Abstract
Leishmaniosis is a vector-borne disease caused by protozoan parasites of the genus Leishmania, which are zoonotic and have an important impact on animal and public health globally. Between 2009 and 2023, blood samples from domestic dogs with clinical suspicion of leishmaniosis were received from 286 veterinary medical centres throughout mainland Portugal. An enzyme-linked immunosorbent assay (ELISA) was utilised to detect antibodies against Leishmania infantum antigens. Additionally, a complete blood count and tests for total proteins, urea, creatinine and alanine aminotransferase, as well as protein electrophoresis, were also performed. No significant relationship between sex and breed was observed. The age distribution was bimodal, with the highest prevalence of disease occurring at 2–5 years of age and a secondary peak occurring at 6 years or over (p < 0.001). No statistical correlation was observed between creatinine and urea across the ELISA serological groups. In contrast, both the gamma globulin levels (r = 0.45; p < 0.001) and the albumin/globulin ratio (r = −0.36; p < 0.001) exhibited moderate correlations with the ELISA. These findings support recent seroprevalence studies in dogs, with some geographical areas in Northern Portugal exhibiting the highest values, which may be the result of geographical shifts in parasite circulation due to climate change.
1. Introduction
Canine leishmaniosis (CanL) is a vector-borne disease caused by protozoan parasites of the genus Leishmania, considerably impacting both human and veterinary public health globally [1,2]. The disease is predominantly observed in the Mediterranean region, South and Central America and selected Asian territories [3,4,5]. In Portugal, CanL is present throughout the mainland, with a higher prevalence in the interior centre and south of Portugal according to the latest studies [6,7]. The complex epidemiological profile is sculpted by an array of factors, including environmental conditions, host–pathogen interactions and vector phenology [8,9,10]. The vectors (phlebotomine sand flies) are influenced by diverse climatic conditions [11,12,13]. This dictates the prevalence of the disease and its spatial distribution, highlighting the complex interplay between vector availability, host genetic susceptibility and environmental exposure [14,15,16,17]. Clinically, CanL manifests with a broad spectrum of clinical signs, ranging from cutaneous lesions and renal dysfunction to systemic involvement, thus complicating its diagnosis and therapeutic management [18]. Previous studies have explored the correlation between clinicopathological parameters and other epidemiological factors, such as age, demonstrating that young dogs develop systemic, renal and haematological abnormalities less frequently compared to older dogs, while dermatologic signs are more common in young and adult dogs. The main clinicopathological abnormalities include hyperglobulinemia, hypoalbuminemia, mild to moderate non-regenerative anaemia and mild to severe proteinuria [19]. The heterogeneity in clinical presentation is intricately associated with the virulence of the infecting Leishmania strain, the immunological status of the host and the potential for co-infections with other vector-borne pathogens, which may further complicate the clinical scenario [20,21,22,23].
Moreover, the (subclinical) carriage and the reservoir potential of domestic and wild canines underscore the complex epidemiology of the disease, posing challenges to control and prevention strategies [24]. The diagnosis of CanL is complex, and multiple parameters need to be considered [8]. Serological procedures are widely used in diagnosing canine leishmaniosis due to their high sensitivity and specificity [18,25,26,27], ability to detect subclinical infections [28,29,30] and practicality in terms of non-invasive sample collection [18,29,30,31]. These tests are essential for early diagnosis and effective treatment and control of the disease [28,29]. Combining serological methods with other diagnostic techniques further enhances their accuracy and reliability [6,31,32].
The expansion of CanL’s geographical distribution in recent years has been notably accelerated by climate change, augmented animal mobility across international borders and urbanisation trends, all contributing to the enlargement of vector habitats and subsequent alterations in disease transmission dynamics [1,13,33,34]. This evolving scenario underscores the need for an integrated approach to health, aligning with the Stockholm Paradigm within the Planetary Health concept, which emphasises the interdependence of human, animal and environmental health [13,35,36,37,38,39]. By situating CanL within this paradigm, we aim to contribute to a more integrated understanding of zoonotic diseases and reinforce the necessity of a comprehensive strategy to mitigate the impact of infectious diseases on a global scale [40,41].
This paper aims to present an update to the distribution patterns and the interplay among the epidemiological and clinicopathological parameters of CanL in the Portuguese canine population during a period of 15 years (2009–2023) and determine possible associations and correlations between different variables related to this disease.
2. Materials and Methods
2.1. Study Area, Sampling and Data Collection
From 2009 to 2023, processed samples of whole blood from domestic dogs were submitted to INNO Veterinary Laboratories (Braga, Portugal), a veterinary diagnostic laboratory in North Portugal. The samples were collected from 286 veterinary medical centres (i.e., clinics and hospitals) from all of the regions of mainland Portugal. Along with the samples, a laboratory requisition was received, which included the complete clinical information for each dog, particularly their breeds, sexes, ages, vaccination and prophylactic statuses, clinical suspicion/clinical signs and requested analyses.
As inclusion criteria, only samples of dogs (Canis lupus familiaris) with clinical suspicion of CanL or those residing in areas where the disease is endemic, cohabiting with another potentially infected animals or having recently returned from a trip to an endemic region, were included. All biological samples were verified for analysis suitability (haemolysis, lipemia, correct anticoagulant ratio to sample volume, date of collection and other factors that may affect the result of the test).
2.2. Blood Analysis and Serological Tests
The tests on complete blood count (CBC), red blood cells (RBC), packed cell volume (PCV), platelets (PLT) and white blood cells (WBC) were conducted using the Sysmex XT-2000iV Analyzer (Sysmex Corporation, Kobe, Japan), with platelet count confirmation being carried out through blood smear stained with Giemsa. The measurements of total protein (TP), urea (UREA), creatinine (CREA) and alanine aminotransferase (ALAT) were performed using the Cobas® 6000 Analyzer (c501) (Roche, Grenzach-Wyhlen, Germany). Protein electrophoretic separation was carried out using the method and materials recommended by the Sebia® Minicap Flex Piercing Analyser (Sebia, Norcross, GA, USA) for capillary electrophoresis.
The samples were tested using the enzyme-linked immunosorbent assay (ELISA)-based quantitative assay, the LEISCAN® Leishmania ELISA Test (Ecuphar, Barcelona, Spain), to detect specific antibodies to Leishmania infantum antigens in dogs. This test has a sensitivity and specificity of 95.3% and 99.8%, respectively [26,42,43,44]. The ELISA methodology is accredited and recommended by the World Organization for Animal Health (WOAH) as one of the reference methods for diagnosing and monitoring infection and disease [45]. The ranges adopted for classifying the animals were those defined by the test: negative (Rz ≤ 0.9), inconclusive (0.9 < Rz ≤ 1.1), low positive (1.1 < Rz ≤ 1.5), positive to high positive (1.5 < Rz ≤ 3) and very high positive (Rz > 3). The ratio (Rz) corresponds to the absorbance ratio of the positive control to the sample absorbance. These intervals were maintained in this manner (instead of being classified merely as positive, for example) for a subsequent analysis of the correlation of Leishmania results with other parameters to examine whether the ELISA test ratio (Rz) had an association with any of these.
2.3. Statistical Analysis
All of the data were available in digital format in Clinidata® (Clinidata XXI version 5.3.25, Maxdata Software, S.A., Carregado, Portugal) and transferred to Microsoft Excel® (Microsoft, Redmond, WA, USA) sheets. Statistical analysis was conducted using the JMP®, version 14.3 SAS Institute, Cary, NC, USA, 1989–2023 SAS and MedCalc® Statistical Software version 20.006 (MedCalc Software Ltd., Ostend, Belgium, 2021). To study the differences between the observed and expected frequencies of categories of a field, nonparametric tests were used, including the binomial test or the Chi-Square test for one-sample analysis, depending on the number of categories in the categorical field. For comparisons among three or more independent groups, the Kruskal–Wallis test was utilised, followed by the Dunn–Bonferroni post hoc test for multiple comparisons when appropriate. Additionally, logistic regression with Tikhonov regularisation was employed to identify breeds with a higher predisposition for testing positive while accounting for a disproportional representation of breeds in the study [46,47,48]. The sample parameters were categorised into two great groups:
Qualitative variables: These involve the district, region (NUTS2), sex, breed and age categories (puppy: <1 year old; young: 1 to <2 years old; adult: 2 to <6 years old; senior: 6 to <11 years old; old: ≥11 years old).
