Annexin A2: A Double-Edged Sword in Pathogen Infection
Abstract
:1. Introduction
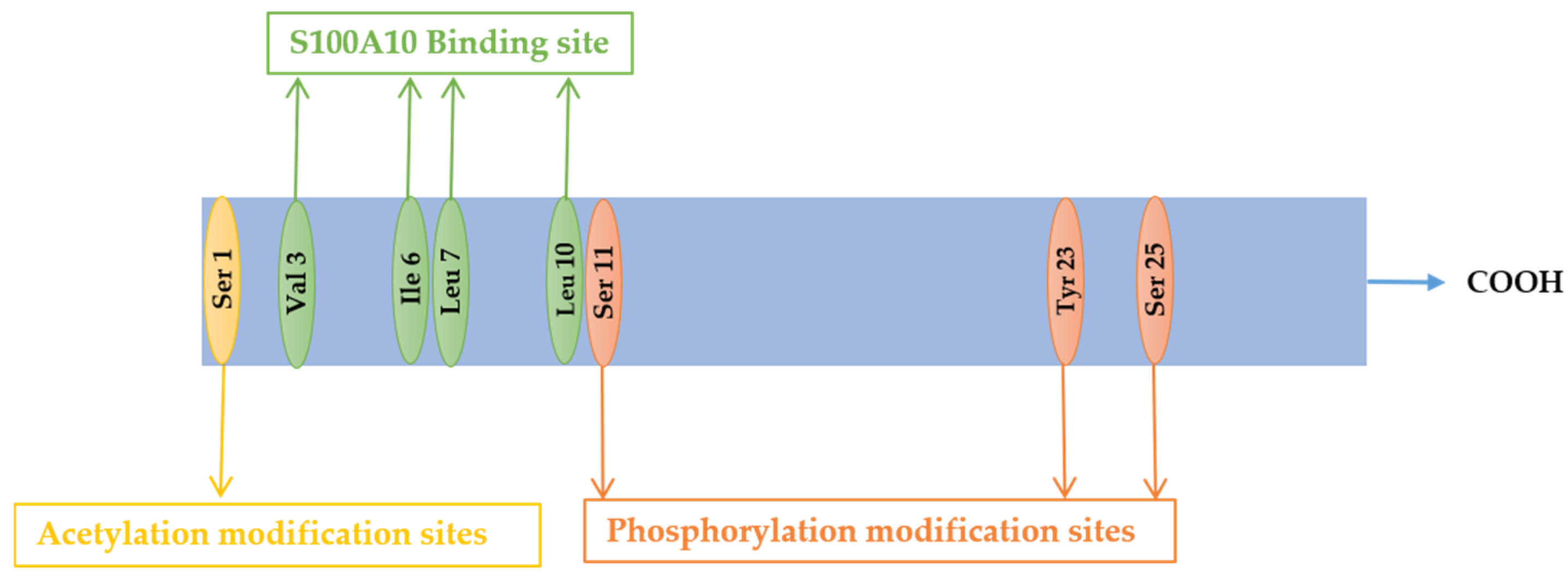
2. Structure and Localization of Annexin A2
3. Mechanisms through Which Annexin A2 Regulates Pathogen Infection
3.1. Annexin A2 Activates Epithelial–Mesenchymal Transition and Promotes Tumor Cell Invasion and Metastasis
3.2. Annexin A2 Is Involved in the Regulation of Innate Immune Responses
3.3. Annexin A2 Regulates Adaptive Immunity
4. Annexin A2 and Bacterial Infection
5. Annexin A2 Protein and Viral Infections
5.1. Annexin A2 Regulates Cytomegalovirus Infection
5.2. Annexin A2 Regulates Influenza Virus Replication
5.3. Annexin A2 Regulates Hepatitis C Virus Assembly and Replication
5.4. Annexin A2 Regulates Pseudorabies Virus Replication
5.5. Annexin A2 Regulates Porcine Reproductive and Respiratory Syndrome Virus Replication
5.6. Annexin A2 Regulates Replication, Assembly, and Release of Classical Swine Fever
5.7. Annexin A2 Regulates Human Papillomavirus Internalization and Infection
5.8. Annexin A2 and other Viruses
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 heterotetramer: Structure and function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef] [PubMed]
- Waisman, D.M. Annexin II tetramer: Structure and function. Mol. Cell. Biochem. 1995, 149–150, 301–322. [Google Scholar] [CrossRef] [PubMed]
- He, K.-L.; Deora, A.B.; Xiong, H.; Ling, Q.; Weksler, B.B.; Niesvizky, R.; Hajjar, K.A. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J. Biol. Chem. 2008, 283, 19192–19200. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Fatimathas, L.; Moss, S.E. Annexins as disease modifiers. Histol. Histopathol. 2010, 25, 6. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Díaz, V.M.; Hurtado, M.; Thomson, T.M.; Reventós, J.; Paciucci, R. Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut 2004, 53, 993–1000. [Google Scholar] [CrossRef]
- Sharma, M.R.; Koltowski, L.; Ownbey, R.T.; Tuszynski, G.P.; Sharma, M.C. Angiogenesis-associated protein annexin II in breast cancer: Selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp. Mol. Pathol. 2006, 81, 146–156. [Google Scholar] [CrossRef]
- Dallacasagrande, V.; Hajjar, K.A. Annexin A2 in Inflammation and Host Defense. Cells 2020, 9, 1499. [Google Scholar] [CrossRef]
- Lim, H.I.; Hajjar, K.A. Annexin A2 in Fibrinolysis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2021, 22, 6836. [Google Scholar] [CrossRef]
- Li, C.; Yu, J.; Liao, D.; Su, X.; Yi, X.; Yang, X.; He, J. Annexin A2: The missing piece in the puzzle of pathogen-induced damage. Virulence 2023, 14, 2237222. [Google Scholar] [CrossRef] [PubMed]
- Liemann, S.; Huber, R. Three-dimensional structure of annexins. Cell. Mol. Life Sci. CMLS 1997, 53, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Erikson, E.; Erikson, R.L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell 1980, 21, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Ween, M.P.; Oehler, M.K.; Ricciardelli, C. The Role of Annexin A2 in Tumorigenesis and Cancer Progression. Cancer Microenviron. 2011, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Glenney, J.R.; Tack, B.F. Amino-terminal sequence of p36 and associated p10: Identification of the site of tyrosine phosphorylation and homology with S-100. Proc. Natl. Acad. Sci. USA 1985, 82, 7884–7888. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Yoon, C.-S.; Jeong, W.; Rhee, S.G.; Waisman, D.M. Annexin A2-S100A10 heterotetramer, a novel substrate of thioredoxin. J. Biol. Chem. 2005, 280, 23584–23592. [Google Scholar] [CrossRef]
