous risk assessment studies have focused on mussels and mussel products to ensure food safety [15,16,17,18,19].
While mussels and mussel products are widely consumed, there is a significant deficiency in evaluating and characterizing stx and eae-carrying E. coli isolates found in fresh raw mussels and RTE stuffed mussels, as well as along the RTE stuffed mussel processing line. A molecular characterization of E. coli serotypes and their AMR genes is of vital importance for the control of the spread of these genes and factors and for the implementation of appropriate prevention and treatment strategies.
The objective of this study was to identify the primary source of contamination of E. coli in fresh raw mussels and RTE stuffed mussels. Additionally, this study aimed to detect the presence of stx and eae, as well as other virulence factors and AMR genes, in a survey of fresh raw mussels and RTE stuffed mussels collected from four different companies in four different regions of Turkey.
2. Materials and Methods
2.1. Sample Collection
During the period between June 2022 and May 2023, RTE stuffed mussels (n = 25) and fresh raw mussels (n = 25) used in the production of these stuffed mussel samples were collected regularly every month (all samples were taken on the same day and were considered as a single batch) from three different companies harvesting and processing mussels from the Marmara Sea, which is an inner sea surrounded by heavily populated cities and industrial areas. All the selected companies applied the Hazard Analysis and Critical Control Points (HACCP) and Good Manufacturing Practices (GMP) systems in their production. The locations of harvesting for these companies were as follows: R1: Balikesir (40°34′41.8″ N 27°35′37.8″ E), R2: Mudanya (40°22′59.8″ N 28°52′45.3″ E), and R3: Gemlik (40°28′18.2″ N 28°54′28.3″ E). In addition, another company (R4: Istanbul [40°59′31.1″ N 29°00′46.1″ E]) was included in this study to investigate point-by-point possible E. coli contamination points. A total of nine sampling points (SP) were selected as follows: SP1: raw mussel, SP2: swab from knives, SP3: shelling step, SP4: swab from handlers’ hands, SP5: stuffing with pre-cooked rice and spices, SP6: cooking with an aromatic blend of rice and spices, SP7: portioning and packaging, SP8: shipment, SP9: ready-to-eat stuffed mussel at selling point. Sterile swabs were used to sample the food handlers’ hands (after routine cleaning procedures) and the knives before they were engaged in food preparation. Swabbing from both the handlers’ hands and the knives was performed on a 10 cm2 area. All the samples were packed in sterile bags and transferred to the laboratory in cold chain within three hours. The cooking periods applied at R4 were 65 °C for 17.6 ± 1.8 min for precooking (rice and spices) and 72 °C for 20.6 ± 0.9 min for main cooking (mussels, rice, and spices together). Detailed information about the regions, sampling points, and processes are provided in Supplementary Table S1.
2.2. Isolation and Enumeration of E. coli
A total of 25 samples were collected on a single day and considered a single batch. The inter-shell contents of each batch were mixed, and 10 g of the mixture from each batch was transferred to sterile stomacher bags (Seward Medical, London, UK). Then, 90 mL of sterile Maximum Recovery Diluent (MRD, Oxoid, ThermoFisher, Milano, Italy) was added and homogenized. Subsequently, 10-fold serial dilutions were prepared from the MRD. E. coli isolation and enumeration were conducted using the pour plate method on Tryptone Bile X-Glucuronide Agar (TBX, Oxoid, Basingstoke, UK). Following inoculation from 1 mL of the appropriate dilutions, the plates were incubated at 37 ± 2 °C for 4 h and then at 44 °C for 20 h. Colonies with a blue–green color were identified and enumerated as log colony-forming units per gram (log CFU/g) [7,20]. Additionally, suspected E. coli colonies were isolated using the Violet Red Bile Agar (VRB, Oxoid, Hampshire, UK) double-layer pour plate method. The inoculated plates were incubated at 37 °C for 24 h. Following incubation, purple colonies (pinkish red colonies with bile precipitate) were considered to be E. coli [21]. The blue–green colonies isolated from the TBX agar and the suspected colonies from the VRB agar were subjected to subculturing in Tryptic Soya Broth (TSB, Oxoid, Thermofisher, Madrid, Spain) and on Tryptic Soya Agar (TSA, Oxoid, Thermofisher, Madrid, Spain) at 37 °C for 24 h. Thereafter, all E. coli isolates were confirmed using standard biochemical tests.
2.3. Identification of E. coli Isolates
The confirmation of E. coli isolates was achieved using polymerase chain reaction (PCR) amplification of the E. coli-specific universal stress protein (uspA) and uidA genes following the procedure outlined by Chen and Griffiths (1998) [22] and Heijnen and Medema (2006) [23], respectively (Table 1).

Table 1.
Primers used for identification and phylogenetic classification of E. coli isolates.
2.4. Determination of Phylogenetic Groups of E. coli Isolates
E. coli isolates were classified into phylogenetic groups (A, B1, B2, and D) in accordance with the Clermont’s method (2000 and 2013) [24,25], which involved the identification of two virulence genes, chuA (encoding a hem transporter protein in E. coli O157: H7), yjaA (encoding a hypothetical protein initially identified in the genome of E. coli K-12), and one DNA fragment (TspE4.C2) and arpa (ankyrin repeat protein) genes [26] (Table 1).