Quantitative variables: These involve CBC parameters such as RBC, PCV, PLT, leukogram (WBC), neutrophils (NEU), lymphocytes (LYM), monocytes (MON), eosinophils (EOS), basophils (BASO), biochemical data (CREA, UREA, ALAT and TP), protein profile (albumin (ALB), alpha 1 and 2, beta 1 and 2, and gamma globulins), albumin/globulin ratio (ALB/GLOB) and results from the quantitative ELISA test classified according to test absorbance (negative, inconclusive, low positive, positive to high positive and very high positive).
3. Results
3.1. Qualitative Variables
3.1.1. Descriptive Data
Of the total of 2910 animals included in this study, only 2801 animals were analysed after the removal of inconclusive ELISA results (n = 109, 3.8%). From the 2801 animals included, 38.8% tested negative (n = 1129), and 57.5% tested positive (n = 1672).
3.1.2. Geographical Distribution
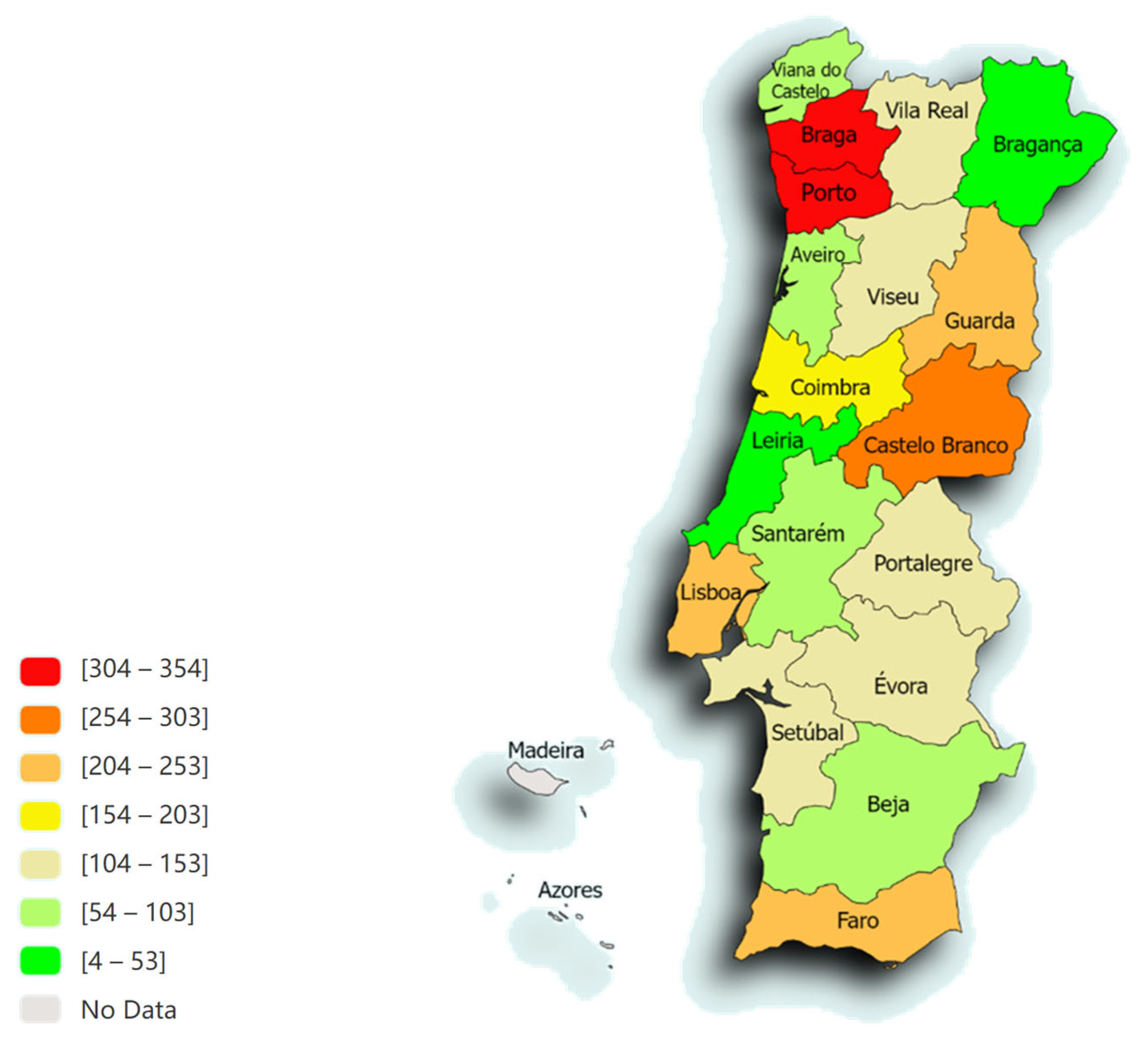
Figure 1 represents the distribution of animals in the different districts of Portugal. The majority of samples originated from Porto (12.6%; n = 354), Braga (11.8%; n = 330), Castelo Branco (9.8%; n = 273) and Lisboa (8.9%; n = 249).
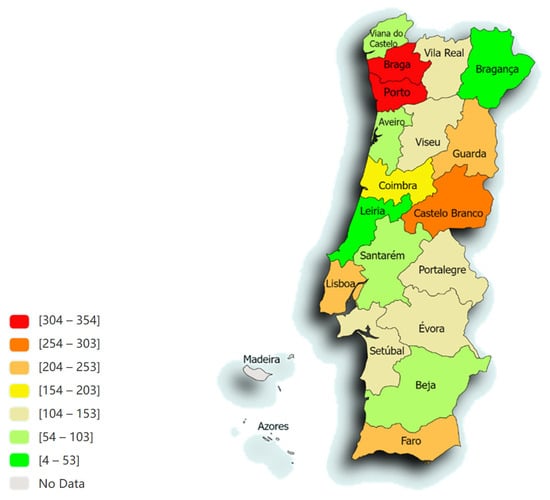
Figure 1.
The spatial distribution, according to the distribution of animals from the different districts of Portugal, of the 2801 animals admitted to the LEISCAN® Leishmania ELISA Test (map drawn in paintmaps.com; accessed on 10 June 2024).
The distribution was divided using the NUTS2 (Nomenclature of Territorial Units for Statistics) regions of mainland Portugal as follows: 33.2% in the north region (n = 930), 32.4% in the centre (n = 906), 10.4% in Alentejo (n = 290), 8.1% in Algarve (n = 226), 7.9% in Greater Lisbon (GL) (n = 220), 4.2% in Oeste e Vale do Tejo (OVT) (n = 118) and 4.0% in Península de Setúbal (PdS) (n = 111). The region with the highest prevalence of positive cases was the OVT region (68.6%), followed by the Alentejo region (68.3%).
The Chi-Square test revealed a statistically significant relationship between the region and the negative and positive outcomes of the LEISCAN® Leishmania ELISA Test (p < 0.001). The average prevalence of the disease in this study across different regions was 59.7%, with an average percentage variation of 9.0% (ranging from 42.3% to 68.6%). The Chi-Square test also indicated a statistically significant relationship between region and prevalence/positivity (p < 0.001). Table 1 illustrates the percentage of positives in the current study during the years 2009 to 2023 across the geographical regions of mainland Portugal.

Table 1.
Negative and positive LEISCAN® Leishmania ELISA Test by regions in the 2801 animals included in this study.
Table 2 illustrates the percentage of positives in the current study during the years 2014 to 2023 across the regions of mainland Portugal.

Table 2.
Evolution of positives over 10 years in mainland Portugal (2014–2023).