- Eberhard, D.A.; Karns, L.R.; VandenBerg, S.R.; Creutz, C.E. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J. Cell Sci. 2001, 114, 3155–3166. [Google Scholar] [CrossRef] [PubMed]
- Chasserot-Golaz, S.; Vitale, N.; Sagot, I.; Delouche, B.; Dirrig, S.; Pradel, L.A.; Henry, J.P.; Aunis, D.; Bader, M.F. Annexin II in exocytosis: Catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J. Cell Biol. 1996, 133, 1217–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rothermund, C.A.; Ayala-Sanmartin, J.; Vishwanatha, J.K. Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 2003, 4, 10. [Google Scholar] [CrossRef]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial-mesenchymal transition: A new target in anticancer drug discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef]
- Wang, T.; Yuan, J.; Zhang, J.; Tian, R.; Ji, W.; Zhou, Y.; Yang, Y.; Song, W.; Zhang, F.; Niu, R. Anxa2 binds to STAT3 and promotes epithelial to mesenchymal transition in breast cancer cells. Oncotarget 2015, 6, 30975. [Google Scholar] [CrossRef]
- Chaudhary, P.; Thamake, S.I.; Shetty, P.; Vishwanatha, J.K. Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by Annexin A2 antibodies. Br. J. Cancer 2014, 111, 2328–2341. [Google Scholar] [CrossRef]
- Rocha, M.R.; Barcellos-de-Souza, P.; Sousa-Squiavinato, A.C.M.; Fernandes, P.V.; Oliveira, I.M.d.; Boroni, M.; Morgado-Diaz, J.A. Annexin A2 overexpression associates with colorectal cancer invasiveness and TGF-ß induced epithelial mesenchymal transition via Src/ANXA2/STAT3. Sci. Rep. 2018, 8, 11285. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, D.; Wu, W.; Wu, S.; Qian, J.; Hao, Y.; Yan, F.; Zhu, P.; Wu, J.; Huang, G.; et al. Mesenchymal Stem Cells Promote Hepatocarcinogenesis via lncRNA-MUF Interaction with ANXA2 and miR-34a. Cancer Res. 2017, 77, 6704–6716. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Trivedi, R.; Tripathi, A.K.; Nandy, R.R.; Wagner, D.C.; Narra, K.; Chaudhary, P. Higher Expression of Annexin A2 in Metastatic Bladder Urothelial Carcinoma Promotes Migration and Invasion. Cancers 2022, 14, 5664. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.P.; Narra, K.; James, J.D.; Jones, H.P.; Tripathi, A.K.; Vishwanatha, J.K. Combination of Small Extracellular Vesicle-Derived Annexin A2 Protein and mRNA as a Potential Predictive Biomarker for Chemotherapy Responsiveness in Aggressive Triple-Negative Breast Cancer. Cancers 2022, 15, 212. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, M.; Guo, Q.; Li, R.; Li, G.; Tan, S.; Li, X.; Wei, Y.; Wu, M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci. Rep. 2015, 5, 15859. [Google Scholar] [CrossRef]
- Stukes, S.; Coelho, C.; Rivera, J.; Jedlicka, A.E.; Hajjar, K.A.; Casadevall, A. The Membrane Phospholipid Binding Protein Annexin A2 Promotes Phagocytosis and Nonlytic Exocytosis of Cryptococcus neoformans and Impacts Survival in Fungal Infection. J. Immunol. 2016, 197, 1252–1261. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Chang, Q.; Su, Z.; Gong, D.; Zhou, Y.; Xiao, J.; Drelich, A.; Liu, Y.; Popov, V.; et al. A new role for host annexin A2 in establishing bacterial adhesion to vascular endothelial cells: Lines of evidence from atomic force microscopy and an in vivo study. Lab. Investig. 2019, 99, 1650–1660. [Google Scholar] [CrossRef]
