Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm
Abstract
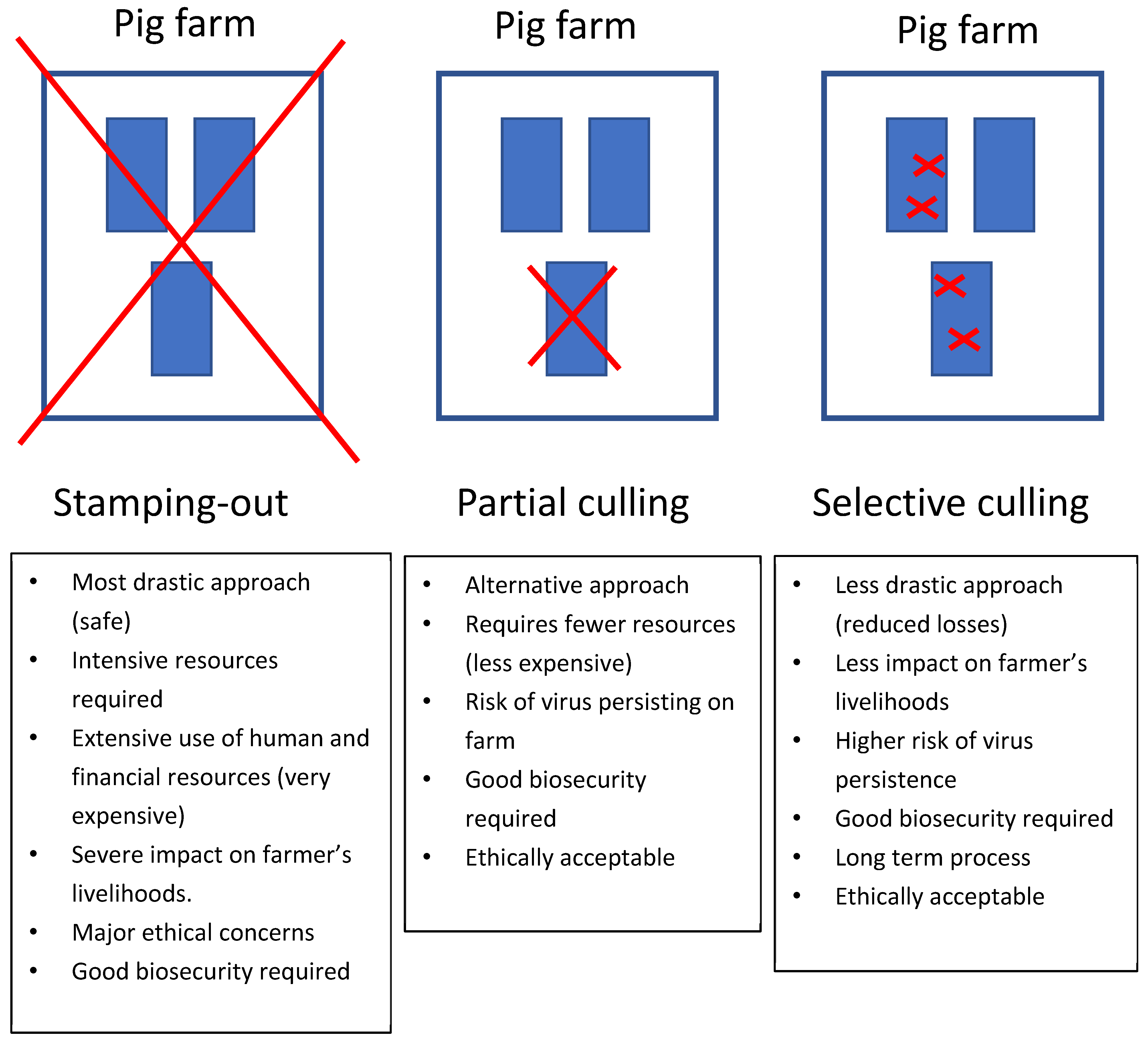
:1. Introduction
2. Materials and Methods