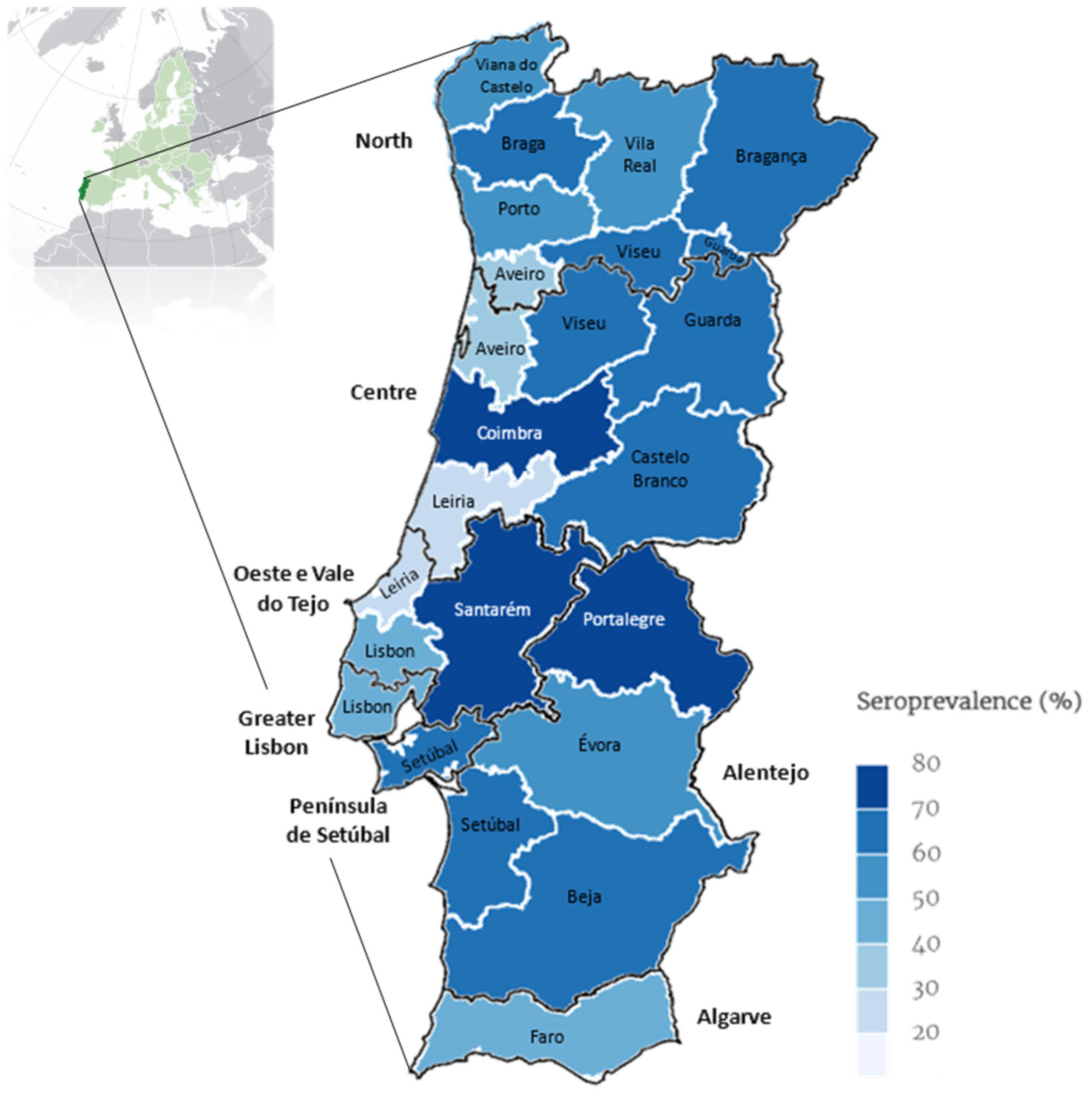
Figure 2 displays the average percentage of positivity over a 15-year period in mainland Portugal (2009–2023).
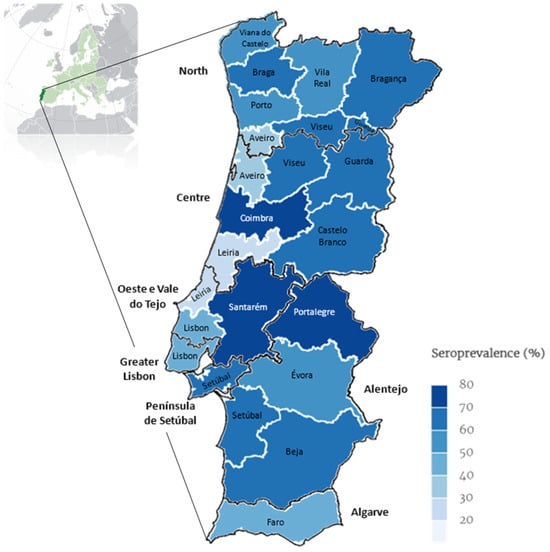
Figure 2.
Map of continental Portugal showing categorical representation of average percentage of prevalence rate over 15 years (2009–2023) for Leishmania seroprevalence determined by ELISA per district and NUTS2 (map drawn in mapinseconds.com; accessed on 10 June 2024).
3.1.3. Sex
From the 2801 animals analysed, 964 (34.4%) were females and 1837 (65.6%) were males. Table 3 represents the percentages of positive and negative tests according to sex. The differences observed between animal sexes were not significant (p = 0.082).

Table 3.
Negative and positive LEISCAN® Leishmania ELISA Test by sex in the 2801 animals included in this study.
3.1.4. Breed
Regarding breed, our study population comprised animals from 83 different dog breeds that included the following: 1365 mixed-breed dogs (48.7%), 306 Labrador Retrievers (10.9%), 147 German Shepherds (5.3%), 85 Boxers (3.0%), 72 Portuguese Podengos (2.6%), 48 Miniature Pinschers (1.7%), 45 Epagneul Bretons (1.6%), 43 Beagles (1.5%), 38 Cocker Spaniels (1.4%), 37 French Bulldogs (1.3%), 37 American Pit Bull Terriers (1.3%), 33 Poodles (1.2%), 32 Estrela Mountain Dogs (1.1%), 31 Golden Retrievers (1.1%), 26 Siberian Huskies (0.9%) and 68 other breeds.
For statistical analysis, the mixed-breed category was excluded. Despite the significant difference among breeds in the LEISCAN® Leishmania ELISA indicated by the Kruskal–Wallis test (p < 0.001), no significant associations were found by the Dunn–Bonferroni test; all adjusted p-values were above 0.05. Therefore, there is insufficient evidence of a significant relationship between dog breed and the negative and positive outcomes of the LEISCAN® Leishmania ELISA Test at the 0.05 significance level. However, logistic regression with Tikhonov regularisation suggested Pugs (coef.: 1.22), Rottweilers (coef.: 1.11), English Bulldogs (coef.: 0.97), Basset Hounds (coef.: 0.97) and Jack Russel Terriers (coef.: 0.96) as having a higher predisposition for testing positive. Among the breeds with the highest prevalence of positives, Labrador Retrievers had 11.2% (n = 187), German Shepherds had 5.0% (n = 83), Boxers had 3.8% (n = 64) and Portuguese Podengos had 2.8% (n = 47).
3.1.5. Age
From the 2801 animals that were analysed, age data were available for only 2513 animals, as for 288 animals (10.3%), requisition documents did not specify their age and were thus excluded from certain analytical processes. The age distribution among these 2513 animals ranged from ≤1 year (6 months) to 23 years, with an average age of 7.2 ± 3.9 years. A total of 1.2% (n = 33) were puppies, 2.6% (n = 73) were young, 30.4% (n = 850) were adults, 41.0% (n = 1149) were seniors and 14.6% (n = 408) were old.
Table 4 displays the percentages of positive and negative results according to age group. The result of the Spearman correlation analysis indicated a significant correlation between age and the negative and positive outcomes of the quantitative ELISA test (p < 0.001). A significance level of p = 0.004 was also observed in the Cochran–Armitage test for the frequency of seropositivity according to the life stage of the individual studied, indicating that the disease predisposition is associated with age. This result suggests that age influences the likelihood of positive results in the serological test, with adult and senior age groups showing a higher incidence of seropositivity than others.