2.5. Antibacterial Resistance Testing of E. coli Isolates
The antibacterial resistance of E. coli isolates was evaluated using the Kirby–Bauer disc diffusion method in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines [25]. A set of antibacterials (Oxoid, Basingstoke, UK) was utilized, including tetracycline (TE; 30 µg), chloramphenicol (CL; 30 µg), trimethoprim-sulfamethoxazole (SXT; 25 µg), streptomycin (STR; 10 µg), ciprofloxacin (CIP; 5 µg), levofloxacin (LEV; 5 µg), and ampicillin (AM; 10 µg). The isolates’ resistance was evaluated according to the CLSI guidance (2023) [27], with E. coli ATCC 25922 used as a quality control strain, and classified as sensitive, intermediate resistant, or resistant.
2.6. Phenotypic Test for the Presence of Extended-Spectrum β-Lactamase (ESβL) in E. coli Isolates
The presence of Extended-Spectrum β-Lactamase (ESβL) in E. coli isolates was also evaluated. Initially, the CLSI-guided (2023) [27] ESβL test was conducted using a cefotaxime (CTX; 30 µg) antibacterial disc, with the isolates classified as sensitive, intermediate, or resistant. The confirmation test was performed according to the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2023) [28] using the double-disc synergy test (DDST) with amoxicillin-clavulanic acid (AMC; 20/10 μg), ceftazidime (CAZ; 30 μg), cefepime (FEP; 30 μg), and cefotaxime (CTX; 30 μg). The presence of a synergistic effect, demonstrated by a clear inhibition zone, indicates ESβL production. The CLSI methods (2023) [27] were employed, with cefotaxime (CTX; 30 µg) and ceftazidime (CAZ; 30 µg) discs plated both individually and in combination with clavulanic acid (CLA; 10 µg) on a Mueller Hinton Agar (MHA, Oxoid, Basingstoke, UK) plate to confirm ESβL. ESβL-positive phenotypes were confirmed by a ≥5 mm enhancement in the zone of inhibition in CTX-CLA or CAZ-CLA compared to the respective antibacterial disc alone (combined disc test, CDT). The enlarged inhibition zone indicated the isolates’ ability to neutralize clavulanic acid.
2.7. Identification of β-Lactamase and Antibacterial Resistance Genes of E. coli Isolates
The investigation of major beta-lactamase genes, namely blaCTX-M, blaTEM, blaSHV, and blaOXA within the E. coli isolates was conducted via PCR in accordance with the methodology outlined by Ogutu et al. (2015) [29]. Furthermore, an analysis was conducted on the isolates to determine the presence of genes associated with resistance to various antibacterial agents, including tetracycline (tetA and tetB), sulfonamides (sul1, sul2, and sul3), florfenicol/chloramphenicol (floR), and quinolones (qnrA and qnrB) [30,31].
2.8. Virulence Factor Genes of E. coli Isolates
E. coli isolates were tested using conventional PCR for the following virulence factors: Shiga-like toxin genes (stx1 and stx2), bundle-forming pilus (bfpA), attaching and effacing factor gene (eae), O157 antigen (rfbE), flagellar antigen (flic), O26 and O103 O-antigen (wzx), O145 (ihp1), and O111 (wbdl) genes, with primers and conditions outlined by ISO (2012) [32] and Clermont et al. (2013) [25].
3. Results
3.1. Isolation, Enumeration, and Identification
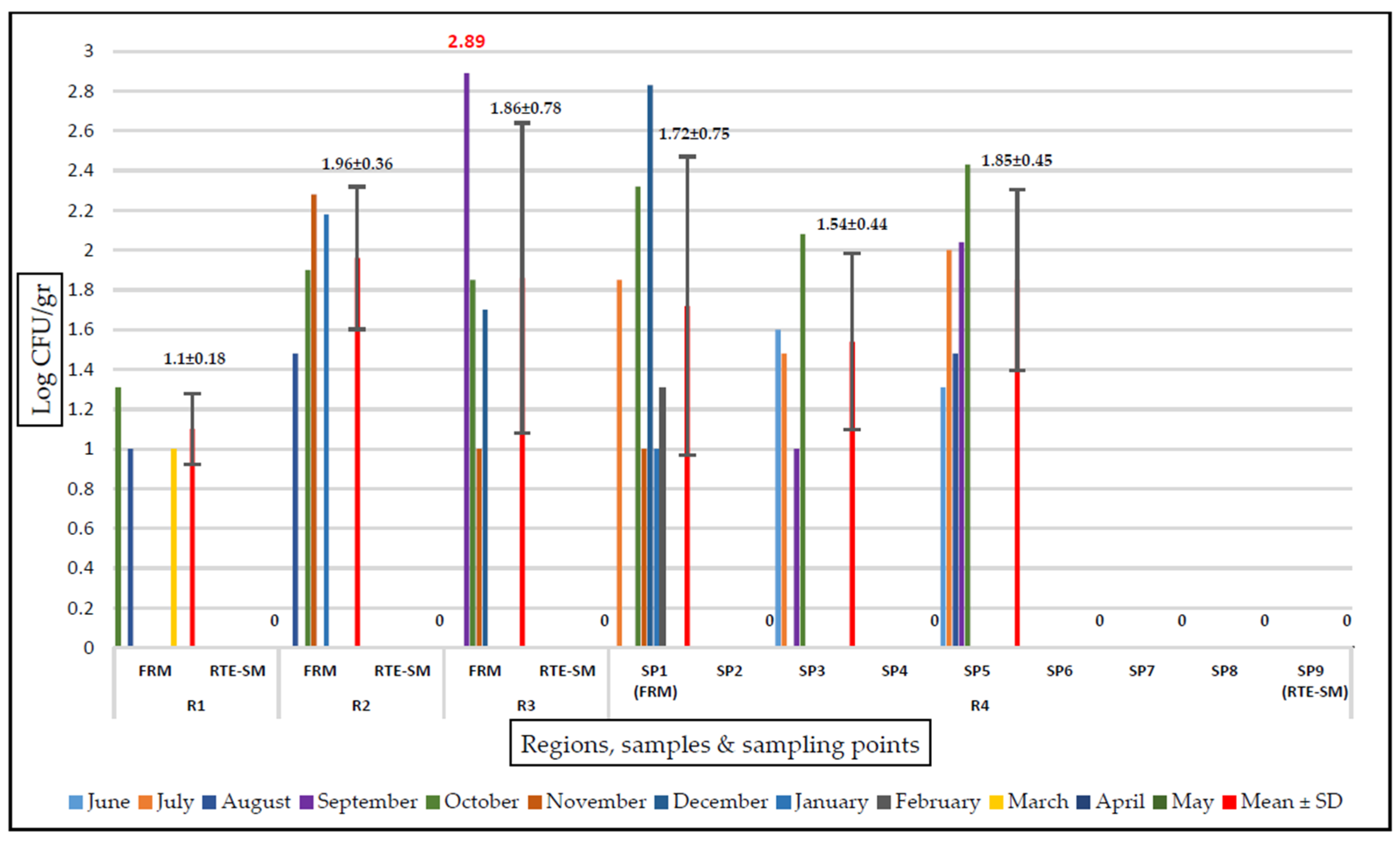
The E. coli counts prior to the cooking process ranged from 1 to 2.89 log CFU/g. The mean E. coli counts in the fresh raw mussel samples from R1, R2, R3, and R4 were 1.10, 1.96, 1.86, and 1.72 log CFU/g, respectively. The highest E. coli count was detected in the fresh raw mussels from R3 in September, followed by 2.83 log CFU/g in the fresh raw mussels from R4. Seasonally, the period with the least contamination was spring, with growth observed only in the fresh raw mussels from R1 in March. No E. coli was detected in any samples during April and May. The highest counts were obtained from samples collected in the autumn period. In December, a single colony from VRB tested positive for E. coli in RTE stuffed mussels from R3.