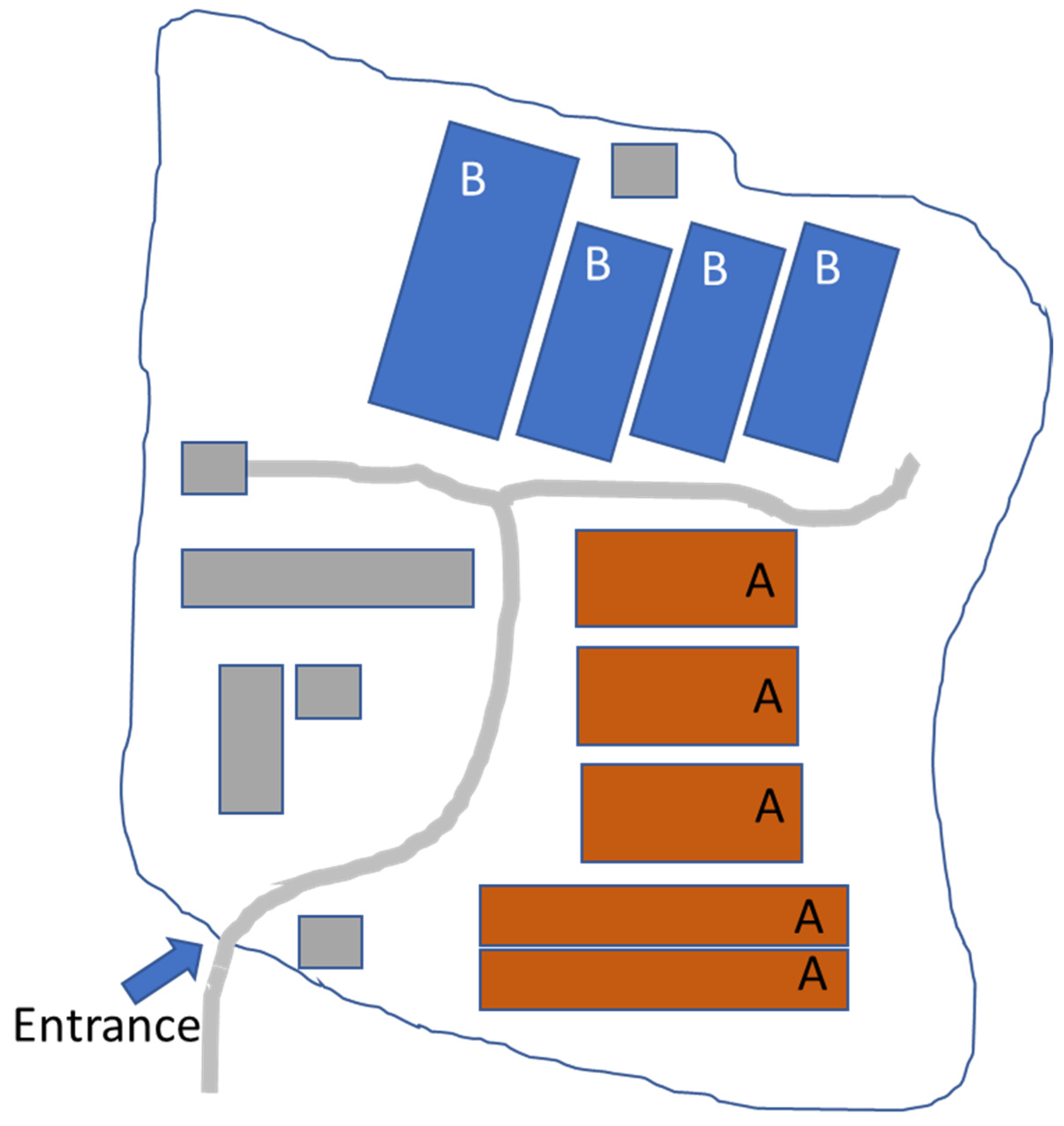
2.1. Farm Description
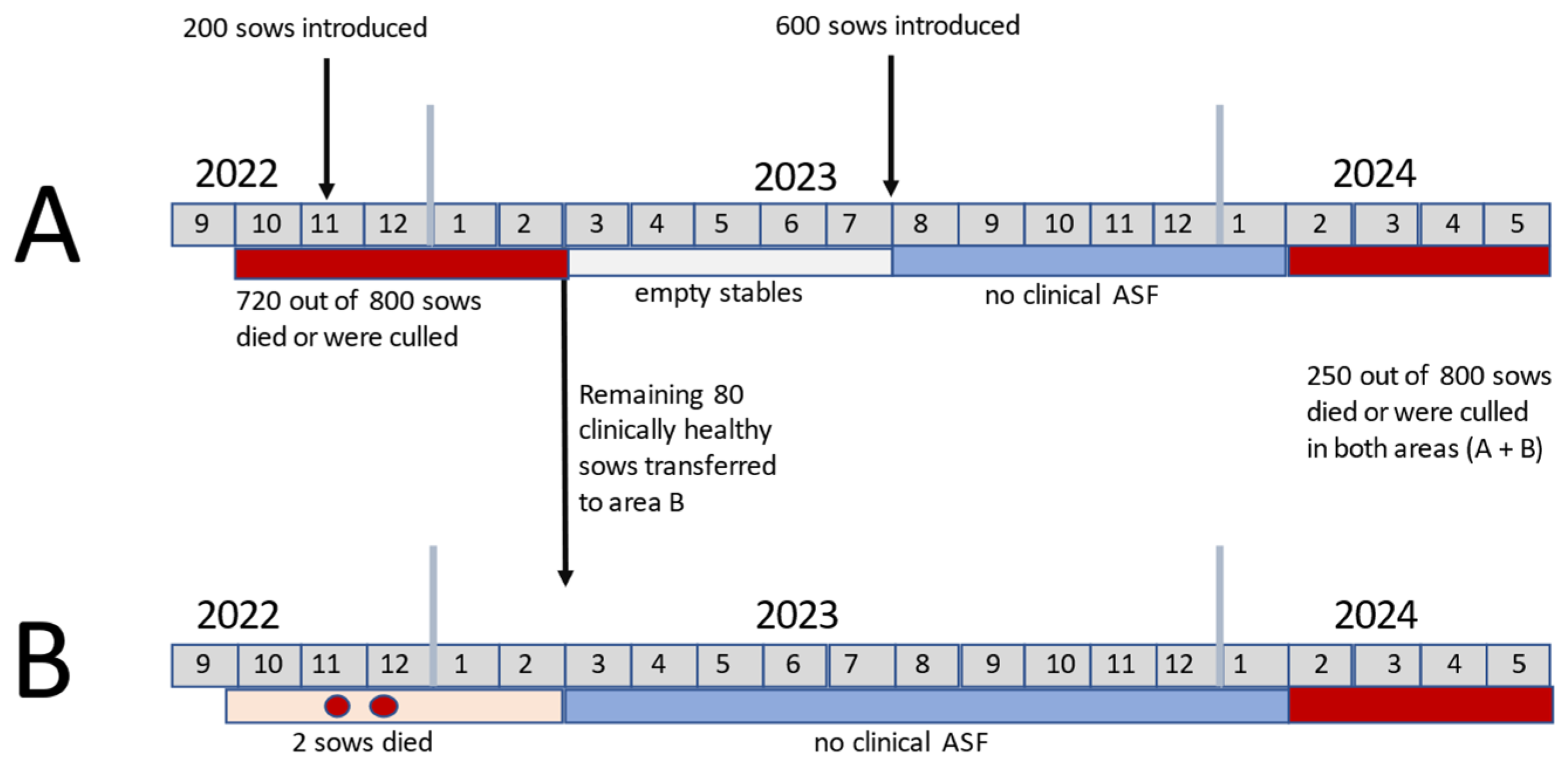
2.2. ASF History
2.3. Pen-Side Tests
- Genome Detection: Using a portable qPCR device (Franklin® Real-Time PCR Thermocycler from Biomeme, Philadelphia, PA, USA, following King et al., 2003 [23]).