- Li, R.; Tan, S.; Yu, M.; Jundt, M.C.; Zhang, S.; Wu, M. Annexin A2 Regulates Autophagy in Pseudomonas aeruginosa Infection through the Akt1-mTOR-ULK1/2 Signaling Pathway. J. Immunol. 2015, 195, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, E.; Hoffmann, A. NF-κB signaling. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Swisher, J.F.; Khatri, U.; Feldman, G.M. Annexin A2 is a soluble mediator of macrophage activation. J. Leukoc. Biol. 2007, 82, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, C.; Hu, K. Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. JASN 2012, 23, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Laumonnier, Y.; Syrovets, T.; Simmet, T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhou, X.; Dong, Q.; Fan, R.; Wu, G.; Ji, B.; Meng, Q.; Zheng, M. Regulation of inflammatory response in human chondrocytes by lentiviral mediated RNA interference against S100A10. Inflamm. Res. 2012, 61, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, J.S.; Kim, W.K.; Oh, K.-J.; Kim, J.-M.; Lee, H.J.; Han, B.S.; Kim, D.S.; Seo, Y.S.; Lee, S.C.; et al. Intracellular annexin A2 regulates NF-κB signaling by binding to the p50 subunit: Implications for gemcitabine resistance in pancreatic cancer. Cell Death Dis. 2015, 6, e1606. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, H.; Zhang, Z.; Gu, Y.; Qiu, H.; He, Z. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. CR 2017, 36, 123. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, C.L.; Tseng, Y.L.; Fang, Y.T.; Lin, Y.S.; Su, W.C.; Chen, C.C.; Chang, K.C.; Wang, Y.C.; Lin, C.F. Annexin A2 silencing induces G2 arrest of non-small cell lung cancer cells through p53-dependent and -independent mechanisms. J. Biol. Chem. 2012, 287, 32512–32524. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, J.; Gu, L.; Bao, H.; Zhao, Y.; Shi, L.; Yao, W.; Tian, G.; Wang, X.; Chen, H. Annexin A2 (ANXA2) interacts with nonstructural protein 1 and promotes the replication of highly pathogenic H5N1 avian influenza virus. BMC Microbiol. 2017, 17, 191. [Google Scholar] [CrossRef]
- Weng, M.; Guo, Z.; Lu, Q.; Jin, Q.; Jiang, Y.; Wang, F.; Guo, J.; Xing, G.; Qiao, S.; Zhang, G. Pseudorabies Virus Regulates the Extracellular Translocation of Annexin A2 To Promote Its Proliferation. J. Virol. 2023, 97, e01545-22. [Google Scholar] [CrossRef]
- Li, J.; Guo, D.; Huang, L.; Yin, M.; Liu, Q.; Wang, Y.; Yang, C.; Liu, Y.; Zhang, L.; Tian, Z.; et al. The interaction between host Annexin A2 and viral Nsp9 is beneficial for replication of porcine reproductive and respiratory syndrome virus. Virus Res. 2014, 189, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, Z.; Guo, H.; Qu, H.; Zhang, Y.; Tu, C. Annexin 2 is a host protein binding to classical swine fever virus E2 glycoprotein and promoting viral growth in PK-15 cells. Virus Res. 2015, 201, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Skeate, J.G.; Kast, W.M. Annexin A2 in Virus Infection. Front. Microbiol. 2018, 9, 2954. [Google Scholar] [CrossRef] [PubMed]
- Laumonnier, Y.; Syrovets, T.; Burysek, L.; Simmet, T. Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood 2006, 107, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, C.; Deora, A.B.; Jacovina, A.T.; Weintraub, R.; Gertler, M.; Khan, K.M.F.; Falcone, D.J.; Hajjar, K.A. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: Enhanced expression by macrophages. Blood 2004, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- McVoy, L.A.; Kew, R.R. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J. Immunol. 2005, 175, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Hanagiri, T.; Shigemats, Y.; Onitsuka, T.; Kuroda, K.; Baba, T.; Mizukami, M.; Ichiki, Y.; Uramoto, H.; Takenoyama, M.; et al. Identification of a tumour associated antigen in lung cancer patients with asbestos exposure. Anticancer. Res. 2010, 30, 2631–2639. [Google Scholar]
