The Establishment of a Novel In Vitro System for Culturing Cytauxzoon felis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Inoculums
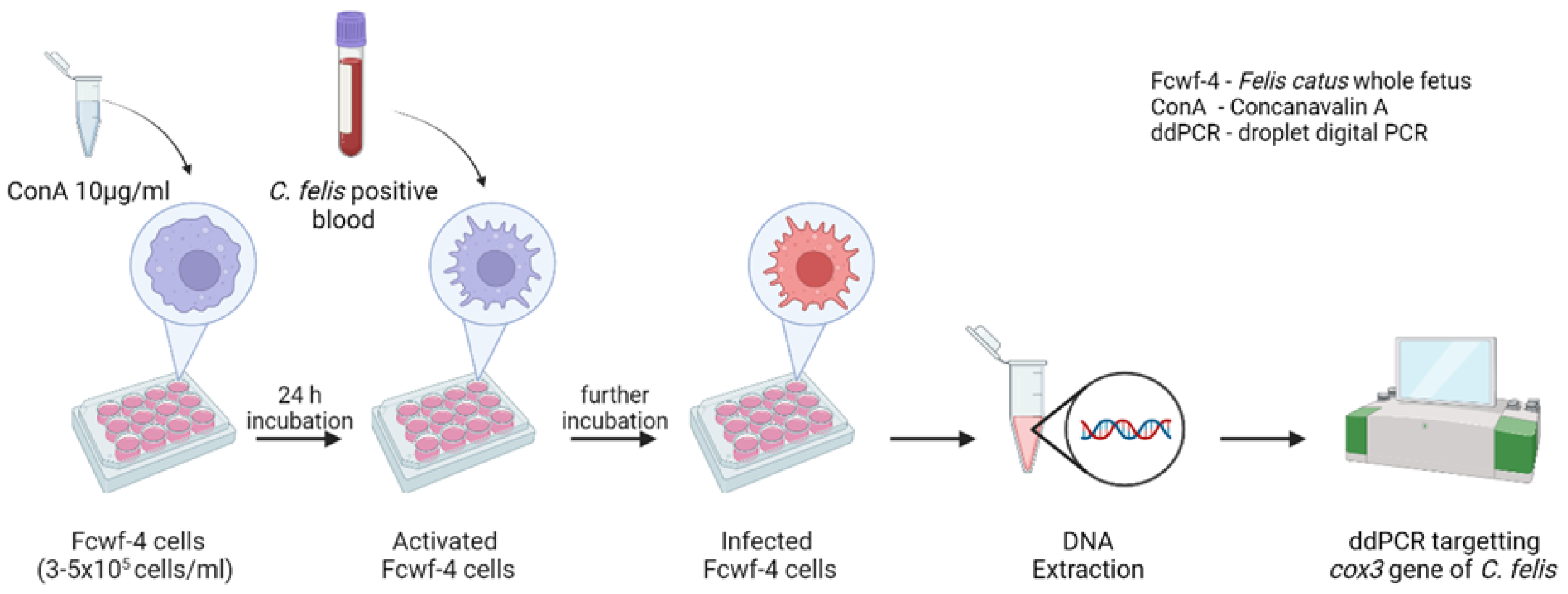
2.3. Experimental Infection of Fcwf-4 Cells with C. felis-Positive Blood
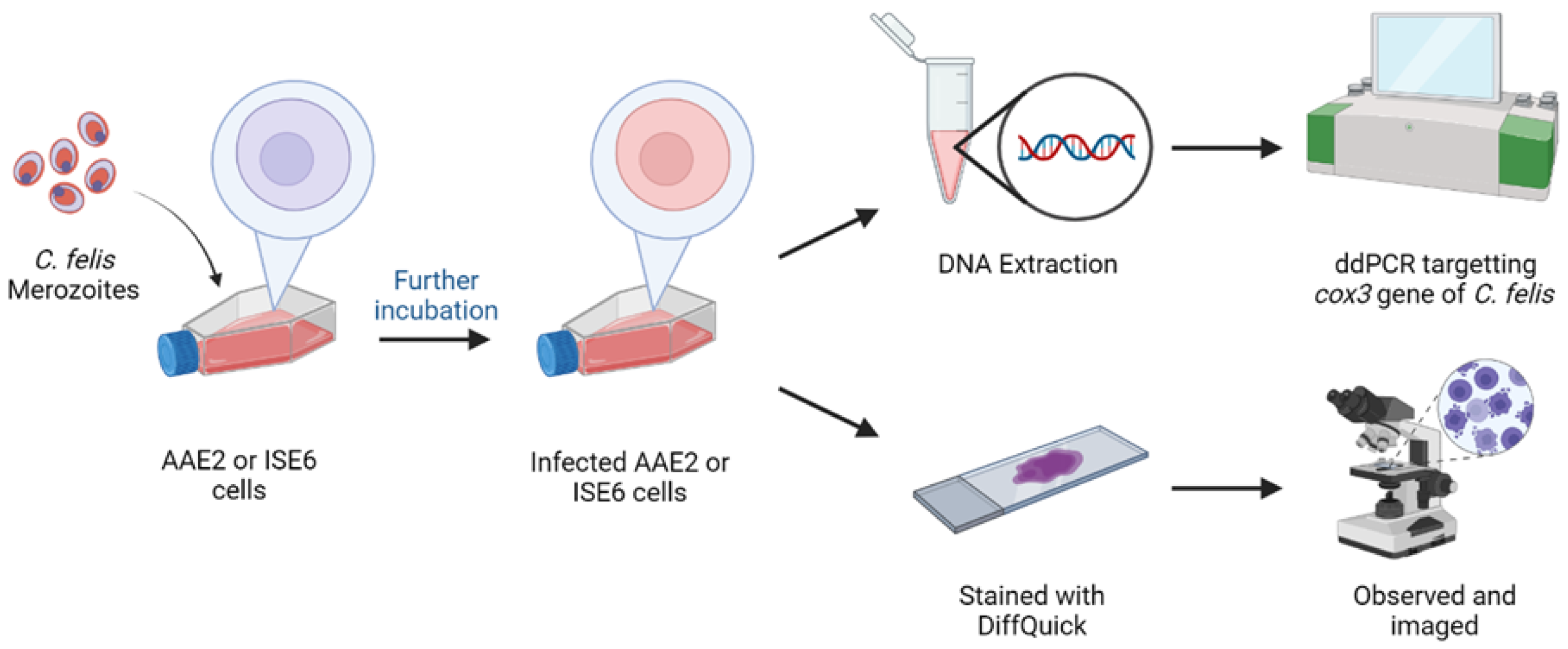
2.4. Experimental Infection of AAE2 Cells with C. felis Merozoites
2.5. In Vitro Inhibition of C. felis
2.6. DNA Extraction
2.7. Parasite Quantification
2.8. Statistical Analysis
3. Results
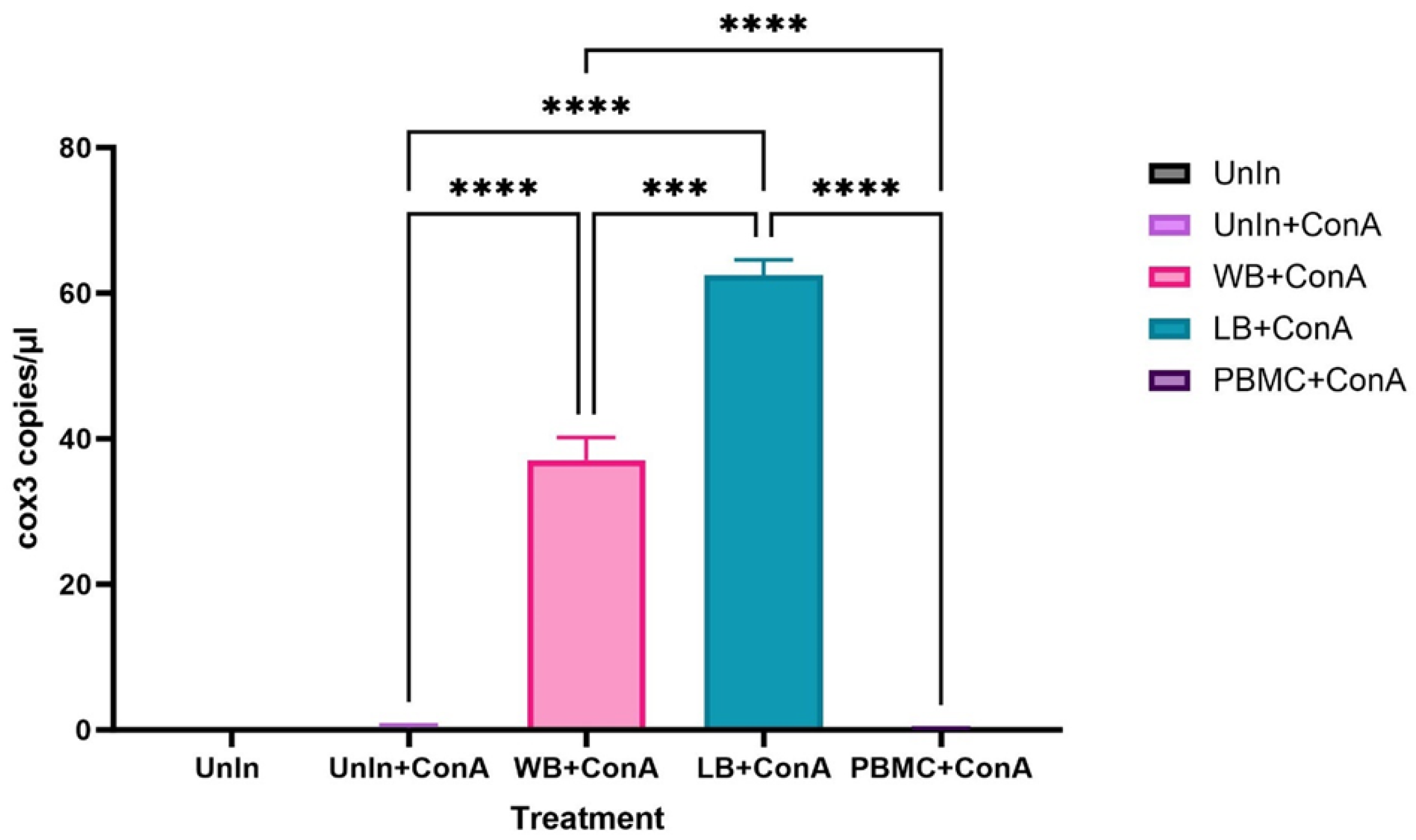
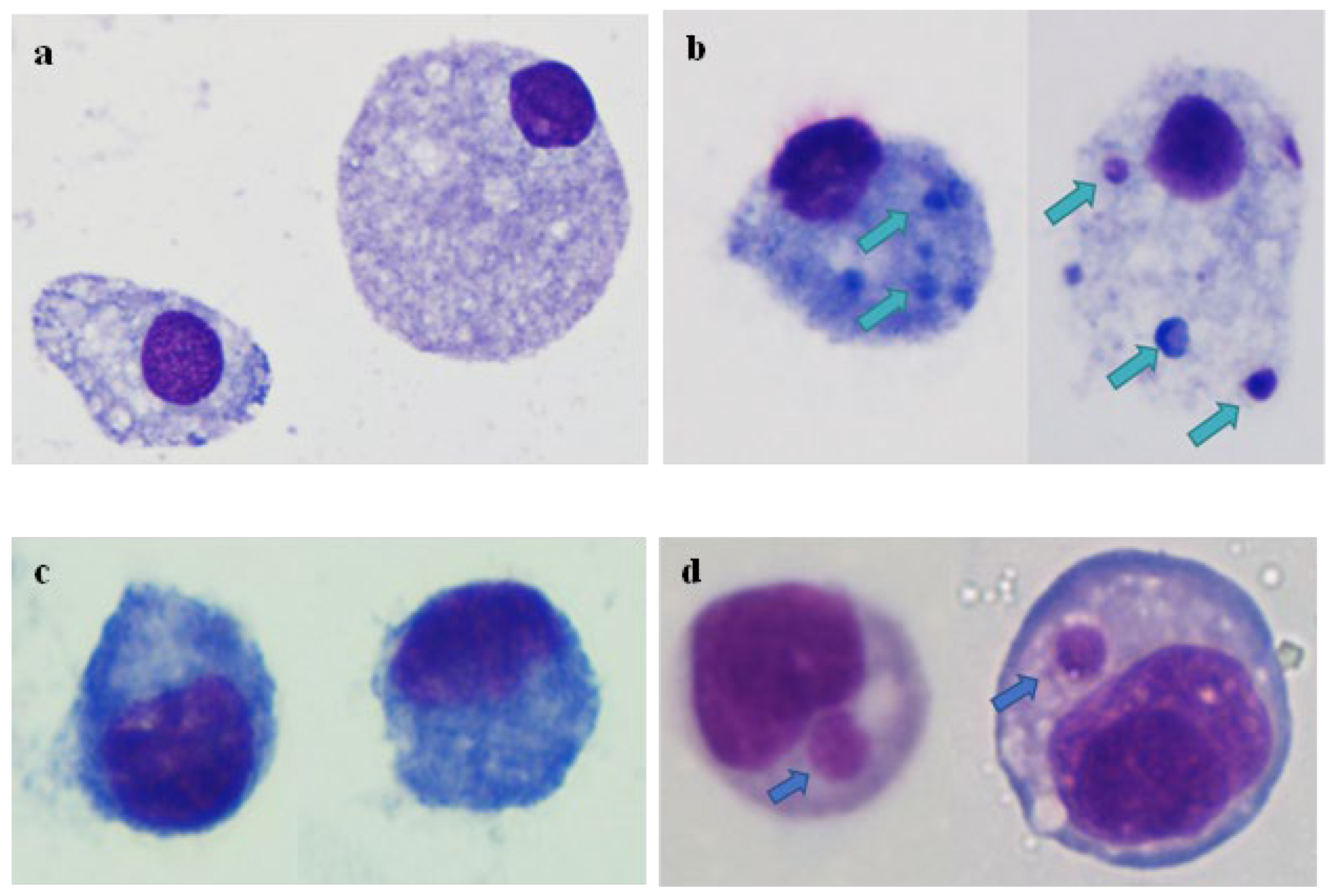
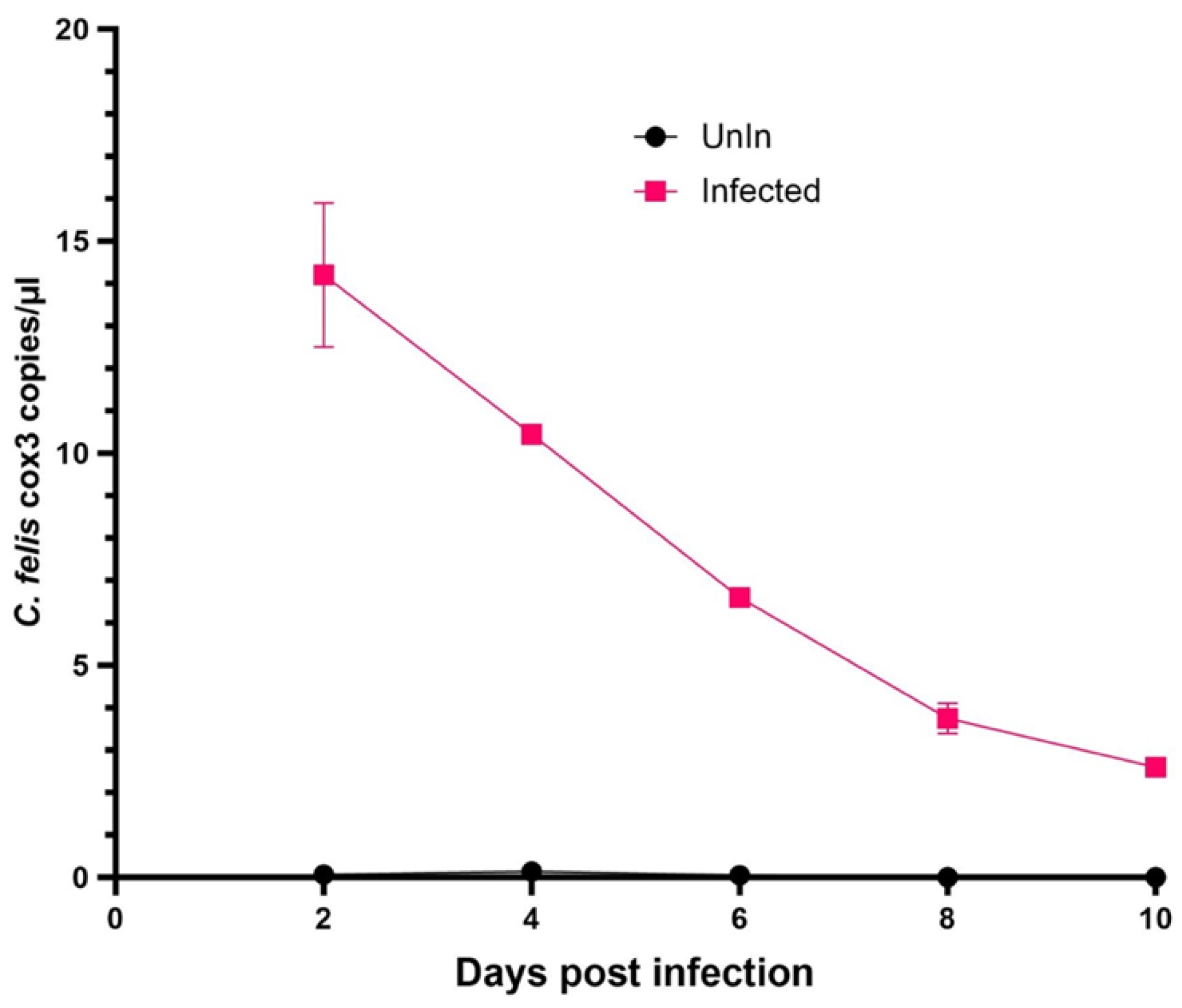
3.1. Inoculation of Fcwf-4 Cells with Chronically Infected Blood Results in Transient Infection, while Acutely Infected Blood Results in Sustained Infection
3.2. Antiprotozoal Drugs and Neutralizing Antibodies Inhibit C. felis Replication In Vitro
3.3. Cytauxzoon felis Merozoites Infects AAE2 and ISE6 Cells In Vitro but Does Not Replicate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichard, M.V.; Sanders, T.L.; Weerarathne, P.; Meinkoth, J.H.; Miller, C.A.; Scimeca, R.C.; Almazán, C. Cytauxzoonosis in North America. Pathogens 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.A.; Birkenheuer, A.J. Cytauxzoonosis. In Greene’s Infectious Diseases of the Dog and Cat; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1218–1229. [Google Scholar]
- Wagner, J. A fatal cytauxzoonosis-like disease in cats. J. Am. Vet. Med. Assoc. 1976, 168, 585–588. [Google Scholar] [PubMed]