Table 4.
Negative and positive LEISCAN® Leishmania ELISA Test by age group in 2513 dogs included in this study.
3.2. Quantitative Variables
3.2.1. Blood and Biochemical Data
Table 5 presents the values of blood and biochemical data recovery from 2801 dogs included in this study, both negative and positive.

Table 5.
Blood and biochemical data recovery from 2801 dogs, both negative and positive, in the LEISCAN® Leishmania ELISA Test.
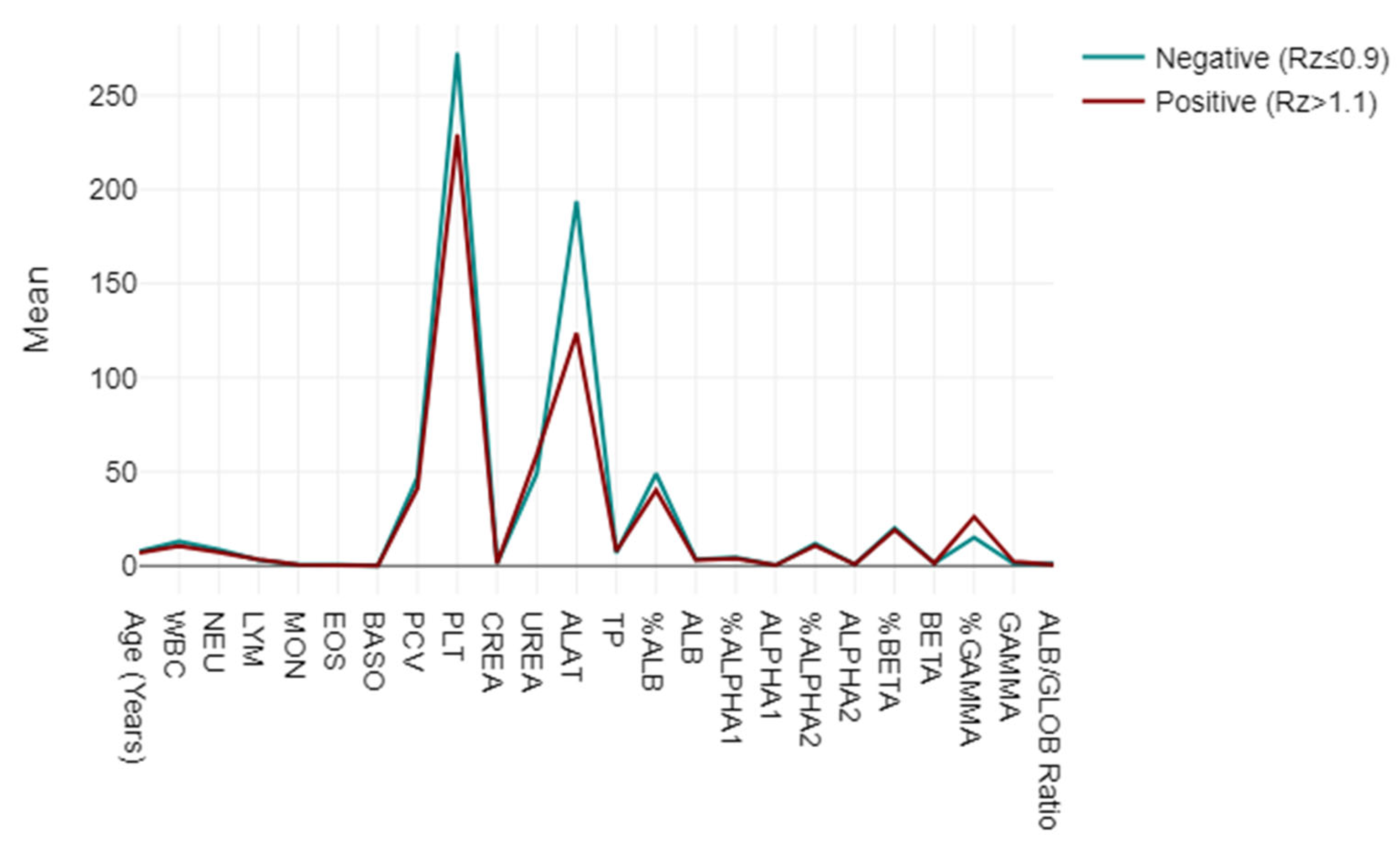
A mixed-model ANOVA was utilised to evaluate blood and biochemical markers, categorising them based on their responses to the LEISCAN® Leishmania ELISA Test as either negative (Rz ≤ 0.9) or positive (Rz > 1.1). The analysis highlighted strong associations in the key parameters, including lower WBC, NEU, PCV and PLT levels in positive cases, alongside elevated UREA and GAMMA globulin levels, suggesting impactful physiopathological correlations. The ALB/GLOB ratio also varied, indicating potential diagnostic utility (p < 0.001) (Figure 3).
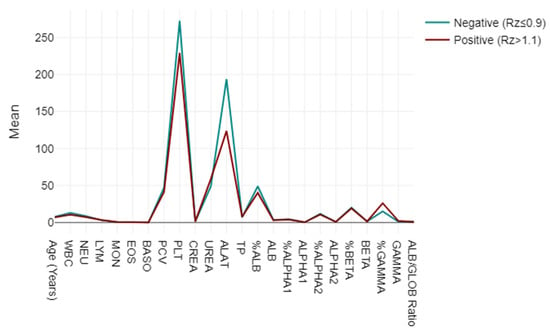
Figure 3.
Mixed-model ANOVA findings: blood and biochemical markers, categorised based on their responses to LEISCAN® Leishmania ELISA Test as either negative (Rz ≤ 0.9) or positive (Rz > 1.1).