- Weiss, R.; Bitton, A.; Ben Shimon, M.; Elhaik Goldman, S.; Nahary, L.; Cooper, I.; Benhar, I.; Pick, C.; Chapman, J. Annexin A2, autoimmunity, anxiety and depression. J. Autoimmun. 2016, 73, 92–99. [Google Scholar] [CrossRef]
- Chen, P.; Yan, H.; Tian, Y.; Xun, Y.; Shi, L.; Bao, R.; Zhang, H.; Chen, G.; Yang, C.; Sun, S.; et al. Annexin A2 as a target endothelial cell membrane autoantigen in Behçet’s disease. Sci. Rep. 2015, 5, 8162. [Google Scholar] [CrossRef]
- Caster, D.J.; Korte, E.A.; Merchant, M.L.; Klein, J.B.; Wilkey, D.W.; Rovin, B.H.; Birmingham, D.J.; Harley, J.B.; Cobb, B.L.; Namjou, B.; et al. Autoantibodies targeting glomerular annexin A2 identify patients with proliferative lupus nephritis. Proteom. Clin. Appl. 2015, 9, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jaffee, E.M. Annexin A2 is a new antigenic target for pancreatic cancer immunotherapy. Oncoimmunology 2012, 1, 112–114. [Google Scholar] [CrossRef]
- Kim, V.M.; Blair, A.B.; Lauer, P.; Foley, K.; Che, X.; Soares, K.; Xia, T.; Muth, S.T.; Kleponis, J.; Armstrong, T.D.; et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J. Immunother. Cancer 2019, 7, 132. [Google Scholar] [CrossRef]
- Rotondo, R.; Bertolotto, M.; Barisione, G.; Astigiano, S.; Mandruzzato, S.; Ottonello, L.; Dallegri, F.; Bronte, V.; Ferrini, S.; Barbieri, O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J. Leukoc. Biol. 2011, 89, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, X.; Friesen, T.J.; Kwak, J.W.; Pisarenko, T.; Mekvanich, S.; Velasco, M.A.; Randolph, T.W.; Kargl, J.; Houghton, A.M. Annexin A2/TLR2/MYD88 pathway induces arginase 1 expression in tumor-associated neutrophils. J. Clin. Investig. 2022, 132, e153643. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Z.; Hsieh, M.S.; Cheng, C.W.; Hsu, T.J.; Lin, Y.T.; Lai, C.H.; Liao, C.C.; Chen, W.Y.; Leung, T.K.; Lee, F.P.; et al. Dendritic cells respond to nasopharygeal carcinoma cells through annexin A2-recognizing DC-SIGN. Oncotarget 2015, 6, 159–170. [Google Scholar] [CrossRef]
- Andersen, B.M.; Xia, J.; Epstein, A.L.; Ohlfest, J.R.; Chen, W.; Blazar, B.R.; Pennell, C.A.; Olin, M.R. Monomeric annexin A2 is an oxygen-regulated toll-like receptor 2 ligand and adjuvant. J. Immunother. Cancer 2016, 4, 11. [Google Scholar] [CrossRef]
- Jolly, C.; Winfree, S.; Hansen, B.; Steele-Mortimer, O. The Annexin A2/p11 complex is required for efficient invasion of Salmonella Typhimurium in epithelial cells. Cell. Microbiol. 2014, 16, 64–77. [Google Scholar] [CrossRef]
- Miyahara, A.; Nakanishi, N.; Ooka, T.; Hayashi, T.; Sugimoto, N.; Tobe, T. Enterohemorrhagic Escherichia coli effector EspL2 induces actin microfilament aggregation through annexin 2 activation. Cell. Microbiol. 2009, 11, 337–350. [Google Scholar] [CrossRef]
- Gong, B.; Shelite, T.; Mei, F.C.; Ha, T.; Hu, Y.; Xu, G.; Chang, Q.; Wakamiya, M.; Ksiazek, T.G.; Boor, P.J.; et al. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc. Natl. Acad. Sci. USA 2013, 110, 19615–19620. [Google Scholar] [CrossRef]
- Su, Z.; Shelite, T.R.; Qiu, Y.; Chang, Q.; Wakamiya, M.; Bei, J.; He, X.; Zhou, C.; Liu, Y.; Nyong, E.; et al. Host EPAC1 Modulates Rickettsial Adhesion to Vascular Endothelial Cells via Regulation of ANXA2 Y23 Phosphorylation. Pathogens 2021, 10, 1307. [Google Scholar] [CrossRef]
- Kannan, T.R.; Baseman, J.B. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 6724–6729. [Google Scholar] [CrossRef] [PubMed]
