Detection of Salmonella Mbandaka Carrying the blaCTX-M-8 Gene Located on IncI1 Plasmid Isolated from a Broiler Flock Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolate, Serotyping, and Antimicrobial Testing
2.2. Whole-Genome Sequencing and Bioinformatical Analysis
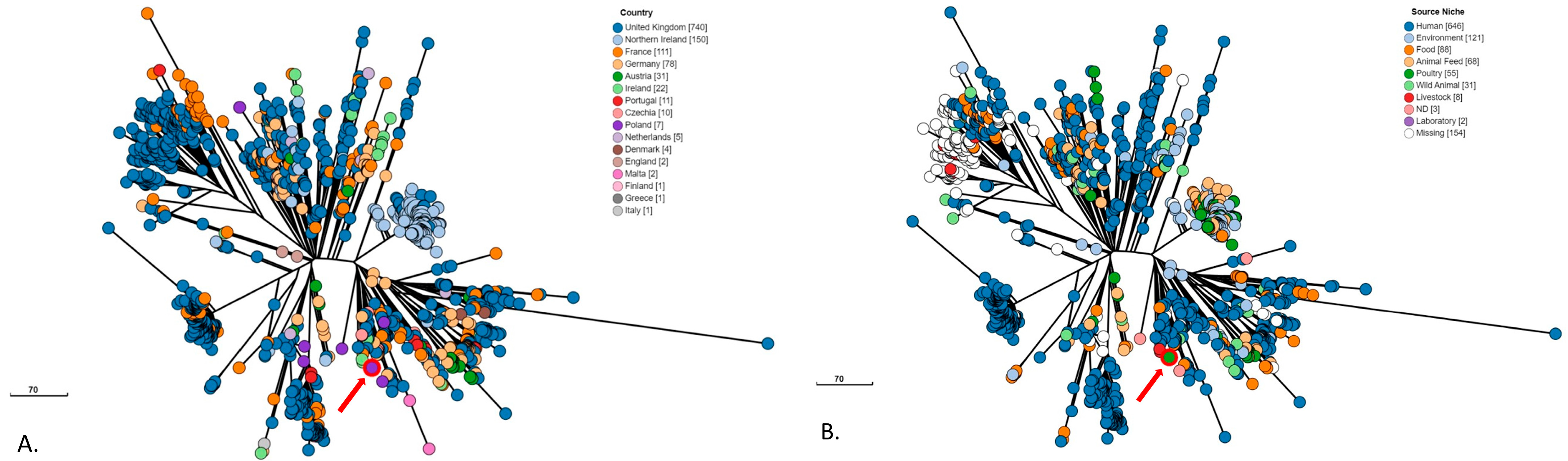
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Hayward, M.R.; Petrovska, L.; Jansen, V.A.A.; Woodward, M.J. Population structure and associated phenotypes of Salmonella enterica serovars Derby and Mbandaka overlap with host range. BMC Microbiol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Hoszowski, A.; Zając, M.; Lalak, A.; Przemyk, P.; Wasyl, D. Fifteen years of successful spread of Salmonella enterica serovar Mbandaka clone ST413 in Poland and its public health consequences. Ann. Agric. Environ. Med. 2016, 23, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Skarżyńska, M.; Hoszowski, A.; Zając, M.; Lalak, A.; Samcik, I.; Kwit, R.; Wasyl, D. Distribution of Salmonella serovars along the food chain in Poland, 2010–2015. J. Vet. Res. 2017, 61, 173–179. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Public Health NIH—National Research Institute, Department of Epidemiology and Surveillance of Infectious Diseases and Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection. Infectious Diseases and Poisonings in Poland in 2022; National Institute of Public Health NIH: Warsaw, Poland, 2023; pp. 1–155. Available online: https://wwwold.pzh.gov.pl/oldpage/epimeld/2022/Ch_2022.pdf (accessed on 15 May 2024).