E. coli was not isolated at any stage after the cooking process (SP6-SP9 in R4). The counts of E. coli were found to be 1.85 log CFU/g before the cooking process and <1 log CFU/g in the RTE stuffed mussel samples by the destruction of 0.90 log after the cooking process. The RTE stuffed mussel samples contained no E. coli, and only one isolate from VRB was E. coli positive (60EM, from R3). No E. coli was detected in any sample from the food contact surfaces (SP2 and SP4) of the RTE stuffed mussel production line (Figure 1).
Figure 1.
Amount of E. coli growth on TBX agar (log CFU/gr). FRM, fresh raw mussel; RTE-SM, ready-to-eat stuffed mussel; SP, sampling point; R, region. A value of 0 on the axis indicates that E. coli was not detected (< 1 log CFU/g) in this region in either the SP samples or the FRM/RTE-SM samples.
The genetic diversity of the isolates we obtained as a result of isolation and PCR identification, which we have provided in Supplementary Table S2, were investigated. To this end, a total of 74 isolates were collected: 44 from fresh raw mussels, 15 from SP3 (shelling step), 14 from SP5 (stuffing with pre-cooked rice and spices), and one from an RTE stuffed mussel sample.
From a seasonal perspective, the highest number of E. coli isolates was obtained from samples in October (16 isolates), followed by September (15 isolates). These isolates, used for phenotypic antibacterial resistance and genetic diversity research, are referred to by a “number and EM” code throughout the remainder of this study.
3.2. Determination of Phylogenetic Groups
Two different groupings were made for the phylogenetic analysis of the E. coli isolates (n = 74) obtained in our study. Phylogroup A was the most dominant (38/74; 51.4%) phylogroup among the isolates, followed by phylogroups B1 (18/74; 24.3%), B2 (13/74; 17.6%), and D (5/74; 6.8%) based on Clermont et al. (2000) [24]. All phylogroup B1 E. coli isolates harbored the arpA gene. All the samples isolated in January and February 2023 (excluding 56EM) belonged to phylogroup A1. In the other months, the isolates showed a heterogeneous distribution (Supplementary Table S3). In the phylogenetic grouping of E. coli isolates, according to Clermont et al. (2013) [25], the most common phylogroup was A (16/74; 21.6%), followed by A or C (11/74; 14.9%), B1 (10/74; 13.5%), B2 (5/74; 6.8%), Clade1 or 2 (2/74; 2.7%), E or Clade1 (2/74; 2.7%), D or E (1/74; 1.4%), and F (1/74; 1.4%). Just over 35.1% (26/74) of the isolates were non-typable (Supplementary Table S3).
3.3. Phenotypic and Genotypic Identification of Antibacterial Resistance and ESBL of E. coli Isolates
The results of the isolates’ phenotypic resistance to antibacterials are provided in Supplementary Table S4. The intermediate resistant and resistant E. coli phenotypes were as follows: ampicillin (35 isolates; 47.3%), streptomycin (21 isolates; 28.4%), cefotaxime (15 isolates; 20.3%), trimethoprim-sulfamethoxazole (11 isolates; 14.9%), tetracycline (10 isolates; 13.5%), chloramphenicol (six isolates; 8.1%); ciprofloxacin (five isolates; 6.8%), and levofloxacin (three isolates; 4.1%). Out of the 74 isolates, 25 isolates (33.8%) exhibited no resistance to any antibacterials. Only one isolate (41EM) isolated in October was ESBL positive. A majority of the E. coli isolates demonstrated high susceptibility rates to levofloxacin and ciprofloxacin, with over 95% of the isolates remaining sensitive.