- Somarajan, S.R.; Al-Asadi, F.; Ramasamy, K.; Pandranki, L.; Baseman, J.B.; Kannan, T.R. Annexin A2 mediates Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin binding to eukaryotic cells. mBio 2014, 5, 01497-14. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, J.; Prysliak, T.; Perez-Casal, J. Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect. Immun. 2010, 78, 4570–4578. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Chen, Y.; Li, Y.; Wang, Z.; Li, G.; Wang, G.; Xin, J. GroEL Protein (Heat Shock Protein 60) of Mycoplasma gallisepticum Induces Apoptosis in Host Cells by Interacting with Annexin A2. Infect. Immun. 2019, 87, 00248-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, D.; Zhang, Y.; Zhao, G.; Raheem, A.; Chen, Y.; Chen, X.; Hu, C.; Chen, H.; Yang, L.; et al. Annexin A2 regulates Mycoplasma bovis adhesion and invasion to embryo bovine lung cells affecting molecular expression essential to inflammatory response. Front. Immunol. 2022, 13, 974006. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Lan, S.; Hao, H.; Jin, X.; Liang, J.; Baz, A.A.; Yan, X.; Gao, P.; Chen, S.; et al. LppA is a novel plasminogen receptor of Mycoplasma bovis that contributes to adhesion by binding the host extracellular matrix and Annexin A2. Vet. Res. 2023, 54, 107. [Google Scholar] [CrossRef]
- Norris, F.A.; Wilson, M.P.; Wallis, T.S.; Galyov, E.E.; Majerus, P.W. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 1998, 95, 14057–14059. [Google Scholar] [CrossRef]
- Vu, K.; Tham, R.; Uhrig, J.P.; Thompson, G.R.; Na Pombejra, S.; Jamklang, M.; Bautos, J.M.; Gelli, A.; Berman, J. Invasion of the Central Nervous System by Cryptococcus neoformans Requires a Secreted Fungal Metalloprotease. mBio 2014, 5, 10–1128. [Google Scholar] [CrossRef]
- Eigenheer, R.A.; Jin Lee, Y.; Blumwald, E.; Phinney, B.S.; Gelli, A. Extracellular glycosylphosphatidylinositol-anchored mannoproteins and proteases of Cryptococcus neoformans. FEMS Yeast Res. 2007, 7, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Eigenheer, R.A.; Phinney, B.S.; Gelli, A. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect. Immun. 2013, 81, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Na Pombejra, S.; Salemi, M.; Phinney, B.S.; Gelli, A. The Metalloprotease, Mpr1, Engages AnnexinA2 to Promote the Transcytosis of Fungal Cells across the Blood-Brain Barrier. Front. Cell. Infect. Microbiol. 2017, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Fa, Z.Z.; Xie, Q.; Wang, G.Z.; Yi, J.; Zhang, C.; Meng, G.X.; Gu, J.L.; Liao, W.Q. Complex Roles of Annexin A2 in Host Blood-Brain Barrier Invasion by Cryptococcus neoformans. CNS Neurosci. Ther. 2017, 23, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Wang, M.L.; Cai, M.; Nan, X.M.; Zhao, Y.G.; Xiong, B.H.; Yang, L. Protein Expression Profiles in Exosomes of Bovine Mammary Epithelial Cell Line MAC-T Infected with Staphylococcus aureus. Appl. Environ. Microbiol. 2023, 89, e0174322. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; McCleery, D.; David, S.; Pet, E.; Stef, D.; Iancu, T.; Pet, I.; Stef, L.; Corcionivoschi, N. The mechanistic role of natural antimicrobials in preventing Staphylococcus aureus invasion of MAC-T cells using an in vitro mastitis model. Ir. Vet. J. 2024, 77, 3. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Cheung AH, K.; Wong, C.C.; Liu, W.; Zhou, Y.; Wang, F.; Huang, P.; Yuan, K.; Coker, O.O.; Pan, Y.; et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 2024, 187, 882–896.e817. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Li, R.; Fang, L.; Sun, L.; Wang, Y.; Wu, M. Annexin A2 Modulates ROS and Impacts Inflammatory Response via IL-17 Signaling in Polymicrobial Sepsis Mice. PLoS Pathog. 2016, 12, e1005743. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.F.; Kurosky, A.; Wasi, S. An Endothelial Cell-Surface Form of Annexin II Binds Human Cytomegalovirus. Biochem. Biophys. Res. Commun. 1994, 198, 983–989. [Google Scholar] [CrossRef]