- Antigen Detection: Using antigen lateral flow tests (INgezim ASFV CROM Ag lateral flow assay (Eurofins Technologies Ingenasa, Madrid, Spain)).
- Antibody Detection: Using an ELISA test (INgezim PPA COMPAC- Blocking ELISA, Eurofins Technologies Ingenasa, Madrid, Spain, for IgG and IgM detection).
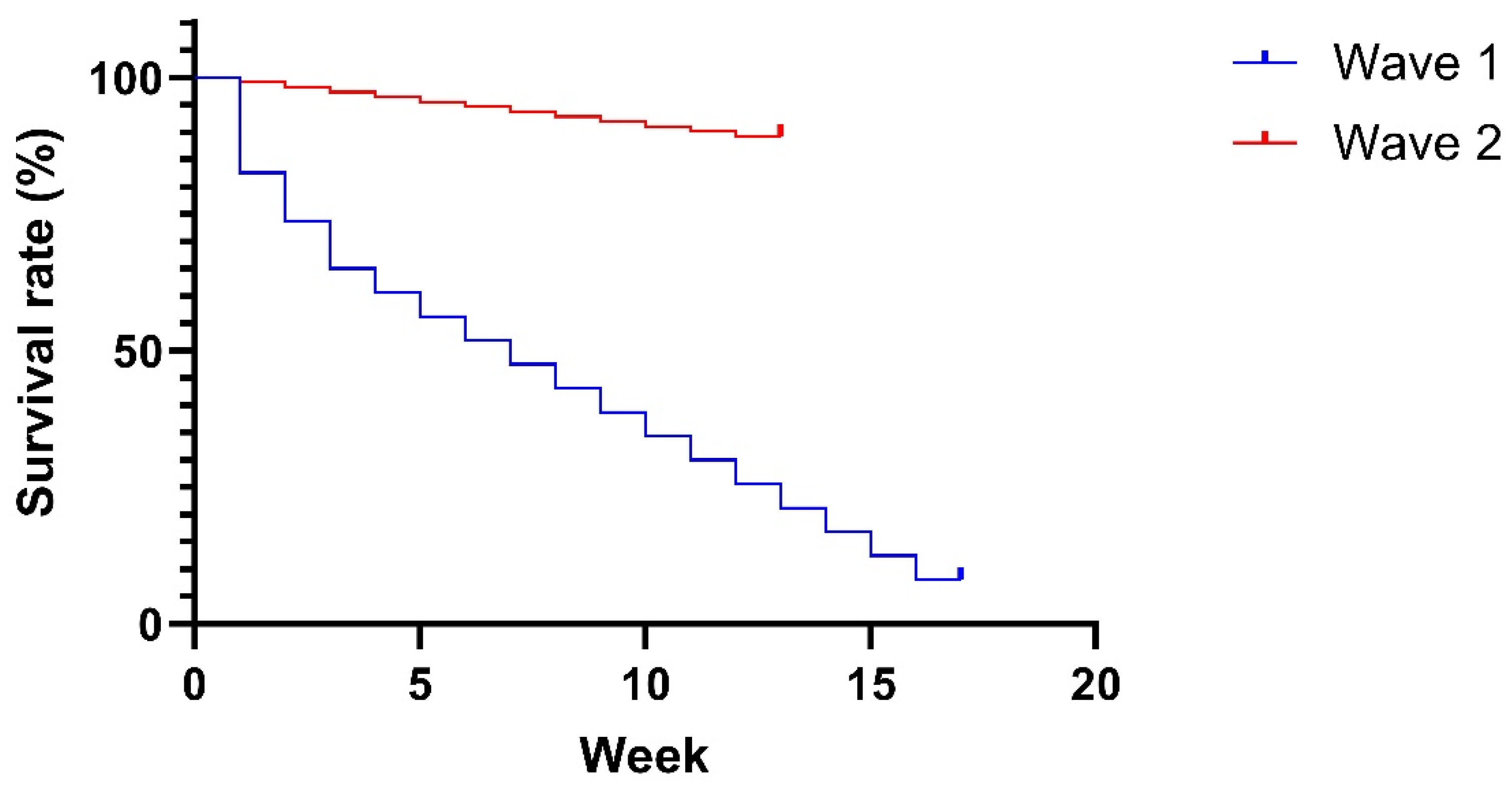
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woonwong, Y.; Do Tien, D.; Thanawongnuwech, R. The Future of the Pig Industry After the Introduction of African Swine Fever into Asia. Anim. Front. 2020, 10, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar—Ecology and Biosecurity, 2nd ed.; FAO Animal Production and Health Manual No. 28; FAO, World Organisation for Animal Health and European Commission: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Bellini, S.; Scaburri, A.; Tironi, M.; Calò, S. Analysis of Risk Factors for African Swine Fever in Lombardy to Identify Pig Holdings and Areas Most at Risk of Introduction in Order to Plan Preventive Measures. Pathogens 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Mighell, E.; Ward, M.P. African Swine Fever Spread Across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). African Swine Fever (ASF) Situation Update in Asia & Pacific. Retrieved from ASF Situation in Asia & Pacific Update. Available online: https://www.fao.org/animal-health/situation-updates/asf-in-asia-pacific/en (accessed on 30 May 2024).