3.2.2. Receiver Operating Characteristic (ROC) Curve Analyses
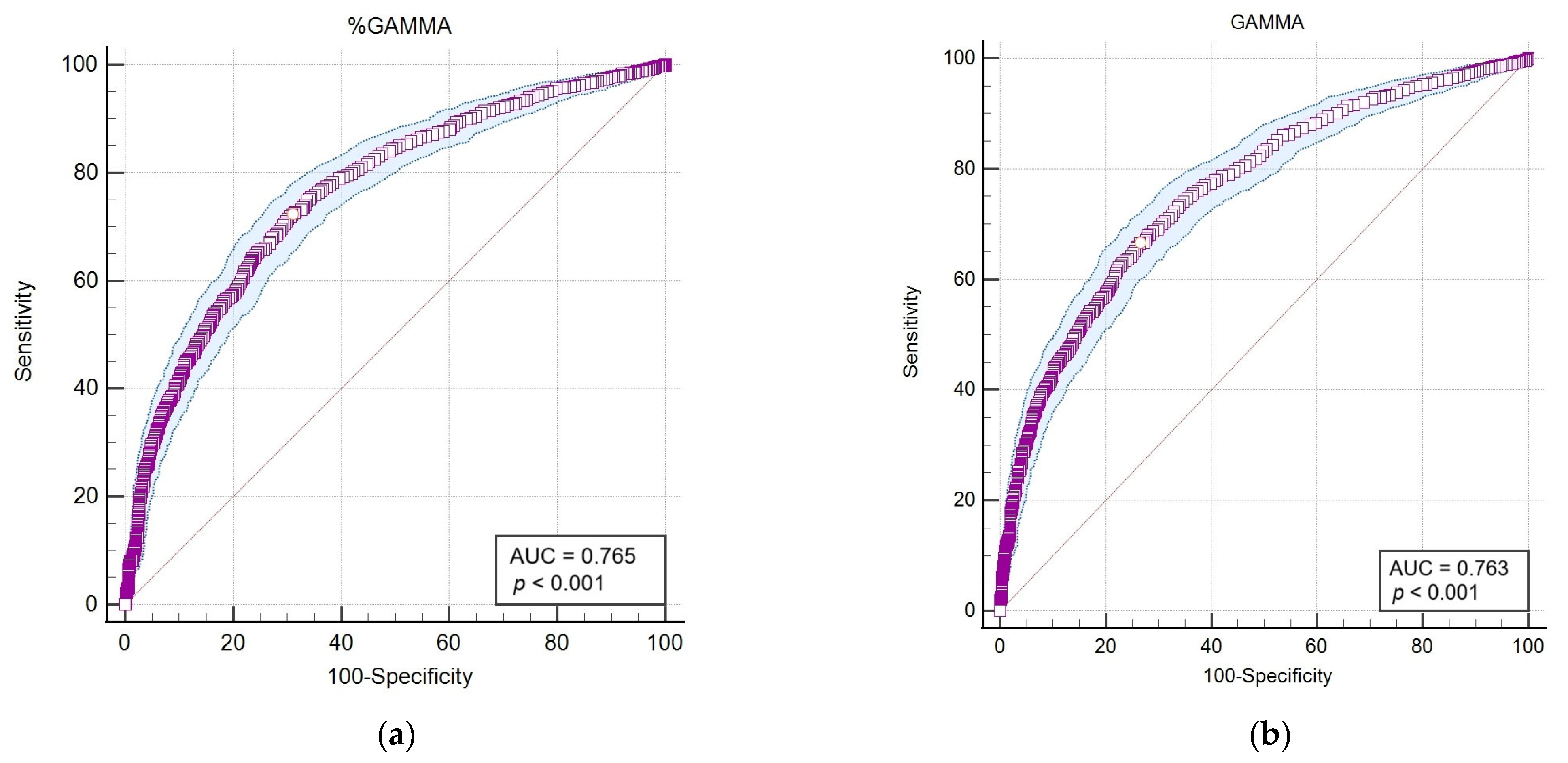
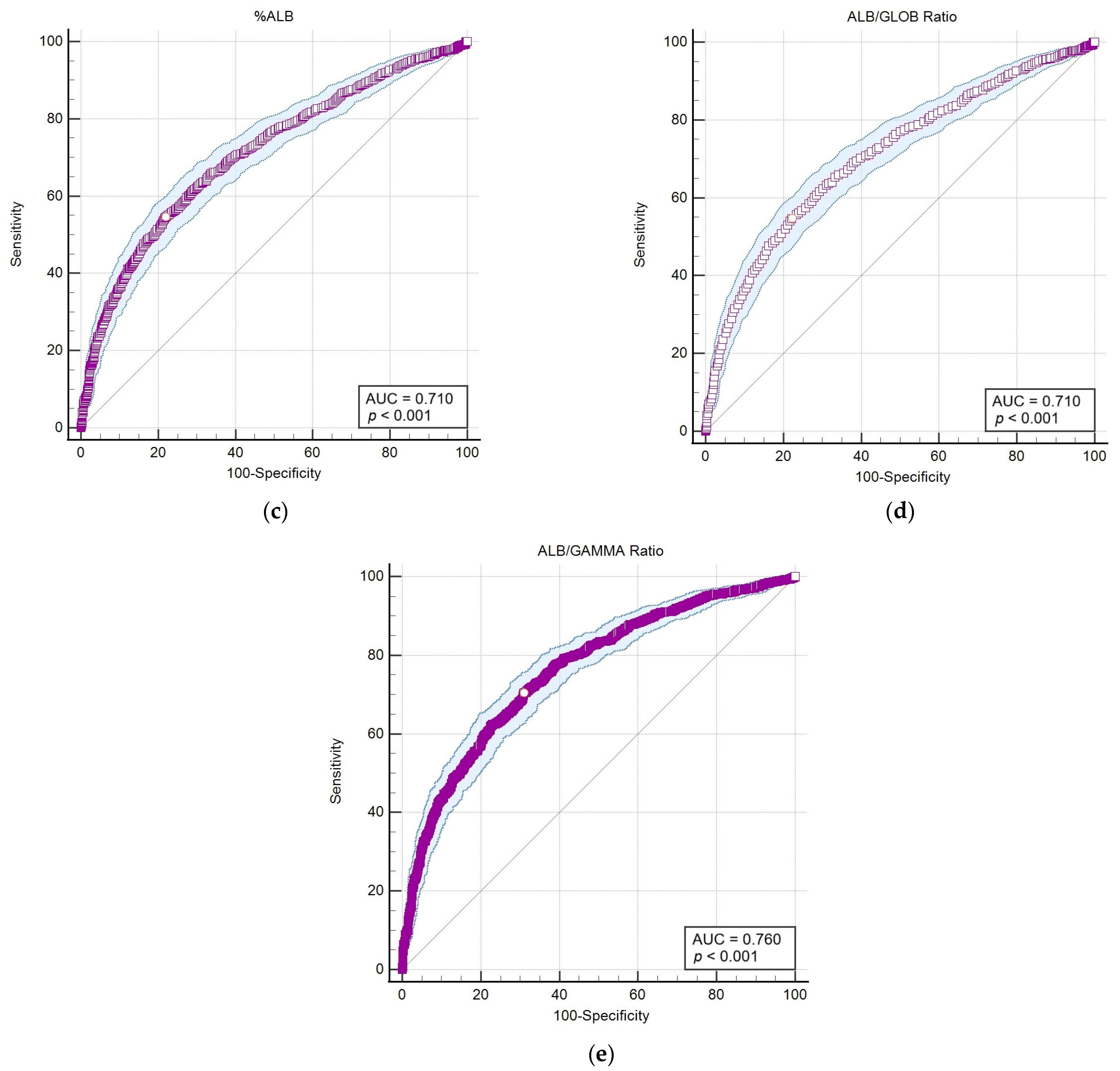
Following the application of Spearman’s statistical correlation analysis, haematological and biochemical markers exhibiting the highest correlations and statistical significance (p < 0.001) with outcomes from the LEISCAN® Leishmania ELISA Test were identified for detailed analysis. Subsequently, an area under the curve–receiver operating characteristic (AUC-ROC) analysis was performed to evaluate the diagnostic sensitivity and specificity of these selected parameters for the positive serology and consequent infection of Leishmania (Table 6 and Figure 4).

Table 6.
Receiver operating characteristic (ROC) curve analyses of markers with the highest correlations and statistical significance (p < 0.001) with outcomes from the LEISCAN® Leishmania ELISA Test.
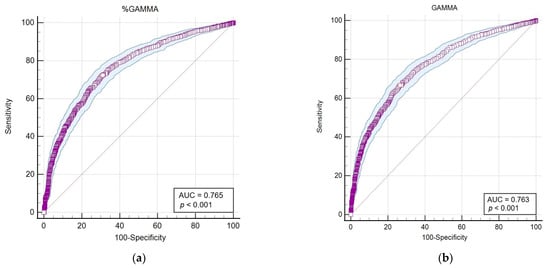
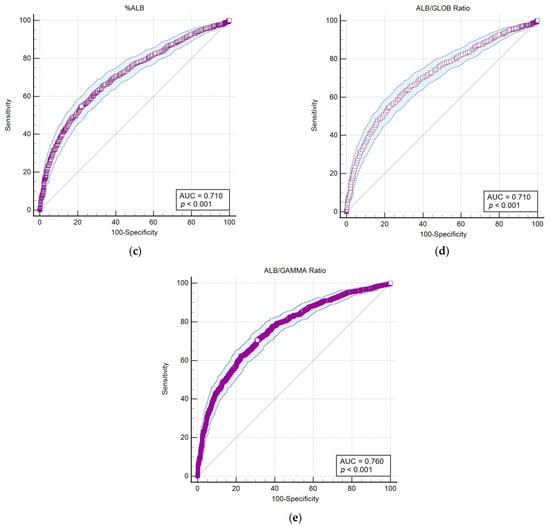
Figure 4.
The area under the curve–receiver operating characteristic (AUC-ROC) of parameters that showed the highest correlation and statistical significance (p < 0.001) with the LEISCAN® Leishmania ELISA Test. (a) The AUC-ROC of %GAMMA; (b) the AUC-ROC of GAMMA (g/dL); (c) the AUC-ROC of %ALB; (d) the AUC-ROC of the ALB/GLOB ratio (g/dL); (e) the AUC-ROC of the ALB/GAMMA ratio (g/dL).