- Wright, J.F.; Kurosky, A.; Pryzdial, E.L.; Wasi, S. Host cellular annexin II is associated with cytomegalovirus particles isolated from cultured human fibroblasts. J. Virol. 1995, 69, 4784–4791. [Google Scholar] [CrossRef]
- Derry, M.C.; Sutherland, M.R.; Restall, C.M.; Waisman, D.M.; Pryzdial, E.L.G. Annexin 2-mediated enhancement of cytomegalovirus infection opposes inhibition by annexin 1 or annexin 5. J. Gen. Virol. 2007, 88, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.-M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.; Marsères, G.; Cosentino, A.; Guerville, F.; Pitard, V.; Fournié, J.; Merville, P.; DéchanetMerville, J.; Couzi, L. Understanding human γδ T cell biology toward a better management of cytomegalovirus infection. Immunol. Rev. 2020, 298, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- LeBouder, F.; Morello, E.; Rimmelzwaan, G.F.; Bosse, F.; Péchoux, C.; Delmas, B.; Riteau, B. Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin. J. Virol. 2008, 82, 6820–6828. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wu, X.; Weng, T.; Cheng, L.; Liu, F.; Wu, H.; Lu, X.; Wu, N.; Sun, S.; Yao, H. Host proteins interact with viral elements and affect the life cycle of highly pathogenic avian influenza A virus H7N9. Heliyon 2024, 10, e28218. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-K.; Jeng, K.-S.; Machida, K.; Lai, M.M.C. Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J. Virol. 2008, 82, 8838–8848. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Lai, C.-K.; Chao, T.-C.; Jeng, K.-S.; Lai, M.M.C. Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft. J. Virol. 2012, 86, 4139–4150. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.B.; Yang, Y.Q.; Gao, J.C.; Zhao, K.; Guo, J.C.; Ye, C.; Jiang, C.G.; Tian, Z.J.; Cai, X.H.; Tong, G.Z.; et al. Annexin A2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication. Vet. Res. 2018, 49, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, L.; Huang, S.; Wang, B.; Li, L.; Li, L.; Gu, W.; Xiao, S.; Liu, G. AMG487 inhibits PRRSV replication and ameliorates lung injury in pig lung xenografts by down-regulating the expression of ANXA2. Antivir. Res. 2022, 202, 105314. [Google Scholar] [CrossRef]
- Sheng, C.; Liu, X.; Jiang, Q.; Xu, B.; Zhou, C.; Wang, Y.; Chen, J.; Xiao, M. Annexin A2 is involved in the production of classical swine fever virus infectious particles. J. Gen. Virol. 2015, 96, 1027–1032. [Google Scholar] [CrossRef]
- Woodham, A.W.; Da, S.D.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.; Langen, R.; Kast, W.M. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef] [PubMed]
- Dziduszko, A.; Ozbun, M. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J. Virol. 2013, 87, 7502–7515. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Fernandez, D.J.; Thornton, S.M.; Skeate, J.G.; Lühen, K.P.; Da, S.D.; Langen, R.; Kast, W.M. Heterotetrameric annexin A2/S100A10 (A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci. Rep. 2018, 8, 11642. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.R.; Wu, Y.Y.; Liao, T.L.; Nielsen, B.L.; Liu, H.J. Cell Entry of Avian Reovirus Modulated by Cell-Surface Annexin A2 and Adhesion G Protein-Coupled Receptor Latrophilin-2 Triggers Src and p38 MAPK Signaling Enhancing Caveolin-1- and Dynamin 2-Dependent Endocytosis. Microbiol. Spectr. 