- Kier, A.B. The Etiology and Pathogenesis of Feline Cytauxzoonosis. Doctoral Dissertation, University of Missouri, Columbia, MO, USA, 1979. [Google Scholar]
- Kier, A.; Wagner, J.; Kinden, D. The pathology of experimental cytauxzoonosis. J. Comp. Pathol. 1987, 97, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Tarigo, J.L.; Scholl, E.H.; Bird, D.M.; Brown, C.C.; Cohn, L.A.; Dean, G.A.; Levy, M.G.; Doolan, D.L.; Trieu, A.; Nordone, S.K.; et al. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS ONE 2013, 8, e71233. [Google Scholar] [CrossRef]
- Blouin, E.; Kocan, A.A.; Glenn, B.L.; Kocan, K.M.; Hair, J.A. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J. Wildl. Dis. 1984, 20, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Blouin, E.F.; Kocan, A.A.; Kocan, K.M.; Hair, J. Evidence of a limited schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J. Wildl. Dis. 1987, 23, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Meinkoth, J.H.; Edwards, A.C.; Snider, T.A.; Kocan, K.M.; Blouin, E.F.; Little, S.E. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 2009, 161, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Edwards, A.C.; Meinkoth, J.H.; Snider, T.A.; Meinkoth, K.R.; Heinz, R.E.; Little, S.E. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J. Med. Entomol. 2010, 47, 890–896. [Google Scholar] [CrossRef]
- Kier, A.; Wagner, J.; Morehouse, L. Experimental transmission of Cytauxzoon felis from bobcats (Lynx rufus) to domestic cats (Felis domesticus). Am. J. Vet. Res. 1982, 43, 97–101. [Google Scholar]