The AUC-ROC analysis serves as an essential statistical instrument for assessing diagnostic test performance. It notably emphasises the GAMMA (%) and ALB/GAMMA ratio as particularly valuable metrics, offering high sensitivity and specificity for the diagnosis of leishmaniosis.
4. Discussion
Although Leishmania spp. can infect a variety of vertebrate species, dogs are the main reservoir for zoonotic L. infantum in the Mediterranean region [6,24]. In Portugal, canine epidemiological studies in different regions have been performed over the years, with the last national seroepidemiological survey being conducted in 2021 [6]. However, no studies have explored the relationships between epidemiological and clinicopathological parameters, nor have any thoroughly described clinicopathologic abnormalities and their clinical usefulness in Portugal, a country where the disease is endemic. In the present study, the authors present an update on CanL in Portugal and its relationship with its clinicopathological parameters. To the authors’ knowledge, this is the first extensive work performed in Portugal and Europe.
The analysis of the data reveals significant physiological differences between groups based on various blood and biochemical markers, highlighting the influence of age and specific conditions on these parameters. With statistically significant p-values indicating robust differences across markers such as WBC, NEU, LYM and the ALB/GLOB ratio, our findings underscore the potential of these parameters as diagnostic tools or indicators of physiological responses. This study contributes to the understanding of the complex interactions between physiological markers and specific health conditions, offering insights for future research and clinical practise. The Spearman correlation analysis reveals a range of relationships between age, various blood parameters (like WBC, NEU, LYM, etc.) and the LEISCAN® Leishmania ELISA Test. While most correlations with age and blood parameters are weak (close to 0), indicating little to no linear relationship, some notable exceptions exist, such as a modest positive correlation between age and certain parameters like PLT and the ALB/GLOB ratio. The negative correlations, though generally weak, suggest slight inverse relationships, such as age with BASO and PCV. The LEISCAN® Leishmania ELISA Test shows a pattern of weak negative correlations with most blood parameters, suggesting that as the condition progresses from negative to positive, some blood parameters may decrease slightly. Overall, the correlations provide insights but also highlight the complexity of relationships between age, blood parameters and disease status, warranting further investigation for a clearer understanding. Consequently, the AUC-ROC analysis emerges as a crucial statistical tool for evaluating the efficacy of diagnostic tests. It particularly underscores the significance of the GAMMA (%) and ALB/GAMMA ratio, identifying them as highly valuable metrics that deliver high sensitivity and specificity in diagnosing leishmaniosis.
4.1. Descriptive Data and Geographical Distribution
In the present study, 59.7% of the analysed animals were positive for antibodies to Leishmania spp., indicating exposure to the parasite. However, this rate does not reflect the true seroprevalence in the general canine population, as the dogs included had clinical suspicions of the disease. Additionally, serological tests can detect antibodies in animals that have been exposed to the parasite but may not necessarily have an active infection. This contrasts with a previous national study that revealed an overall true seroprevalence of 12.5% in dogs [6,49] and 4.8% in humans [50]. The data obtained in this study challenge the previously held belief that the Leishmania frequency in Portugal was predominantly confined to the southern regions and in small endemic foci in the north of the country [16,51,52,53], unveiling a broader geographical distribution across the mainland, which aligns with findings from the most recent epidemiological studies [6,7]. The Oeste e Vale do Tejo and Alentejo regions reported the highest positivity rates, with 68.6% and 68.3%, respectively, highlighting the north region, which surprisingly exhibits a notable prevalence of 60.1%, indicating that no geographical area is free from CanL [52,54]. These findings support seroprevalence studies in humans, where sub-regions in the northern part of the country exhibited the highest prevalences, which may be the result of geographical shifts in parasite circulation due to climate change [39,55].
There is a significant correlation between the geographical region and Leishmania infection outcomes (p < 0.001), highlighting the influence of location on disease prevalence. This finding is pivotal as it aligns with the Stockholm Paradigm, which posits that pathogens can expand beyond their traditional ecological niches, adapting to new hosts and environments [38,39,56].
4.2. Sex
The distribution of positive samples between females (34.4%) and males (65.6%) in this study presents a substantial skew towards males. However, that difference was not statistically significant (p = 0.082). This finding suggests that, contrary to certain diseases where sex might play a role in susceptibility due to hormonal, genetic or behavioural factors, the Leishmania infection values in this population do not significantly differ between males and females [57,58].
The lack of significant difference in infection rates by sex supports the notion that preventive measures and clinical interventions should be uniformly applied across sexes, focusing instead on other risk factors. The observed data are consistent with what has been reported over the past two decades [57].
4.3. Breed
The significant diversity observed among breeds, with mixed breeds constituting a significant portion (48.7%), underlines the heterogeneity of the study population. Despite this diversity, the Kruskal–Wallis test indicated a significant difference among breeds in their response to the LEISCAN® Leishmania ELISA Test (p < 0.001). However, the Dunn–Bonferroni post hoc test showed no significant associations between particular breeds and the ELISA outcomes, indicating that despite there being breed diversity, it does not significantly influence the likelihood of a positive or negative test result. Our results confirm that the seroprevalence of L. infantum among different canine breeds is not associated with antibody titres, which aligns with the findings of authors from recent studies [57,59,60].
4.4. Age
The age distribution was bimodal, with the highest prevalence of the disease occurring at 2–5 years of age and a secondary peak occurring at 6 years or over. The age analysis revealed a significant correlation between age and Leishmania infection outcomes (p < 0.001), with a notable predisposition towards adult and senior dogs. This trend is further supported by the Cochran–Armitage test, highlighting a significant association between age and seropositivity frequency (p < 0.004), suggesting that older animals are more likely to develop the disease. The clear correlation between age and infection frequency emphasises the need for targeted surveillance and preventive measures for older dogs, who are at a higher risk. However, it is crucial to implement preventive measures from an early age. The observed data are consistent with what has been reported [6,57,61].
4.5. Quantitative Variables
Spearman’s correlation analysis (Supplementary Materials: Table S1) was performed on the data obtained. To facilitate interpretation, the authors chose to expound the most salient associations, with correlations manifesting robust (either positive or negative) relational magnitudes underscored by their statistical significance.
Drawing from the Spearman correlation coefficients at hand, the interpretations for the most pronounced positive correlations are as follows:
- PCV vs. ALB (%) (r = 0.59; p < 0.001) and PCV vs. ALB (r = 0.61; p < 0.001): Both show a moderate positive correlation, suggesting that as the packed cell volume increases, there is an increase in both the percentage and absolute levels of albumin.
- TP vs. GAMMA (%) (r = 0.59; p < 0.001) and TP vs. GAMMA (g/dL) (r = 0.71; p < 0.001): Both show a moderate to strong positive correlation, suggesting that as the total protein level increases, there is an increase in both the percentage and absolute levels of gamma globulins.
- CREA vs. UREA (r = 0.53; p < 0.001): There is a moderate positive correlation, suggesting that as the creatinine increases, there is an increase in urea levels.
Such positive correlations may be indicative of typical physiological interactions; alternatively, they might signal specific pathological states when deviating from established normative ranges.
The following is an interpretation of the most significant negative correlations, as determined by the provided correlation coefficients (r):
- ALB/GLOB Ratio vs. GAMMA (%) (r = −0.77; p < 0.001) and ALB/GLOB Ratio vs. GAMMA (g/dL) (r = −0.77; p < 0.001): Both show a similarly strong inverse relationship, indicating that higher albumin/globulin ratios are associated with lower overall gamma globulin levels.
- GAMMA (%) vs. ALB (%) (r = −0.77; p < 0.001) and GAMMA (g/dL) vs. ALB (%) (r = −0.77; p < 0.001): There is a strong negative correlation, indicating that a higher percentage of albumin in the blood is associated with lower overall gamma globulins.
- ALB vs. GAMMA (%) (r = −0.54; p < 0.001) and ALB vs. GAMMA (g/dL) (r = −0.46; p < 0.001): These indicate a moderate inverse relationship, suggesting that higher total albumin levels are associated with lower overall gamma globulins.