2023, 11, e0000923. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus infection. Semin. Perinatol. 2018, 42, 30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Asha, K.; Khanna, M.; Ronsard, L.; Meseko, C.A.; Sanicas, M. The emerging influenza virus threat: Status and new prospects for its therapy and control. Arch. Virol. 2018, 163, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Unravelling hepatitis C virus replication from genome to function. Nature 2005, 436, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Backes, P.; Quinkert, D.; Reiss, S.; Binder, M.; Zayas, M.; Rescher, U.; Gerke, V.; Bartenschlager, R.; Lohmann, V. Role of annexin A2 in the production of infectious hepatitis C virus particles. J. Virol. 2010, 84, 5775–5789. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Gu, Z.; Hou, C.; Sun, H.; Yang, W.; Dong, J.; Bai, J.; Jiang, P. Emergence of highly virulent pseudorabies virus in southern China. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2015, 79, 221–228. [Google Scholar]
- Rao, P.; Pham, H.T.; Kulkarni, A.; Yang, Y.; Liu, X.; Knipe, D.M.; Cresswell, P.; Yuan, W. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J. Virol. 2011, 85, 8093–8104. [Google Scholar] [CrossRef]
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef]
- Sha, H.; Zhang, H.; Chen, Y.; Huang, L.; Zhao, M.; Wang, N. Research Progress on the NSP9 Protein of Porcine Reproductive and Respiratory Syndrome Virus. Front. Vet. Sci. 2022, 9, 872205. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Bai, J.; Liu, X.; Nauwynck, H.; Jiang, P. ZAP, a CCCH-Type Zinc Finger Protein, Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication and Interacts with Viral Nsp9. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Zhao, S.; Ge, X.; Wang, X.; Liu, A.; Guo, X.; Zhou, L.; Yu, K.; Yang, H. The DEAD-box RNA helicase 5 positively regulates the replication of porcine reproductive and respiratory syndrome virus by interacting with viral Nsp9 in vitro. Virus Res. 2015, 195, 217–224. [Google Scholar] [CrossRef]
- Moennig, V.; Plagemann, P. The pestiviruses. Adv. Virus Res. 1992, 41, 53–98. [Google Scholar] [CrossRef]
- Ruggli, N.; Tratschin, J.D.; Mittelholzer, C.; Hofmann, M.A. Nucleotide sequence of classical swine fever virus strain Alfort/187 and transcription of infectious RNA from stably cloned full-length cDNA. J. Virol. 1996, 70, 3478–3487. [Google Scholar] [CrossRef]
- Risatti, G.R.; Borca, M.V.; Kutish, G.F.; Lu, Z.; Holinka, L.G.; French, R.A.; Tulman, E.R.; Rock, D.L. The E2 glycoprotein of classical swine fever virus is a virulence determinant in swine. J. Virol. 2005, 79, 3787–3796. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Francesci, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- De Sanjose, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Gräßel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Spoden, G.A.; Tenzer, S.; Boller, K.; Bago, R.; Rajesh, S.; Overduin, M.; et al. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 32337. [Google Scholar] [CrossRef]
- Martínez-Costas, J.; Grande, A.; Varela, R.; García-Martínez, C.; Benavente, J. Protein architecture of avian reovirus S1133 and identification of the cell attachment protein. J. Virol. 1997, 71, 59–64. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Mai, H.; Lou, S.; Luo, M.; Xie, H.; Zhou, B.; Hou, J.; Jiang, D.K. A functional variant of CD40 modulates clearance of hepatitis B virus in hepatocytes via regulation of the ANXA2/CD40/BST2 axis. Hum. Mol. Genet. 2023, 32, 1334–1347. [Google Scholar] [CrossRef]