- Sherrill, M.K.; Cohn, L.A. Cytauxzoonosis: Diagnosis and treatment of an emerging disease. J. Feline Med. Surg. 2015, 17, 940–948. [Google Scholar] [CrossRef]
- Rizzi, T.E.; Reichard, M.V.; Cohn, L.A.; Birkenheuer, A.J.; Taylor, J.D.; Meinkoth, J.H. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasites Vectors 2015, 8, 13. [Google Scholar] [CrossRef]
- Meinkoth, J.H.; Kocan, A.A. Feline cytauxzoonosis. Vet. Clin. Small Anim. Pract. 2005, 35, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Wikander, Y.M.; Reif, K.E. Cytauxzoon felis: An overview. Pathogens 2023, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Meinkoth, J.; Kocan, A.A.; Whitworth, L.; Murphy, G.; Fox, J.C.; Woods, J.P. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J. Vet. Intern. Med. 2000, 14, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Birkenheuer, A.J.; Brunker, J.D.; Ratcliff, E.R.; Craig, A.W. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J. Vet. Intern. Med. 2011, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Munderloh, U.G.; Kurtti, T.J. Formulation of medium for tick cell culture. Exp. Appl. Acarol. 1989, 7, 219–229. [Google Scholar] [CrossRef]
- Sugimoto, C.; Sato, M.; Kawazu, S.; Kamio, T.; Fujisaki, K. Purification of merozoites of Theileria sergenti from infected bovine erythrocytes. Parasitol. Res. 1991, 77, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Weerarathne, P.; Maker, R.; Huang, C.; Taylor, B.; Cowan, S.R.; Hyatt, J.; Tamil Selvan, M.; Shatnawi, S.; Thomas, J.E.; Meinkoth, J.H.; et al. A Novel