- PCV vs. GAMMA (%) (r = −0.50; p < 0.001) and PCV vs. GAMMA (g/dL) (r = −0.45; p < 0.001): There is a moderate negative correlation, suggesting that higher packed cell volume (PCV) values are associated with lower overall gamma globulins.
- MON vs. CREA (r = −0.22; p < 0.001): There is a weak negative correlation, indicating that as the monocyte counts increase, the creatinine levels tend to decrease slightly.
- PLT vs. GAMMA (g/dL) (r = −0.23; p < 0.001) and PLT vs. GAMMA (%) (r = −0.22; p < 0.001): Both indicate weak negative correlations, suggesting that higher platelet counts are associated with slightly lower gamma globulin levels and percentages.
Positive correlations may be indicative of typical physiological interactions; alternatively, they might signal specific pathological states when deviating from established normative ranges. These correlations highlight important relationships between different blood markers and liver functions, reflecting how biological variables interact. Strong and significant correlations (p < 0.001) are particularly relevant for understanding the pathophysiology underlying these measurements.
4.5.1. Serological Correlations
In the context of the LEISCAN® Leishmania ELISA Test, we conducted an analysis to explore serological correlations based on age, blood and biochemical data from 2801 animals. Table 7 serves as a critical reference for understanding the complex interactions between various physiological markers used in our study and Leishmania infection.

Table 7.
Age, blood and biochemical data correlations from the 2801 animals included in this study with the LEISCAN® Leishmania ELISA Test in descending order of correlation strength.
The data reveal significant correlations between various haematological and biochemical parameters and Leishmania seropositivity. Gamma globulins (GAMMA) and total protein (TP) show strong and moderate positive correlations, respectively, while urea (UREA) and creatinine (CREA) exhibit weaker positive correlations. Conversely, monocytes (MON), basophils (BASO), alanine aminotransferase (ALAT), eosinophils (EOS), neutrophils (NEU), total leukocytes (WBC), platelets (PLT), albumin (ALB), and packed cell volume (PCV) demonstrate significant negative correlations with seropositivity. The overall trend indicates that Leishmania-seropositive dogs tend to have higher levels of gamma globulins and total protein but lower levels of several other haematological and biochemical parameters. This suggests a complex interaction between age, immune response and disease susceptibility.
4.5.2. Haematological and Serum Protein Markers
Regarding leukocyte profiling, a similar inverse relationship is noted in the WBC count, with a correlation coefficient of −0.17, indicating a weak yet statistically significant negative association with positive ELISA outcomes. This trend is paralleled in the NEU counts, which mirror the WBC results in both magnitude and significance. The MON and BASO counts display minimal inverse correlations with the ELISA results with statistical significance. The EOS counts are weakly negatively correlated, suggesting a trend towards negative ELISA outcomes with increased eosinophil counts. A more pronounced negative correlation is observed with PCV, where Spearman’s rho of −0.29 suggests that as the PCV levels decrease (or are lower), the likelihood of a positive test for Leishmania increases. The PLT counts also demonstrate a negative correlation with the ELISA outcomes, supporting the hypothesis that specific haematological parameters can play a crucial role in the understanding of Leishmania infection. We hypothesise that in the early stages of leishmaniosis, dogs exhibit lower leukocyte counts, including polymorphonuclear cells (PMNC) and monocytes, with mild leukocytosis with neutrophilia or monocytosis developing as the disease progresses. Additionally, eosinophilia, occasionally reported in leishmaniosis and associated with parasitic infestation or an allergic component, could explain the weak negative correlation between eosinophil counts and ELISA outcomes. The pronounced negative correlation between PCV and ELISA outcomes may indicate that as the PCV levels decrease, reflecting moderate non-regenerative anaemia often seen in advanced stages of leishmaniosis, the likelihood of a positive ELISA test increases. This aligns with our hypothesis that dogs with lower PCV levels are more likely to test positive for Leishmania due to the disease’s impact on blood cell production. These observations align with what has been previously observed in other studies [62,63,64].
In the data, it is possible to observe a nuanced interplay between the serum protein levels and ELISA outcomes, employing the LEISCAN® ELISA methodology for serodiagnosis. These significant correlations between the serum protein levels and ELISA outcomes reveal a complex biochemical interplay. Elevated TP levels are moderately positively correlated with ELISA positivity, indicative of an active humoral immune response to Leishmania, suggesting a substantial immunologic interaction with the pathogen, with elevated serum proteins being part of the inflammatory response. In contrast, both the percentage of ALB and the ALB/GLOB ratio exhibit moderate negative correlations, pointing to an inverse relationship between specific protein distribution patterns and serological evidence of infection, where albumin decreases and globulins increase due to the inflammatory response and antibody production. These findings are consistent with the available literature indicating the typical serum protein profiles in infection [32,65]. This suggests that certain biochemical profiles, particularly those involving ALB and GLOB ratios, may be inversely associated with the likelihood of infection.
Moreover, we observed a moderate positive correlation in the percentages of GAMMA and absolute GAMMA with ELISA positivity, underscoring the critical role of specific immunoglobulins in the disease’s pathophysiology and the adaptive immune system’s response. Additionally, our analysis extended to other serum proteins such as ALPHA 1 (%), ALPHA 1 (g/dL), ALPHA 2 (%), ALPHA 2 (g/dL), BETA (%) and BETA (g/dL), which showed correlations ranging from very weak to weak with ELISA outcomes, each with distinct statistical significance. This suggests a nuanced and complex relationship between serum protein profiles and Leishmania seropositivity, illustrating the diverse ways in which protein fractions may be linked to the presence of Leishmania antigens [29,66].
4.5.3. Renal and Hepatic Biochemical Markers
In our study, we observed no statistical correlation of azotaemia across various LEISCAN® Leishmania ELISA serological groups ranging from negative (Rz ≤ 0.9) to very high positive (Rz > 3). Interestingly, the creatinine levels showed a very weak positive correlation with ELISA positivity, suggesting that renal function, as indicated by creatinine levels, may only marginally impact the serological detection of Leishmania. Furthermore, the urea levels were weakly positively correlated, while ALAT, a marker of hepatic damage, exhibited a weak negative correlation with the ELISA outcomes.
These findings suggest a relatively low prevalence of cases in the advanced stages of the disease, characterised by elevated ALAT, UREA and/or CREA levels, primarily due to renal dysfunction or hepatic failure. This observation is contrary to the expectation set by many published studies [67,68,69,70,71], which often associate kidney disease with glomerular damage due to the deposition of immune complexes, leading to a progressive reduction in the perfusion of the peritubular capillaries and subsequent tubular and interstitial damage [53]. The lack of a strong association between high concentrations of circulating blood antibodies to Leishmania and increased levels of creatinine and/or urea in our study could be attributed to the large sample size or the relatively low sensitivity of serum creatinine and urea as early indicators of kidney disease [65,71,72]. These analytes mainly reflect filtration capacity rather than specific injury markers, which may explain why this serious disorder is infrequently described in the literature, likely because the disease is typically detected in less advanced phases before significant lesions develop [65,71,73,74,75,76].
In light of these observations, urinary biomarkers of proteinuria, such as Cystatin C (CisC) and neutrophil gelatinase-associated lipocalin (NGAL), emerge as more sensitive and specific indicators of glomerular and tubular damage, representing the optimal choice for renal evaluation [74,77]. A possible use in routine clinical practise, which is widely available, is symmetric dimethylarginine (SDMA), which may be a useful adjunct to serum creatinine (sCr) and the urine protein/creatinine ratio (Up/c), aligning with the International Renal Interest Society (IRIS) guidelines, established by the LeishVet group, for the early detection of CanL-associated nephropathy [8,71,77,78,79].
4.5.4. Planetary Health in Leishmaniosis
Implementing a “One Health” approach in the fight against leishmaniosis includes integrated surveillance of human and animal health, vaccination campaigns, vector control, and community education on prevention and treatment. This strategy enhances the effectiveness of interventions and promotes sustainability in public and environmental health, which is crucial for managing zoonotic diseases in a connected world. The importance of this approach is reinforced by the Planetary Health framework, which acknowledges the interconnectedness of human health and natural systems, and the Stockholm Paradigm, which emphasises the ecological and evolutionary interactions in disease dynamics. Integrating these principles is vital for understanding and addressing the complex epidemiology of leishmaniosis, involving various hosts, vectors, and environmental factors, ultimately fostering a sustainable and health-conscious interaction among humans, animals, and the environment [80,81].