| Virus | Genome | Interoperating Protein | Observations | References |
|---|---|---|---|---|
| CMV | dsDNA | -- | -ANXA2 promotes viral infection -CMV can promote ANXA2 expression -ANXA2-mediated activation of fibrinogen promotes viral replication | [79,80,81,82,83] |
| IAV | ssRNA | NS1 (H5N1) and NP, PA (H7N9) | -ANXA2 interacts with NS1 protein in IAV H5N1 -ANXA2 overexpression increases the titer of IAV H5N1 -ANXA2 protein is involved in the growth cycle of HPAI H7N9 | [40,84,85] |
| HCV | ssRNA | NS | -ANXA2 interacts with nonstructural (NS) proteins -ANXA2 promotes HCV assembly and replication | [86,87] |
| PRV | ssRNA | US3 | -ANXA2 interacts with US3 -ANXA2 promotes PRV replication | [41] |
| PRRSV | ssRNA | NSP9 | -ANXA2 interacts with vimentin -ANXA2 interacts with NSP9 -ANXA2 promotes PRRSV replication | [42,88,89] |
| CSFV | ssRNA | E2 and NS5A | -ANXA2 interacts with E2 -ANXA2 interacts with NS5A -ANXA2 promotes CSFV replication, assembly, and release | [43,90] |
| HPV | dsDNA | -- | -A2t promotes HPV internalization and infection -Knockdown of ANXA2 suppresses HPV16 internalization | [91,92,93] |
| ARV | dsRNA | σC | Inhibition of ANXA2 leads to significant reduction in viral load | [94] |
| Pathogens | In Vivo/Vitro | Cell/Animal Models | References | |
|---|---|---|---|---|
| Bacterium | Klebsiella pneumoniae | vivo | Mice | [28] |
| Rickettsia | vivo | Mice | [30] | |
| vitro | BMECs (mice) and HUVECs (human) | [30,61,62] | ||
| Staphylococcus aureus | vitro | HUVECs (human) and MAC-T cells (human) | [30,75,76] | |
| Mycoplasma pneumoniae | vitro | A549 airway cells (human) | [64] | |
| Mycoplasma bovis | vitro | EBL (cow) | [67,68] | |
| Mycoplasma gallisepticum | vitro | PBMCs (chicken), HEK293T (human), and DF-1 cells (human) | [66] | |
| Salmonella | vitro | MDCK cells (dog) and HeLa cells (human) | [59] | |
| Escherichia coli | vitro | COS-7 cells (monkey) | [60] | |
| Streptococcus anginosus | vitro | Ges-1 (human) and AGS cells (human) | [77] | |
| Fungus | Cryptococcal | vivo | Mice | [29] |
| vitro | hCMEC/D3 cells (human) and BMEC cells (human) | [72,73] | ||
| Virus | CMV | vitro | Human foreskin fibroblast cell lines (human) | [80] |
| IAV | vitro | MDCK cells (dog) and A549 cells (human) | [40,84] | |
| HCV | vitro | Huh-7 cells (human), Huh-7.5 cells (human), and HEK293 cells (human) | [87,98] | |
| PRV | vivo | Mice | [41] | |
| vitro | PK-15 cells (pig) and 3D4/21 cells (pig) | [41] | ||
| PRRSV | vitro | Marc-145 cells (monkey), PAMs (pig), and 293T cells (human) | [42,88] | |
| CSFV | vitro | PK-15 cells (pig) | [43,90] | |
| HPV | vitro | HeLa cells (human) and HaCaT cells (human) | [91,92,93] | |
| ARV | vitro | Vero cells (monkey) and DF-1 cells (chicken) | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhao, D.; Zhang, Y.; Yu, D.; Liu, G.; Zhang, K. Annexin A2: A Double-Edged Sword in Pathogen Infection. Pathogens 2024, 13, 564. https://doi.org/10.3390/pathogens13070564
Wang T, Zhao D, Zhang Y, Yu D, Liu G, Zhang K. Annexin A2: A Double-Edged Sword in Pathogen Infection. Pathogens. 2024; 13(7):564. https://doi.org/10.3390/pathogens13070564
Chicago/Turabian StyleWang, Tianyu, Dengshuai Zhao, Yuanhang Zhang, Dixi Yu, Guoping Liu, and Keshan Zhang. 2024. "Annexin A2: A Double-Edged Sword in Pathogen Infection" Pathogens 13, no. 7: 564. https://doi.org/10.3390/pathogens13070564
APA StyleWang, T., Zhao, D., Zhang, Y., Yu, D., Liu, G., & Zhang, K. (2024). Annexin A2: A Double-Edged Sword in Pathogen Infection. Pathogens, 13(7), 564. https://doi.org/10.3390/pathogens13070564