- De Sousa Violante, M.; Michel, V.; Romero, K.; Bonifait, L.; Baugé, L.; Perrin-Guyomard, A.; Feurer, C.; Radomski, N.; Mallet, L.; Mistou, M.-Y.; et al. Tell me if you prefer bovine or poultry sectors and I’ll tell you who you are: Characterization of Salmonella enterica subsp. enterica serovar Mbandaka in France. Front. Microbiol. 2023, 14, 1130891. [Google Scholar] [CrossRef]
- Keaton, A.A.; Schwensohn, C.A.; Brandenburg, J.M.; Pereira, E.; Adcock, B.; Tecle, S.; Hinnenkamp, R.; Havens, J.; Bailey, K.; Applegate, B.; et al. Multistate outbreak of Salmonella Mbandaka infections linked to sweetened puffed wheat cereal—United States, 2018. Epidemiol. Infect. 2022, 150, e135. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC) and European Food Safety Authority (EFSA). Multi-country outbreak of Salmonella Mbandaka ST413 linked to consumption of chicken meat products in the EU/EEA and the UK-first update. EFSA Support. Publ. 2024, 21, 8749E. [Google Scholar] [CrossRef]
- World Health Organization. WHO List of Medically Important Antimicrobials A Risk Management Tool for Mitigating Antimicrobial Resistance due to Non-Human Use; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Gierczyński, R.; Szych, J.; Rastawicki, W.; Jagielski, M. The molecular characterisation of the extended spectrum beta-lactamase (ESBL) producing strain of Salmonella enterica serovar Mbandaka isolated in Poland. Acta Microbiol. Pol. 2003, 52, 183–190. [Google Scholar]
- Eller, C.; Leistner, R.; Guerra, B.; Fischer, J.; Wendt, C.; Rabsch, W.; Werner, G.; Pfeifer, Y. Emergence of extended-spectrum-lactamase (ESBL)CTX-M-8 in Germany. J. Antimicrob. Chemother. 2014, 69, 562–564. [Google Scholar] [CrossRef]
- Norizuki, C.; Wachino, J.I.; Suzuki, M.; Kawamura, K.; Nagano, N.; Kimura, K.; Arakawa, Y. Specific blaCTX-M-8/IncI1 Plasmid Transfer among Genetically Diverse Escherichia coli Isolates between Humans and Chickens. Antimicrob. Agents Chemother. 2017, 61, e00663-17. [Google Scholar] [CrossRef]
- Soares, F.B.; Camargo, C.H.; Cunha, M.P.V.; de Almeida, E.A.; Bertani, A.M.J.; de Carvalho, E.; de Paiva, J.B.; Fernandes, S.A.; Tiba-Casas, M.R. Co-occurrence of qnrE1 and blaCTX-M-8 in IncM1 transferable plasmids contributing to MDR in different Salmonella serotypes. J. Antimicrob. Chemother. 2019, 74, 1155–1156. [Google Scholar] [CrossRef]
- Sartori, L.; Sellera, F.P.; Moura, Q.; Cardoso, B.; Fontana, H.; Côrtes, L.; Cerdeira, L.; Lincopan, N. Genomic features of a polymyxin-resistant Klebsiella pneumoniae ST491 isolate co-harbouring blaCTX-M-8 and qnrE1 genes from a hospitalised cat in São Paulo, Brazil. J. Glob. Antimicrob. Resist. 2020, 21, 186–187. [Google Scholar] [CrossRef]
- PN-EN ISO 6579-1:2017-04/A1:2020; Food Chain Microbiology—Horizontal Method for Detection, Enumeration and Serotyping of Salmonella. ISO: Geneva, Switzerland, 2020.
- Grimont, P.A.D.; Weill, F.X. Antigenic Formulae of Salmonella Serovars, 9th ed.; WHO Collaborating Centre for Research on Salmonella, Institute Pasteur: Paris, France, 2017; Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 20 February 2024).