Vaccine Strategy to Prevent Cytauxzoonosis in Domestic Cats. Vaccines 2023, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-F.; Peake, B.; Madden, R.; Cowan, S.R.; Scimeca, R.C.; Thomas, J.E.; Reichard, M.V.; Ramachandran, A.; Miller, C.A. A probe-based droplet digital polymerase chain reaction assay for early detection of feline acute cytauxzoonosis. Vet. Parasitol. 2021, 292, 109413. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Shindel, N.; Dardiri, A.; Ferris, D. An indirect fluorescent antibody test for the detection of Cytauxzoon-like organisms in experimentally infected cats. Can. J. Comp. Med. 1978, 42, 460. [Google Scholar] [PubMed]
- Frontera-Acevedo, K. Feline immune response to infection by Cytauxzoon felis and the role of CD18 in the pathogenesis of cytauxzoonosis. Doctoral Dissertation, University of Georgia, Athens, GA, USA, 2013. [Google Scholar]
- Loyola, W.; Gaziri, D.A.; Gaziri, L.C.J.; Felipe, I. Concanavalin A enhances phagocytosis and killing of Candida albicans by mice peritoneal neutrophils and macrophages. FEMS Immunol. Med. Microbiol. 2002, 33, 201–208. [Google Scholar] [CrossRef]
- Lukehart, S.A. Activation of macrophages by products of lymphocytes from normal and syphilitic rabbits. Infect. Immun. 1982, 37, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, R.; Paul, S.; Chauhan, A.K.; Kang, S.C. Morin hydrate augments phagocytosis mechanism and inhibits LPS induced autophagic signaling in murine macrophage. Int. Immunopharmacol. 