- Cooper, T.L.; Smith, D.; Gonzales, M.J.C.; Maghanay, M.T.; Sanderson, S.; Cornejo, M.R.J.C.; Pineda, L.L.; Sagun, R.A.A.; Salvacion, O.P. Beyond Numbers: Determining the Socioeconomic and Livelihood Impacts of African Swine Fever and Its Control in the Philippines. Front. Vet. Sci. 2022, 8, 734236. [Google Scholar] [CrossRef] [PubMed]
- Lamberga, K.; Depner, K.; Zani, L.; Oļševskis, E.; Seržants, M.; Ansonska, S.; Šteingolde, Ž.; Bērziņš, A.; Viltrop, A.; Blome, S.; et al. A practical guide for strategic and efficient sampling in African swine fever-affected pig farms. Transbound. Emerg. Dis. 2022, 69, e2408–e2417. [Google Scholar] [CrossRef]
- Nga, B.T.T.; Padungtod, P.; Depner, K.; Chuong, V.D.; Duy, D.T.; Anh, N.D.; Dietze, K. Implications of partial culling on African swine fever control effectiveness in Vietnam. Front. Vet Sci. 2022, 9, 957918. [Google Scholar] [CrossRef]
- Mai, N.T.A.; Trinh, T.B.N.; Nguyen, V.T.; Lai, T.N.H.; Le, N.P.; Nguyen, T.T.H.; Nguyen, T.L.; Ambagala, A.; Do, D.L.; Le, V.P. Estimation of basic reproduction number (R0) of African swine fever (ASF) in mid-size commercial pig farms in Vietnam. Front. Vet. Sci. 2022, 9, 918438. [Google Scholar] [CrossRef]
- van Rensburg, L.J.; Penrith, M.-L.; van Heerden, J.; Heath, L.; Etter, E.M.C. Investigation into eradication of African swine fever in domestic pigs from a previous outbreak (2016/17) area of South Africa. Res. Vet. Sci. 2020, 133, 42–47. [Google Scholar] [CrossRef]
- Ito, S.; Kawaguchi, N.; Bosch, J.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M. What can we learn from the five-year African swine fever epidemic in Asia? Front. Vet. Sci. 2023, 10, 1273417. [Google Scholar] [CrossRef]
- Duong, D.T. Sustainable Development for Vietnam Agriculture. E3S Web Conf. 2020, 175, 01015. [Google Scholar] [CrossRef]
- Ngoc Que, N.; Thi Ngoc Linh, P.; Cong Thang, T.; Thi Thuy, N.; Thi Thinh, N.; Rich, K.M.; Nguyen-Viet, H. Economic Impacts of African Swine Fever in Vietnam. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/bd455275-1c09-4137-af89-10d75255aabe/content (accessed on 2 May 2024).