- Shifu, C. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Larsen, M.; Cosentino, S.; Rasmussen, S.; Rundsten, C.; Hasman, H.; Marvig, R.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.; Aarestrup, F.; et al. Multilocus Sequence Typing of Total Genome Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Volby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; the Agama Study Group; Achtman, M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Manageiro, V.; Clemente, L.; Romão, R.; Silva, C.; Vieira, L.; Ferreira, E.; Caniça, M. IncX4 Plasmid Carrying the New mcr-1.9 Gene Variant in a CTX-M-8-Producing Escherichia coli Isolate Recovered From Swine. Front. Microbiol. 2019, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.P.R.; Gonzalez, I.H.L.; Ramos, P.L.; Stehling, E.G. International high-risk clone of multidrug-resistant CTX-M-8-producing Escherichia coli C-ST410 infecting an elephant (Loxodonta africana) in a zoo. J. Glob. Antimicrob. Resist. 2020, 22, 643–645. [Google Scholar] [CrossRef]
- Suzuki, M.; Norizuki, C.; Wachino, J.I.; Kawamura, K.; Nagano, N.; Nagano, Y.; Hayashi, W.; Kimura, K.; Doi, Y.; Arakawa, Y. Dissecting the clonality of I1 plasmids using ORF-based binarized structure network analysis of plasmids (OSNAp). J. Infect. Chemother. 2022, 28, 473–479. [Google Scholar] [CrossRef]
- Fernandes, M.R.; McCulloch, J.A.; Vianello, M.A.; Moura, Q.; Pérez-Chaparro, P.J.; Esposito, F.; Sartori, L.; Dropa, M.; Matté, M.H.; Lira, D.P.A.; et al. First Report of the Globally Disseminated IncX4 Plasmid Carrying the mcr-1 Gene in a Colistin-Resistant Escherichia coli Sequence Type 101 Isolate from a Human Infection in Brazil. Antimicrob. Agents Chemother. 2016, 60, 6415–6417. [Google Scholar] [CrossRef]
- Wasyl, D.; Kern-Zdanowicz, I.; Domańska-Blicharz, K.; Zając, M.; Hoszowski, A. High-level fluoroquinolone-resistant Salmonella enterica serovar Kentucky ST198 epidemic clone with IncA/C conjugative plasmid carrying bla(CTX-M-25) gene. Vet. Microbiol. 2015, 175, 85–91. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021–2022. EFSA J. 2024, 22, e8583. [Google Scholar] [CrossRef]
- Szych, J.; Gierczyński, R.; Wardak, S.; Cieślik, A. Wystepowanie i charakterystyka szczepów opornych na antybiotyki oksy-imino-betalaktamowe wśród szczepów Salmonella enterica subsp. enterica izolowanych w Polsce [The occurrence and characterisation of oxy-imino-beta-lactams resistant strains among Salmonella enterica subsp. enterica isolated in Poland]. Med. Dosw. Mikrobiol. 2005, 57, 115–130. [Google Scholar]
- Bonnet, R.; Sampaio, J.L.; Labia, R.; De Champs, C.; Sirot, D.; Chanal, C.; Sirot, J. A novel CTX-M beta-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob. Agents. Chemother. 2000, 44, 1936–1942. [Google Scholar] [CrossRef]
- Vinué, L.; Saenz, Y.; Martinez, S.; Somalo, S.; Moreno, M.A.; Torres, C.; Zarazaga, M. Prevalence and diversity of extended-spectrum β-lactamases in faecal Escherichia coli isolates from healthy humans in Spain. Clin. Microbiol. Infect. 2009, 15, 954–957. [Google Scholar] [CrossRef]
- Foley, S.L.; Kaldhone, P.R.; Ricke, S.C.; Han, J. Incompatibility Group I1 (IncI1) Plasmids: Their Genetics, Biology, and Public Health Relevance. Microbiol. Mol. Biol. Rev. 2021, 85, e00031-20. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. IS26 cannot move alone. J. Antimicrob. Chemother. 2021, 76, 1428–1432. [Google Scholar] [CrossRef]
- Lalak, A.; Wasyl, D.; Zając, M.; Skarżyńska, M.; Hoszowski, A.; Samcik, I.; Woźniakowski, G.; Szulowski, K. Mechanisms of cephalosporin resistance in indicator Escherichia coli isolated from food animals. Vet. Microb. 2016, 194, 69–73. [Google Scholar] [CrossRef]