2014, 22, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Hill, D.R. Antiparasitic Agent Atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Siregar, J.E.; Kurisu, G.; Kobayashi, T.; Matsuzaki, M.; Sakamoto, K.; Mi-ichi, F.; Watanabe, Y.I.; Hirai, M.; Matsuoka, H.; Syafruddin, D.; et al. Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex. Parasitol. Int. 2015, 64, 295–300. [Google Scholar] [CrossRef]
- Baneth, G. Antiprotozoal treatment of canine babesiosis. Vet. Parasitol. 2018, 254, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Nathan, H.C.; Hutner, S.H.; Duch, D.S.; Nichol, C.A. Prevention by polyamines of the curative effect of amicarbalide and imidocarb for Trypanosoma brucei infections in mice. Biochem. Pharmacol. 1981, 30, 883–886. [Google Scholar] [CrossRef]
- Plumb, D.C. Plumb’s Veterinary Drug Handbook: Desk; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Greene, C.E.; Latimer, K.; Hopper, E.; Shoeffler, G.; Lower, K.; Cullens, F. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J. Am. Vet. Med. Assoc. 1999, 215, 497–500. [Google Scholar] [CrossRef]
- Lu, F.; Xin-Long, H.E.; Richard, C.; Jun, C.A.O. A brief history of artemisinin: Modes of action and mechanisms of resistance. Chin. J. Nat. Med. 2019, 17, 331–336. [Google Scholar] [CrossRef]
- Eastman, R.T.; Fidock, D.A. Artemisinin-based combination therapies: A vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 2009, 7, 864–874. [Google Scholar] [CrossRef]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P.; Kennedy, T.J. Determination of the activity of ponazuril against Sarcocystis neurona in cell cultures. Vet. Parasitol. 2000, 92, 165–169. [Google Scholar] [CrossRef]