Cephalothin-blaSHV was not detected in any E. coli isolates. tetB (10/74; 13.5%), sul1 (3/74; 4.1%), sul2 (9/74; 12.2%), sul3 (2/74; 2.7%), qnrA (1/74; 1.4%), qnrB (1/74; 1.4%), and floR (1/74; 1.4%) were detected (Supplementary Table S5).
3.4. Identification of Virulence Factor Genes of E. coli Isolates
The Shiga-like toxin (stx1 and stx2), attaching and effacing factor (eae), bundle-forming pilus structural gen (bfpA), O157 antigen (rfbE), O26 and O103 O-antigen (wzx), and O145 (ihp1) genes were not detected. wbdl (O111 gene) was detected in only one isolate (23EM). flicH7 (flagellar antigen) was found in three isolates (7EM and 10EM [June and August, respectively] and 12EM [September]).
4. Discussion
E. coli species, which serve as an indicator of fecal contamination and are consequently an important food safety indicator, can reach prevalences of up to 30% in shellfish [33]. In India, Singh et al. (2020) [34] isolated a total of 150 E. coli isolates from four different types of fresh shellfish. Our results showed that E. coli was enumerated in 35.4% (17/48) of fresh raw samples and none of the stuffed mussels, with a mean count of 2.15 log CFU/g. Raw mussels can harbor many important pathogens such as E. coli through municipal sewage discharge, industrial wastewater loads, rainfall or irrigation water runoff over land, and the release of contaminants into streams, lakes, or coastal waters [17].
In the current study, a comprehensive monthly sampling approach allowed for monitoring and ensuring the mussels’ microbiological safety and quality throughout their preparation, handling, and distribution processes. The absence of E. coli in the hand and knife swab samples showed that the food handlers complied with hygiene and sanitation rules. A study conducted in Istanbul Province in Turkey examined the microbiological quality of RTE stuffed mussels according to the Turkish Food Codex. The results revealed that 77% of the samples had coliform bacteria and 22% had E. coli [35]. In another study conducted in Ankara Province in Turkey, 30% of the analyzed stuffed mussel samples were not suitable for consumption due to the presence of E. coli [13]. Unlike other studies, which detected very high levels of E. coli contamination in RTE stuffed mussel, in our study, only one (2.1%) E. coli isolate was identified in the RTE stuffed mussel samples. This can be due to the inactivation of the bacteria during heating processes, the prevention of contamination, or any other difference in the processing steps. E. coli contamination in RTE stuffed mussels can be significantly reduced under stringent processing conditions. Strict hygiene protocols should be followed, including regular hand washing, wearing gloves, and using sanitized equipment by all food handlers involved in the processing. The processing environment should be controlled, with limited access to prevent external contamination, and with regular cleaning and disinfection conducted. All equipment and utensils used in the processing should be sterilized before use. Mussel products should be cooked at temperatures exceeding 70 °C to ensure the inactivation of E. coli and other potential pathogens. Additionally, the final product should be sold under appropriate conditions to prevent post-processing contamination. It is also important to consider that the levels of contamination could vary due to changes in pollution rates in the marine environments where the mussels are harvested. Implementing these comprehensive measures can help ensure the safety of RTE stuffed mussels [17,36,37].
Bazzoni et al. (2019) [38] reported that the presence of E. coli in raw mollusk samples was more pronounced in the fall and winter seasons (270 and 330 MPN/100 g, respectively). They noted that they did not encounter contamination during the summer season. Similarly, Sferlazzo et al. (2018) [39] mentioned that E. coli contamination tended to be lower during the summer season. In a study conducted in Italy, E. coli was detected in all samples of M. galloprovincialis and Ruditapes decussatus [40]. In another study conducted in Italy, which examined 600 raw mussels, E. coli was detected in 3.5% of the samples [41]. Notably, E. coli was not detected during these months, indicating either successful microbial control measures or seasonal conditions not conducive to E. coli survival.
Phylogenetic groups of E. coli have also been associated with different ecological niches, virulence factors, and antibacterial resistance patterns [42,43]. Therefore, the presence and diversity of E. coli phylogenetic groups in mussels may vary depending on the origin, season, and treatment of the products. In addition, PCR detection of phylogenetic groups of E. coli from fresh raw mussels and RTE stuffed mussels can provide useful information about the microbial quality and safety of these products. It can also help to identify the possible sources of fecal contamination and to monitor the effectiveness of processing methods. Furthermore, it can contribute to the understanding of the epidemiology and ecology of E. coli in aquatic environments and food chains. E. coli isolates categorized within phylogroup A are primarily commensal [44]. In our study, 6.8% and 1.4% of the identified isolates belonged to groups B2 and D, respectively, which are considered pathogenic. Based on the classification, 26 of the isolates belonged to an unknown phylogroup, which requires different typing methods such as MLST [23]. Phylogroups B1 and A are the most prevalent across multiple months, suggesting these are the common isolates in the environment studied. Groups B2 and D also appeared but were less frequent. The dominance of Group A and occasional appearances of Group D between December and February suggests that some isolates are more adapted to colder conditions.