- Nguyen, M.; Thuy, T.; Dorny, P.; Lebailly, P.; Le, C.; Minh, T.; Nguyen, H.; Thu, T.; Dermauw, V.; Phong, G.; et al. Mapping the Pork Value Chain in Vietnam: A Systematic Review. Trop. Anim. Health Prod. 2020, 52, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, M.; Mun, H.-S.; Ampode, K.M.B.; Lagua, E.B.; Park, H.-R.; Kim, Y.-H.; Hasan, M.K.; Yang, C.-J. Smart Pig Farming—A Journey Ahead of Vietnam. Agriculture 2024, 14, 555. [Google Scholar] [CrossRef]
- Dat, P.M. Export of Some Key Agricultural Products of Vietnam. Int. J. Prof. Bus. Rev. 2023, 8, e0417. [Google Scholar] [CrossRef]
- Yen, V.H.; Nhung, N.H.; Dung, T.A. Vietnam Food Security Policy Review; Petersen, E.H., Ed.; The Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2017. Available online: https://www.aciar.gov.au/sites/default/files/legacy/vietnam_food_security_policy_web.pdf (accessed on 2 May 2024).
- Nguyen-Thi, T.; Pham-Thi-Ngoc, L.; Nguyen-Ngoc, Q.; Dang-Xuan, S.; Lee, H.S.; Nguyen-Viet, H.; Padungtod, P.; Nguyen-Thu, T.; Nguyen-Thi, T.; Tran-Cong, T.; et al. An Assessment of the Economic Impacts of the 2019 African Swine Fever Outbreaks in Vietnam. Front. Vet. Sci. 2021, 8, 686038. [Google Scholar] [CrossRef] [PubMed]
- Le, V.P.; Jeong, D.G.; Yoon, S.W.; Kwon, H.M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, V.T.; Cho, K.H.; Mai, N.T.A.; Park, J.Y.; Trinh, T.B.N.; Jang, M.K.; Nguyen, T.T.H.; Vu, X.D.; Nguyen, T.L.; Nguyen, V.D.; et al. Multiple Variants of African Swine Fever Virus Circulating in Vietnam. Arch. Virol. 2022, 167, 1137–1140. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development. Công văn 5169/BNN-TY 2019 Hướng Dẫn Bổ Sung Biện Pháp Phòng Chống Bệnh Dịch Tả Lợn Châu Phi. Thư Viện Pháp Luật. 22 July 2019. Available online: https://thuvienphapluat.vn/cong-van/Tai-nguyen-Moi-truong/Cong-van-5169-BNN-TY-2019-huong-dan-bo-sung-bien-phap-phong-chong-benh-Dich-ta-lon-Chau-Phi-428874.aspx (accessed on 2 June 2024).
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan PCR Assay with Internal Amplification Control for the Detection of African Swine Fever Virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, P.; Pikalo, J.; Beer, M.; Blome, S. Lateral Flow Assays for the Detection of African Swine Fever Virus Antigen Are Not Fit for Field Diagnosis of Wild Boar Carcasses. Transbound. Emerg. Dis. 2022, 69, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Pikalo, J.; Deutschmann, P.; Fischer, M.; Roszyk, H.; Beer, M.; Blome, S. African Swine Fever Laboratory Diagnosis—Lessons Learned from Recent Animal Trials. Pathogens 2021, 10, 177. [Google Scholar] [CrossRef]
- Lamberga, K.; Ardelean, F.; Blome, S.; Busauskas, P.; Djuric, B.; Globig, A.; Guberti, V.; Miteva, A.; Olševskis, E.; Seržants, M.; et al. African Swine Fever Outbreak Investigations—The Significance of Disease-Related Anecdotal Information Coming from Laypersons. Pathogens 2022, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L.; van Heerden, J.; Pfeiffer, D.U.; Oļševskis, E.; Depner, K.; Chenais, E. Innovative research offers new hope for managing African swine fever better in resource-limited smallholder farming settings: A timely update. Pathogens 2023, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.L.; Guberti, V.; Depner, K.; Lubroth, J. (Eds.) Preparation of African Swine Fever Contingency Plans; FAO: Rome, Italy, 2009; Volume 8. [Google Scholar]