- Mitchell, M.A. Ponazuril. J. Exot. Pet Med. 2008, 17, 228–229. [Google Scholar] [CrossRef]
- Hackstein, J.H.; Mackenstedt, U.; Mehlhorn, H.; Meijerink, J.P.; Schubert, H.; Leunissen, J.A. Parasitic apicomplexans harbor a chlorophyll a-D1 complex, the potential target for therapeutic triazines. Parasitol. Res. 1995, 81, 207–216. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Zajac, A.M.; Davis, W.L.; Kennedy, T.J.; Lindsay, D.S. The effects of ponazuril on development of apicomplexans in vitro. J. Eukaryot. Microbiol. 2005, 52, 231–235. [Google Scholar] [CrossRef]
- Yang, T.S.; Reichard, M.V.; Thomas, J.E.; Marr, H.S.; Karounos, M.; Hyatt, J.; Miller, C.; Birkenheuer, A.J. Transmission of Cytauxzoon felis by injection of Amblyomma americanum salivary glands. Parasitol. Int. 2023, 95, 102753. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Spainhour, R.; Cowan, S.R.; Nafe, L.; Birkenheuer, A.; Reichard, M.V.; Miller, C.A. A Serodiagnostic IgM ELISA to Detect Acute Cytauxzoonosis. Pathogens 2022, 11, 1183. [Google Scholar] [CrossRef]
- Cowell, R.L.; Fox, J.C.; Panciera, R.J.; Tyler, R.D. Detection of anticytauxzoon antibodies in cats infected with a Cytauxzoon organism from bobcats. Vet. Parasitol. 1988, 28, 43–52. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Zweygarth, E.; Blouin, E.F.; Gould, E.A.; Jongejan, F. Tick cell lines: Tools for tick and tick-borne disease research. Trends Parasitol. 2007, 23, 450–457. [Google Scholar] [CrossRef]