The emergence of multidrug-resistant (MDR) foodborne pathogens, defined as acquired resistance to at least one antibacterial agent in three or more antibacterial categories, is considered a significant challenge in public health, with MDR E. coli recognized as a prominent issue in ensuring food safety [12,45,46]. In the present study, the antibacterial resistance profiles and resistance genes of the E. coli isolates were evaluated using disk diffusion and PCR methods. Our findings revealed that 66.2% (49/74) of the isolates exhibited resistance to at least one antibacterial, with 32.4% having resistance to two or more classes of antibacterials and thus being classified as MDR. The resistance included resistance to CTX, which is utilized as a last-resort option for treating severe infections caused by E. coli and other Gram-negative bacteria. The highest resistance rates were observed for AMP (47.3%), STR (28.4%), and CFX (20.3%). This resistance is particularly alarming due to the role of CTX in treating severe bacterial infections and its implications for the selection of ESβL producers. These antibacterials are widely used in human and veterinary medicine, and their overuse may select for resistant bacteria that can be transmitted through the food chain. The emergence and spread of resistance to these antibacterials may compromise the effectiveness of the available therapeutic options and increase the risk of treatment failure and mortality [47]. The resistance patterns exhibited seasonal fluctuations, with a notable increase in AMP and CTX resistance during the cooler months (October and November). This could be attributed to seasonal changes in antibacterial drug usage patterns in agriculture and human medicine, which often influence environmental reservoirs of resistance. Our data also revealed significant resistance to commonly used antibacterials among E. coli isolates, with a marked seasonality in resistance patterns. The high susceptibility to fluoroquinolones offers some therapeutic reprieve, although the emergence of ESβL producers and resistance to critical beta-lactam antibacterials paints a complex picture of the resistance landscape. These findings highlight the importance of tailored antibacterial stewardship and proactive public health strategies to manage and mitigate antibacterial resistance effectively.
E. coli species may contain many antibacterial resistance genes and may adversely affect human health. Some of these genes include blaTEM, which encodes a beta-lactamase enzyme that confers resistance to penicillin and some cephalosporins; tetA, which encodes a tetracycline efflux pump that confers resistance to tetracycline and doxycycline; and sul1, a gene that encodes a sulfonamide-resistant dihydropteroate synthase enzyme that confers resistance to sulfonamides [10,11,48]. The results of the present study revealed that 11 different resistance genes were contained in the E. coli isolates. The most common resistance genes were blaTEM (10/74; 13.5%), blaOXA (5/74; 6.8%), and blaCTX-M (1/74; 1.4%) which confer resistance to beta-lactams, and tetA (9/74; 12.2%) and tetB (10/74; 13.5%), which confer resistance to tetracyclines. These genes are often located on mobile genetic elements, such as plasmids and transposons, that can facilitate their horizontal transfer among bacteria [49,50]. We also detected ESβL in only one isolate with the blaCTX-M gene, as well as the quinolone resistance genes qnrA and qnrB in only one isolate each. These genes confer resistance to third-generation cephalosporins and fluoroquinolones, respectively, and their presence in E. coli isolates from food sources is of great concern for public health. The EsβL-associated genes blaTEM and blaCTX-M were detected in the isolates, underscoring the presence of significant resistance mechanisms that complicate treatment options.
In this study, a comprehensive molecular analysis was conducted to assess the presence of virulence and resistance genes in E. coli isolates. Our findings indicate a low prevalence of virulence factors among the isolates, with significant implications for food safety and public health. Strains designated as STEC are characterized by their ability to produce Shiga toxins, which are encoded by the stx1 and stx2 genes. The key distinguishing factor between pathogenic and non-pathogenic E. coli strains lies in the presence of virulence-associated genes [51]. In this study, these virulence-associated genes were not detected in any of the isolates. The non-detection of critical virulence genes such as Shiga-like toxins (stx1 and stx2), the attaching and effacing factor (eae), and the bundle-forming pilus structural gene (bfpA) in the majority of the isolates suggests a reduced potential for causing severe enteropathogenic or enterohemorrhagic infections in consumers. This absence is particularly notable, as these genes are commonly associated with severe gastrointestinal diseases including hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS). Balière et al. (2015) [52] found the presence of the stx gene in 35% of various shellfish samples they collected, and specifically in mussels, the presence of the stx gene was determined as 36.5%. Martin et al. (2019) [53] identified the virulence genes stx1, stx2, and eae at a lower frequency (7%) in Shiga toxin-producing E. coli isolates obtained from Norwegian bivalves in marine environments. This holds paramount significance for consumers of shellfish, as insufficient heat treatment during the preparation of edible shellfish species can result in foodborne infections [51]. The identification of the O111 antigen (wbdl) in only one isolate (23EM) and the flagellar antigen H7 (flicH7) in three isolates (7EM, 10EM, and 12EM) highlights the presence of specific pathogenic isolates that warrant closer attention. While the prevalence of these antigens is low, their presence indicates a potential risk for pathogenicity and necessitates continuous surveillance. The O111 and H7 antigens are particularly concerning due to their association with outbreaks and severe illness in humans [4]. The detection of these antigens, even in a small number of isolates, underscores the importance of rigorous food safety protocols in the processing of mussels. RTE mussel products pose a higher risk of contamination, emphasizing the crucial need for rigorous microbial testing and control measures in food production facilities.
Our study revealed the presence of E. coli isolates with diverse serotypes, phylogenetic groups, virulence factors, and AMR profiles in fresh raw mussels from the Marmara Sea in Turkey. Some of these isolates may have the potential to cause human infections and pose a challenge for the treatment of these infections. In addition, future studies could focus on comparing various contamination prevention methods to determine the most effective strategies for reducing bacterial counts in RTE mussels. Such research would provide valuable insights into optimizing processing techniques to enhance food safety.