- de Carvalho Ferreira, H.C.; Weesendorp, E.; Elbers, A.R.W.; Bouma, A.; Quak, S.; Stegeman, J.A.; Loeffen, W.L.A. African Swine Fever Virus Excretion Patterns in Persistently Infected Animals: A Quantitative Approach. Vet. Microbiol. 2012, 160, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and Transmission Characteristics of Oral Low-Dose Infection of Domestic Pigs and European Wild Boar with a Caucasian African Swine Fever Virus Isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef]
- Guinat, C.; Gubbins, S.; Vergne, T.; Gonzales, J.L.; Dixon, L.; Pfeiffer, D.U. Experimental Pig-to-Pig Transmission Dynamics for African Swine Fever, Georgia 2007/1 Strain. Epidemiol. Infect. 2016, 144, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nurmoja, I.; Motus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological Analysis of the 2015–2017 African Swine Fever Outbreaks in Estonia. Prev. Vet. Med. 2018, 181, 104556. [Google Scholar] [CrossRef] [PubMed]
- Lamberga, K.; Olševskis, E.; Seržants, M.; Berzinš, A.; Viltrop, A.; Depner, K. African Swine Fever in Two Large Commercial Pig Farms in Latvia—Estimation of the High-Risk Period and Virus Spread within the Farm. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Swine Health Information Center. SHIC-Funded ASF Study on Test-and-Remove Protocol in Vietnam Finds It Unreliable. Swine Health Information Center, 30 June 2021. Available online: https://www.swinehealth.org/shic-funded-asf-study-on-test-and-remove-protocol-in-vietnam-finds-it-unreliable/ (accessed on 2 May 2024).
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Lamberga, K.; Viltrop, A.; Nurmoja, I.; Masiulis, M.; Bušauskas, P.; Oļševskis, E.; Seržants, M.; Laddomada, A.; Ardelean, F.; Depner, K. The Effectiveness of Protection and Surveillance Zones in Detecting Further African Swine Fever Outbreaks in Domestic Pigs—Experience of the Baltic States. Viruses 2024, 16, 334. [Google Scholar] [CrossRef]
- Ambagala, A.; Goonewardene, K.; Kanoa, I.E.; Than, T.T.; Nguyen, V.T.; Lai, T.N.H.; Nguyen, T.L.; Erdelyan, C.N.G.; Robert, E.; Tailor, N.; et al. Characterization of an African Swine Fever Virus Field Isolate from Vietnam with Deletions in the Left Variable Multigene Family Region. Viruses 2024, 16, 571. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Podgórska, K. African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends. Viruses 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
| Sow | Days Since Stopped Eating | Body Temperature °C | qPCR (Ct-Value) | AG-LFD | Ab-Elisa |
|---|---|---|---|---|---|
| 1 (7879) | 4 | 38.03 | pos (28) | neg | pos |
| 2 (7973) | 14 | 38.5 | pos (24) | neg | pos |
| 3 (8008) | 13 | 38.0 | pos (30) | neg | pos |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nga, B.T.T.; Auer, A.; Padungtod, P.; Dietze, K.; Globig, A.; Rozstalnyy, A.; Hai, T.M.; Depner, K. Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm. Pathogens 2024, 13, 567. https://doi.org/10.3390/pathogens13070567
Nga BTT, Auer A, Padungtod P, Dietze K, Globig A, Rozstalnyy A, Hai TM, Depner K. Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm. Pathogens. 2024; 13(7):567. https://doi.org/10.3390/pathogens13070567
Chicago/Turabian StyleNga, Bui Thi To, Agathe Auer, Pawin Padungtod, Klaas Dietze, Anja Globig, Andriy Rozstalnyy, Tran Minh Hai, and Klaus Depner. 2024. "Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm" Pathogens 13, no. 7: 567. https://doi.org/10.3390/pathogens13070567
APA StyleNga, B. T. T., Auer, A., Padungtod, P., Dietze, K., Globig, A., Rozstalnyy, A., Hai, T. M., & Depner, K. (2024). Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm. Pathogens, 13(7), 567. https://doi.org/10.3390/pathogens13070567