- Kocan, K.M.; Halbur, T.; Blouin, E.F.; Onet, V.; de la Fuente, J.; Garcia-Garcia, J.C.; Saliki, J.T. Immunization of cattle with Anaplasma marginale derived from tick cell culture. Vet. Parasitol. 2001, 102, 151–161. [Google Scholar] [CrossRef]
- Banerjee, K.; Guru, P.; Dhanda, V. Growth of arboviruses in cell cultures derived from the tick Haemaphysalts spinigera. Indian J. Med. Res. 1977, 66, 530–536. [Google Scholar]
- Bell-Sakyi, L.; Paxton, E.A.; Munderloh, U.G.; Sumption, K.J. Growth of Cowdria ruminantium, the causative agent of heartwater, in a tick cell line. J. Clin. Microbiol. 2000, 38, 1238–1240. [Google Scholar] [CrossRef]
- Obonyo, M.; Munderloh, U.G.; Fingerle, V.; Wilske, B.; Kurtti, T.J. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 1999, 37, 2137–2141. [Google Scholar] [CrossRef]
- Woldehiwet, Z.; Horrocks, B.K.; Scaife, H.; Ross, G.; Munderloh, U.G.; Bown, K.; Edwards, S.W.; Hart, C.A. Cultivation of an Ovine Strain of Ehrlichia phagocytophila in Tick Cell Cultures. J. Comp. Pathol. 2002, 127, 142–149. [Google Scholar] [CrossRef]
- Bekker, C.P.; Bell-Sakyi, L.; Paxton, E.A.; Martinez, D.; Bensaid, A.; Jongejan, F. Transcriptional analysis of the major antigenic protein 1 multigene family of Cowdria ruminantium. Gene 2002, 285, 193–201. [Google Scholar] [CrossRef]
- Hart, C.E.; Thangamani, S. Tick-virus interactions: Current understanding and future perspectives. Parasite Immunol. 2021, 43, e12815. [Google Scholar] [CrossRef]