5. Conclusions
In conclusion, while the prevalence of E. coli in RTE products appears low, the unauthorized harvesting and sale of mussels in polluted coastal areas of Turkey present significant food safety risks. To address these concerns, adherence to quality and safety standards during cultivation, thorough cleaning, proper cooking, and timely consumption or storage of mussels and stuffed mussels are crucial. Additionally, strict hygiene measures in production, continuous pathogen monitoring, and responsible use of antibacterials are essential to protect public health and prevent antibacterial resistance. Collaborative efforts among stakeholders in the seafood industry, regulatory agencies, and healthcare professionals are essential to effectively mitigate these risks and ensure the safety of seafood consumers. This study demonstrated that, in addition to phenotypic and PCR-based classification, genome-based classification and serotype determination should be included in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13070532/s1. Supplementary Table S1: Regions, sampling points, and types of samples taken from four mussel processing and sale companies around Marmara Sea; Supplementary Table S2: E. coli isolates used in phenotypic antibacterial resistance and genetic diversity investigation; Supplementary Table S3: Genes for phylogenetic group detection of E. coli isolates; Supplementary Table S4: Antibacterial resistance and ESBL profile of E. coli isolates; Supplementary Table S5: Antibacterial resistance genes of E. coli isolates.
Author Contributions
Conceptualization, A.Y., I.B.S. and M.D.; methodology, A.Y., I.B.S. and M.D.; formal analysis, A.Y., I.B.S. and M.D.; investigation, A.Y., I.B.S., N.A. and M.D.; resources, A.Y., I.B.S., N.A. and M.D.; data curation, A.Y., I.B.S. and M.D.; writing—original draft preparation, A.Y., I.B.S. and M.D.; writing—review and editing, A.Y., I.B.S., N.A. and M.D.; visualization, A.Y., I.B.S. and M.D.; supervision, A.Y., I.B.S. and M.D.; project administration, A.Y., I.B.S. and M.D.; funding acquisition, A.Y., I.B.S. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bursa Uludag University, Scientific Research Project Association Research Grant, grant number TOA-2022-668. The Article Processing Charge was entirely covered by the authors themselves, without external funding or support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data available is included in the manuscript.
Acknowledgments
We would like to express our appreciation to Nedret Guclu for her valuable assistance during the laboratory work. Additionally, we are grateful to Bayram Suzer for his contribution to the preparation of the figure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yaghubi, E.; Carboni, S.; Snipe, R.M.J.; Shaw, C.S.; Fyfe, J.J.; Smith, C.M. Farmed mussels: A nutritive protein source, rich in omega-3 fatty acids, with a low environmental footprint. Nutrients 2021, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Neacșu, S.M.; Oprea, E.; Dumitrescu, D.-E.; Nedelescu, M.; Drăgănescu, D.; Nicolescu, T.O.; Roșca, A.C.; Ghica, M. Black Sea mussels qualitative and quantitative chemical analysis: Nutritional benefits and possible risks through consumption. Nutrients 2022, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Kisla, D.; Uzgun, Y. Microbiological evaluation of stuffed mussels. J. Food Prot. 2008, 7, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Yohans, H.; Mitiku, B.A.; Tassew, H. Levels of Escherichia coli as bio-indicator of contamination of fish food and antibiotic resistance pattern along the value chain in Northwest Ethiopia. Vet. Med. 2022, 13, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.; Velebit, B.; Teodorovic, V.; Djordjevic, V.; Karabasil, N.; Vasilev, D.; Djuric, S.; Adzic, B.; Dimitrijevic, M. Influence of environmental conditions on Norovirus presence in mussels harvested in Montenegro. Food Environ. Virol. 2017, 9, 406–414. [Google Scholar] [CrossRef]
- Mannas, H.; Mimouni, R.; Chaouqy, N.; Hamadi, F.; Martinez-Urtaza, J. Occurrence of Vibrio and Salmonella species in mussels (Mytilus galloprovincialis) collected along the Moroccan Atlantic coast. Springerplus 2014, 3, 265. [Google Scholar] [CrossRef] [PubMed]
- Jozić, S.; Vukić, L.D.; Aljinović, A.; Vlakančić, W.; Cenov, A.; Vrdoljak, T.A.; Rakić, A.; Šolić, M. Is TBX agar a suitable medium for monitoring Escherichia coli in bathing water using the membrane filtration method? Environ. Monit. Assess. 2019, 191, 558. [Google Scholar] [CrossRef] [PubMed]
- Kijewska, A.; Koroza, A.; Grudlewska-Buda, K.; Kijewski, T.; Wiktorczyk-Kapischke, N.; Zorena, K.; Skowron, K. Molluscs—A ticking microbial bomb. Front. Microbiol. 2023, 13, 1061223. [Google Scholar] [CrossRef] [PubMed]
- Shuping, L.S.; Human, I.S.; Lues, J.F.R.; Paulse, A.N. The prevalence of viruses related to the production of mussels and oysters in Saldanha Bay: A systematic review. Aquac. J. 2023, 3, 90–106. [Google Scholar] [CrossRef]
- Bunduki, G.K.; Heinz, E.; Phiri, V.S.; Noah, P.; Feasey, N.; Musaya, J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 753. [Google Scholar] [CrossRef]