- Wasyl, D.; Zając, M.; Lalak, A.; Skarżyńska, M.; Samcik, I.; Kwit, R.; Jabłoński, A.; Bocian, A.; Woźniakowski, G.; Hoszowski, A.; et al. Antimicrobial resistance in Escherichia coli isolated from wild animals in Poland. Microb. Drug Resist. 2018, 24, 807–815. [Google Scholar] [CrossRef]
- Zając, M.; Sztromwasser, P.; Bortolaia, V.; Leekitcharoenphon, P.; Cavaco, L.M.; Ziȩtek-Barszcz, A.; Hendriksen, R.S.; Wasyl, D. Occurrence and Characterization of mcr-1-Positive Escherichia coli Isolated From Food-Producing Animals in Poland, 2011–2016. Front. Microbiol. 2019, 10, 1753. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zaja̧c, M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wia̧cek, J.; Wasyl, D. Antimicrobial Resistance Glides in the Sky—Free-Living Birds as a Reservoir of Resistant Escherichia coli With Zoonotic Potential. Front. Microbiol. 2021, 12, 656223. [Google Scholar] [CrossRef]


| No. | Plasmid | Species | Source | Geographic Location | Collection Year | Plasmid Size (bp) | pMLST | Accession No. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | pS22_2161_IncI1 | Salmonella Mbandaka | Broiler flock environment | Poland | 2022 | 89,439 | pST113 | CP146619.1 | This study |
| 2 | pLV23529-CTX-M-8 | Escherichia coli | Sus scrofa | Portugal | 2015 | 89,458 | pST113 | KY964068.1 | [27] |
| 3 | pA117-CTX-M-8 | Escherichia coli | Loxodonta africana | Brazil | 2019 | 101,273 | pST113 | MN816371.1 | [28] |
| 4 | pN23 | Escherichia coli | Homo sapiens | Japan | No data | 91,831 | pST113 | AP017892.1 | [12] |
| 5 | pS11 | Escherichia coli | Chicken meat | Japan | No data | 101,377 | pST113 | AP017893.1 | [12] |
| 6 | pMTY12368_IncI1-I | Escherichia coli | Chicken meat | Japan | 2012 | 84,532 | pST113 | CP134369.1 | unpublished |
| 7 | pHU493 | Escherichia coli | Homo sapiens | Japan | 2010 | 94 912 | pST113 | LC567073.1 | [29] |
| 8 | pCH41 | Escherichia coli | Chicken meat | Japan | 2010 | 86,204 | pST113 | LC567085.1 | [29] |
| 9 | pP44 | Escherichia coli | Canis lupus familiaris | Japan | 2015 | 89,476 | pST113 | LC567053.1 | [29] |
| 10 | pHU485 | Escherichia coli | Homo sapiens | Japan | 2010 | 86,204 | pST114 | LC567072.1 | [29] |
| 11 | pCH110 | Escherichia coli | Chicken meat | Japan | 2010 | 97,607 | pST235 | LC567091.1 | [29] |
| 12 | pCH365 | Escherichia coli | Chicken meat | Japan | 2010 | 108,776 | pST132 | LC567098.1 | [29] |
| 13 | pICBEC72Hctx | Escherichia coli | Homo sapiens | Brazil | 2016 | 92,070 | pST131 | KX443694.1 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, M.; Skarżyńska, M.; Lalak, A.; Iwan, E.; Wasyl, D. Detection of Salmonella Mbandaka Carrying the blaCTX-M-8 Gene Located on IncI1 Plasmid Isolated from a Broiler Flock Environment. Pathogens 2024, 13, 723. https://doi.org/10.3390/pathogens13090723
Zając M, Skarżyńska M, Lalak A, Iwan E, Wasyl D. Detection of Salmonella Mbandaka Carrying the blaCTX-M-8 Gene Located on IncI1 Plasmid Isolated from a Broiler Flock Environment. Pathogens. 2024; 13(9):723. https://doi.org/10.3390/pathogens13090723
Chicago/Turabian StyleZając, Magdalena, Magdalena Skarżyńska, Anna Lalak, Ewelina Iwan, and Dariusz Wasyl. 2024. "Detection of Salmonella Mbandaka Carrying the blaCTX-M-8 Gene Located on IncI1 Plasmid Isolated from a Broiler Flock Environment" Pathogens 13, no. 9: 723. https://doi.org/10.3390/pathogens13090723
APA StyleZając, M., Skarżyńska, M., Lalak, A., Iwan, E., & Wasyl, D. (2024). Detection of Salmonella Mbandaka Carrying the blaCTX-M-8 Gene Located on IncI1 Plasmid Isolated from a Broiler Flock Environment. Pathogens, 13(9), 723. https://doi.org/10.3390/pathogens13090723