- Blouin, E.F.; de la Fuente, J.; Garcia-Garcia, J.C.; Sauer, J.R.; Saliki, J.T.; Kocan, K.M. Applications of a cell culture system for studying the interaction of Anaplasma marginale with tick cells. Anim. Health Res. Rev. 2002, 3, 57–68. [Google Scholar] [CrossRef]
- Al-Rofaai, A.; Bell-Sakyi, L. Tick cell lines in research on tick control. Front. Physiol. 2020, 11, 152. [Google Scholar] [CrossRef]





| Antiprotozoal | Final Concentration in Cell Culture |
|---|---|
| Atovaquone | 0.1 nM, 1 nM, 10 nM |
| Imidocarb dipropionate | 2 nM, 200 nM |
| Artemisinin | 10 nM, 500 nM |
| Ponazuril | 5 µg/mL, 25 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerarathne, P.; Reichard, M.; Miller, C.; Scimeca, R.C. The Establishment of a Novel In Vitro System for Culturing Cytauxzoon felis. Pathogens 2024, 13, 565. https://doi.org/10.3390/pathogens13070565
Weerarathne P, Reichard M, Miller C, Scimeca RC. The Establishment of a Novel In Vitro System for Culturing Cytauxzoon felis. Pathogens. 2024; 13(7):565. https://doi.org/10.3390/pathogens13070565
Chicago/Turabian StyleWeerarathne, Pabasara, Mason Reichard, Craig Miller, and Ruth C. Scimeca. 2024. "The Establishment of a Novel In Vitro System for Culturing Cytauxzoon felis" Pathogens 13, no. 7: 565. https://doi.org/10.3390/pathogens13070565
APA StyleWeerarathne, P., Reichard, M., Miller, C., & Scimeca, R. C. (2024). The Establishment of a Novel In Vitro System for Culturing Cytauxzoon felis. Pathogens, 13(7), 565. https://doi.org/10.3390/pathogens13070565