- de Nies, L.; Lopes, S.; Busi, S.B.; Galata, V.; Heintz-Buschart, A.; Laczny, C.C.; May, P.; Wilmes, P. PathoFact: A pipeline for the prediction of virulence factors and antimicrobial resistance genes in metagenomic data. Microbiome 2021, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Koga, V.L.; Rodrigues, G.R.; Scandorieiro, S.; Vespero, E.C.; Oba, A.; de Brito, B.G.; de Brito, K.C.; Nakazato, G.; Kobayashi, R.K. Evaluation of the antibiotic resistance and virulence of Escherichia coli strains isolated from chicken carcasses in 2007 and 2013 from Paraná, Brazil. Foodborne Pathog. Dis. 2015, 12, 479–485. [Google Scholar] [CrossRef]
- Ates, M.; Ozkizilcik, A.; Tabakoglu, C. Microbiological analysis of stuffed mussels sold in the streets. Indian J. Med. 2011, 51, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Karademir, F.; Kahraman, T. Microbiological quality of stuffed mussels consumed in Istanbul. Kocatepe Vet. J. 2021, 14, 436–443. [Google Scholar] [CrossRef]
- Kafa, B.; Kilinc, B. Microbiological quality of frozen black mussels (Mytilus galloprovincialis, Lamarck, 1819) purchased from markets in the Izmir Province of Turkey. EgeJFAS 2021, 38, 167–172. [Google Scholar] [CrossRef]
- Mudadu, A.; Spanu, C.; Pantoja, J.; Dos Santos, M.; De Oliveira, C.; Salza, S.; Piras, G.; Uda, M.T.; Virgilio, S.; Giagnoni, L.; et al. Association between Escherichia coli and Salmonella spp. food safety criteria in live bivalve molluscs from wholesale and retail markets. Food Cont. 2022, 137, 108942. [Google Scholar] [CrossRef]
- Nuñal, S.N.; Jane, M.; Monaya, K.; Rose, T.; Mueda, C.; Mae Santander-De Leon, S. Microbiological quality of oysters and mussels along its market supply chain. J. Food Prot. 2023, 86, 100063. [Google Scholar] [CrossRef]
- Selegean, J.P.; Kusserow, R.; Patel, R.; Heidtke, T.M.; Ram, J.L. Using zebra mussels to monitor Escherichia coli in environmental waters. J. Environ. Qual. 2011, 30, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-García, A.; Oliveira, A.P.S.C.D.; Mejia-Ballesteros, J.E.; Godoy, S.H.S.D.; Barbieri, E.; Sousa, R.L.M.D.; Fernandes, A.M. Escherichia coli detection and identification in shellfish from southeastern Brazil. Aquaculture 2019, 504, 158–163. [Google Scholar] [CrossRef]
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli. Part 2: Colony-Count Technique at 44 °C Using 5-bromo-4-chloro-3-indolyl Beta-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- Davidson, P.M.; Roth, L.A.; Gambrel-Lenarz, S.A.; Bruhn, J. Chapter 7, Coliform and other indicator bacteria. In Standard Methods for the Examination of Dairy Products, 17th ed.; Wehr, H.M., Frank, J.F., Eds.; American Public Health Association: Washington, DC, USA, 2004; ISBN 978-0-87553-002-4. [Google Scholar]
- Chen, J.; Griffiths, M.W. PCR Differentiation of Escherichia coli from other gram-negative bacteria using primers derived from the nucleotide sequences flanking the gene encoding the universal stress protein. Lett. Appl. Microbiol. 1998, 27, 369–371. [Google Scholar] [CrossRef]
- Heijnen, L.; Medema, G. Quantitative Detection of E. coli, E. coli O157 and other Shiga toxin producing E. coli in water samples using a culture method combined with real-time PCR. J. Water Health 2006, 4, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsis, S.; Bingen, E. Rapid and simple determination of Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-group. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Alfinete, N.W.; Bolukaoto, J.Y.; Heine, L.; Potgieter, N.; Barnard, T.G. Virulence and phylogenetic analysis of enteric pathogenic Escherichia coli isolated from children with diarrhoea in South Africa. Int. J. Infect. Dis. 2022, 114, 226–232. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.1. 2023. Available online: https://acslm.ie/?page_id=13220 (accessed on 4 April 2024).
- Ogutu, J.O.; Zhang, Q.; Huang, Y.; Yan, H.; Su, L.; Gao, B.; Zhang, W.; Zhao, J.; Cai, W.; Li, W.; et al. Development of a multiplex PCR system and its application in detection of blaSHV, blaTEM, blaCTX-M-1, blaCTX-M-9 and blaOXA-1 group genes in clinical Klebsiella pneumoniae and Escherichia coli strains. J. Antibiot. 2015, 68, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Saticioglu, I.B.; Buyukekiz, A.G.; Balta, F.; Altun, S. Molecular characterization and antimicrobial resistance profile of atypical Citrobacter gillenii and Citrobacter sp. isolated from diseased rainbow trout (Oncorhynchus mykiss). J. Global Antimicrob. Resist. 2017, 10, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Saticioglu, I.B.; Duman, M.; Smith, P.; Wiklund, T.; Altun, S. Antimicrobial resistance and resistance genes in Flavobacterium psychrophilum isolates from Turkey. Aquaculture 2019, 512, 734293. [Google Scholar] [CrossRef]
- ISO/TS 13136:2012; Microbiology of Food and Animal Feed. Real-Time Polymerase Chain Reaction (PCR)-Based Method for the Detection of Food-Borne Pathogens. Horizontal Method for the Detection of Shiga Toxin-Producing Escherichia coli (STEC) and the Determination of O157, O111, O26, O103, and O145 Serogroups. International Organization for Standardization: Geneva, Switzerland, 2012.
- Bennani, M.; Badri, S.; Baibai, T.; Oubrim, N.; Hassar, M.; Cohen, N.; Amarouch, H. First detection of Shiga toxin-producing Escherichia coli in shellfish and coastal environments of Morocco. Appl. Biochem. Biotechnol. 2011, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Nayak, B.B.; Kumar, S.H. High Prevalence of multiple antibiotic-resistant, extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli in fresh seafood sold in retail markets of Mumbai, India. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Bingol, B.E.; Colak, H.; Muratoglu, K.; Hampikyan, H. The microbiological quality of stuffed mussels (Midye Dolma) sold in Istanbul. Brit. Food J. 2008, 110, 1079–1087. [Google Scholar] [CrossRef]
- Hunt, J.M. Shiga Toxin-Producing Escherichia coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Allahyari, S.; Amani, Z.; Bruck, W.M.; Mahmoudi, R.; Peymani, A. Prevalence, phylogroups, and antimicrobial susceptibility of Escherichia coli isolates from food products. Antibiotics 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, A.M.; Mudadu, A.G.; Esposito, G.; Urru, R.; Ortu, S.; Mara, L.; Uda, M.T.; Arras, I.; Lorenzoni, G.; Sanna, G.; et al. Bacterial and viral investigations combined with determination of phytoplankton and algal biotoxins in mussels and water from a Mediterranean coastal lagoon (Sardinia, Italy). J. Food Prot. 2019, 82, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Sferlazzo, G.; Meloni, D.; Lamon, S.; Marceddu, M.; Mureddu, A.; Consolati, S.G.; Pisanu, M.; Virgilio, S. Evaluation of short purification cycles in naturally contaminated Mediterranean mussels (Mytilus galloprovincialis) harvested in Sardinia (Italy). Food Microbiol. 2018, 74, 86. [Google Scholar] [CrossRef] [PubMed]
- Lamon, S.; Piras, F.; Meloni, D.; Agus, V.; Porcheddu, G.; Pes, M.; Cambula, M.G.; Esposito, G.; Fois, F.; Consolati, S.G.; et al. Enumeration of Escherichia coli and determination of Salmonella spp. and verotoxigenic Escherichia coli in shellfish (Mytilus galloprovincialis and Ruditapes decussatus) harvested in Sardinia, Italy. Ital. J. Food Saf. 2020, 9, 195–200. [Google Scholar]
- Normanno, G.; Parisi, A.; Addante, N.; Quaglia, N.C.; Dambrosio, A.; Montagna, C.; Chiocco, D. Vibrio parahaemolyticus, Vibrio vulnificus and microorganisms of fecal origin in mussels (Mytilus galloprovincialis) sold in the Puglia region (Italy). Int. J. Food Microbiol. 2006, 106, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Katongole, P.; Bulwadda, K.D.; Kyobe, B.H.; Patrick, K.D.; Florence, N.C. Phylogenetic groups and antimicrobial susceptibility patterns of uropathogenic Escherichia coli clinical isolates from patients at Mulago National Referral Hospital, Kampala, Uganda. F1000Research 2019, 8, 1828. [Google Scholar] [CrossRef]
- Redha, M.A.; Al Sweih, N.; Albert, M.J. Virulence and phylogenetic groups of Escherichia coli cultured from raw sewage in Kuwait. Gut Pathog. 2022, 14, 18. [Google Scholar] [CrossRef]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018, 4, e000192. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rashid, M.; Kotwal, S.K.; Malik, M.; Singh, M. Prevalence, genetic profile of virulence determinants and multidrug resistance of Escherichia coli isolates from foods of animal origin. Vet. World 2013, 6, 139–142. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liao, C.; Peng, J.; Tao, S.; Zhang, T.; Dai, Y.; Ding, Y.; Ma, Y. Resistance and virulence gene analysis and molecular typing of Escherichia coli from duck farms in Zhanjiang, China. Front. Cell. Infect. Microbiol. 2023, 13, 1202013. [Google Scholar] [CrossRef] [PubMed]
- Mwakyoma, A.A.; Kidenya, B.R.; Minja, C.A.; Mushi, M.F.; Sandeman, A.; Sabiti, W.; Holden, M.T.G.; Mshana, S.E. Comparison of horizontal blaCTX-M gene transfer via conjugation among extended spectrum β-lactamases producing Escherichia coli isolates from patients with urinary tract infection, their animals, and environment. Arch. Mol. Biol. Genet. 2023, 2, 1–8. [Google Scholar] [CrossRef]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef] [PubMed]
- Al Qabili, D.M.A.; Aboueisha, A.M.; Ibrahim, G.A.; Youssef, A.I.; El-Mahallawy, H.S. Virulence and antimicrobial-resistance of shiga toxin-producing E. coli (STEC) isolated from edible shellfish and its public health significance. Arch. Microbiol. 2022, 204, 510. [Google Scholar] [CrossRef] [PubMed]
- Baliere, C.; Rince, A.; Blanco, J.; Dahbi, G.; Harel, J.; Vogeleer, P.; Giard, J.C.; Mariani-Kurkdjian, P.; Gourmelon, M. Prevalence and characterization of shiga toxin-producing and enteropathogenic Escherichia coli in shellfish-harvesting areas and their watersheds. Front. Microbiol. 2015, 6, 1356. [Google Scholar] [CrossRef]
- Martin, C.C.; Svanevik, C.S.; Lunestad, B.T.; Sekse, C.; Johannessen, G.S. Isolation and characterisation of Shiga toxin-producing Escherichia coli from Norwegian bivalves. Food Microbiol. 2019, 84, 103268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).