4.5.5. Recommendations
For future articles investigating the epidemiology and clinicopathology of vector-borne diseases, such as canine leishmaniosis, it is imperative to adopt a holistic and integrative research methodology. This includes advanced statistical techniques and comprehensive geographic information systems (GIS) to analyse environmental and climatic impacts on disease vectors. Future studies should consider incorporating molecular diagnostics with conventional serological assays to improve detection accuracy. Collaboration with global health institutions and adherence to the One Health approach will be essential in understanding zoonotic diseases within the broader context of interconnected human, animal and environmental health. Detailed analyses of regional variations and specific risk factors are vital for developing targeted control measures and informing policy decisions. In this study, the authors regret not obtaining data on the urine protein/creatinine ratio (Up/c) and symmetric dimethylarginine (SDMA), which could have provided crucial insights into the understanding of the disease’s impact on renal function. Future research should incorporate these parameters to enhance the interpretation and management of leishmaniosis.
5. Conclusions
In this study, the authors analyse the seroprevalence and geographical distribution of Leishmania infection across mainland Portugal, challenging previous perceptions that the disease is confined to specific regions. The analysis, based on a dataset of 2801 dogs, shows a 59.7% positivity rate for Leishmania antibodies, indicating a widespread distribution of the disease, potentially influenced by shifts in parasite circulation due to climate change. However, these findings do not represent the true seroprevalence in the general canine population, as the included dogs may be biassed because the submitted samples come from animals with a higher suspicion of Leishmaniosis than the general population. As a result, these outcomes significantly differ from earlier studies in Portugal, which used random sampling techniques and reported an overall true seroprevalence of 12.5% in dogs and 4.8% in humans. This highlights the critical need for enhanced surveillance and preventive measures across all regions of Portugal.
Our analysis revealed significant variances in seroprevalence, notably in the centre and northern regions, challenging prior studies and suggesting a more extensive disease spread. These data are further corroborated by a significant correlation between geographical location and infection outcomes, aligning with the Stockholm Paradigm that pathogens are capable of expanding beyond their traditional ecological confines. Dog age emerged as a significant factor in Leishmania infection, with a noted bimodal predisposition towards adult and senior dogs.
Despite the lack of a statistical correlation between the azotaemia and ELISA serological groups, the observed weak positive correlations with the creatinine and urea levels suggest renal function’s limited impact on Leishmania detection. This challenges the traditional linkage between kidney disease and Leishmania infection, which has traditionally been attributed to glomerular damage due to immune complex deposition, highlighting the importance of considering renal and hepatic function markers as indirect indicators of disease severity. The data obtained in this study emphasise the necessity of considering renal and hepatic markers in the broader context of disease stage distribution within the population.
Significantly, this study underscores the diagnostic value of examining albumin and globulin ratios, with the albumin percentage and the albumin/globulin ratio (ALB/GLOB) showing moderate negative correlations with serological evidence of infection. The application of AUC-ROC analysis has further identified the albumin/gamma globulin ratio (ALB/GAMMA) as a valuable diagnostic metric, offering high sensitivity and specificity for Leishmania detection and the diagnosis of CanL.
In conclusion, this study elucidates the complex seroprevalence patterns and biochemical markers associated with Leishmania infection, underscoring the imperative for a sophisticated approach to diagnostics, surveillance and disease management. Our findings significantly enrich the current understanding of Leishmania, advocating for an approach that integrates localised research efforts within a One Health framework to efficiently address the disease’s multifaceted impact.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens13080635/s1, Table S1. Spearman correlation analysis: age, blood and biochemical data from 2801 dogs across negative and positive LEISCAN® Leishmania ELISA Test.
Author Contributions
Conceptualization, R.L., A.S. and L.C.; methodology, R.L., A.S. and L.C.; software, R.L., A.G. and A.S.; validation, R.L., A.G., A.S., P.B.-S., Â.M., E.L.D., A.C.C. and L.C.; formal analysis, R.L. and Â.M.; investigation, R.L.; resources, R.L., A.S. and P.B.-S.; data curation, R.L. and A.S.; writing—original draft preparation, R.L. and A.G.; writing—review and editing, R.L., A.G., A.S., P.B.-S., Â.M., E.L.D., A.C.C. and L.C.; visualisation, R.L.; supervision, E.L.D., A.C.C. and L.C.; project administration, E.L.D., A.C.C. and L.C.; funding acquisition, R.L., E.L.D., A.C.C. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by projects UIDB/00772/2020 (Doi:10.54499/UIDB/00772/2020), UIDP/00681/2020 (Doi:10.54499/UIDP/00681/2020) and LA/P/0059/2020, funded by the Portuguese Foundation for Science and Technology (FCT).
Institutional Review Board Statement
All procedures complied with the Portuguese legislation for the protection of animals used for scientific purposes (i.e., Decree-Law no. 113/2013 of 7th August 2013), which transposes European legislation (i.e., Directive 2010/63/EU of the European Parliament and of the Council, of 22 September 2010). This study project was approved by the Institutional Review Board of INNO Veterinary Laboratories (protocol codes INNO.007 and INNO.0026; approved on 29 September 2021), which ensures that the analysed samples of veterinary medical centres can be used anonymously in studies and scientific research works related with this project.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
The authors extend their sincere gratitude to the INNO Veterinary Laboratories (Braga, Portugal) for their generous provision of the results that facilitated the research conducted in this study.
Conflicts of Interest
Authors A.S. and P.B.-S. are employed by the company INNO Veterinary Laboratories. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; Da Silva, S.M. Canine leishmaniasis: An overview of the current status and strategies for control. Biomed. Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Tamponi, C.; Scarpa, F.; Carta, S.; Knoll, S.; Sanna, D.; Gai, C.; Pipia, A.P.; Dessì, G.; Casu, M.; Varcasia, A.; et al. Seroprevalence and risk factors associated with Leishmania infantum in dogs in Sardinia (Italy), an endemic island for leishmaniasis. Parasitol. Res. 2021, 120, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, P.; Barlozzari, G.; Carvelli, A.; Scarpulla, M.; Iacoponi, F.; Macrì, G. Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic mediterranean region. PLoS ONE 2021, 16, e0244923. [Google Scholar] [CrossRef] [PubMed]
- Vaselek, S. Canine leishmaniasis in Balkan—A review of occurrence and epidemiology. Acta Trop. 2021, 224, 106110. [Google Scholar] [CrossRef]
- Almeida, M.; Maia, C.; Cristóvão, J.M.; Morgado, C.; Barbosa, I.; Ibars, R.F.; Campino, L.; Gonçalves, L.; Cortes, S. Seroprevalence and risk factors associated with Leishmania infection in dogs from Portugal. Microorganisms 2022, 10, 2262. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.; Coelho, A.C.; Quintas, H.; Cardoso, L. Leishmania seroprevalence in dogs: Comparing shelter and domestic communities. Animals 2023, 13, 2352. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Martinković, F.; Popović, M.; Smolec, O.; Mrljak, V.; Eckersall, P.D.; Horvatić, A. Data independent acquisition reveals in-depth serum proteome changes in canine leishmaniosis. Metabolites 2023, 13, 365. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-Time PCR applications for diagnosis of leishmaniasis. Parasit Vectors 2018, 11, 273. [Google Scholar] [CrossRef]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef] [PubMed]
- Gage, K.L.; Burkot, T.R.; Eisen, R.J.; Hayes, E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008, 35, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Rejali, M.; Mohammadi Dashtaki, N.; Ebrahimi, A.; Heidari, A.; Maracy, M. Cutaneous leishmaniasis based on climate regions in Iran (1998-2021): A systematic review and meta-analysis. Adv. Biomed. Res. 2022, 11, 120. [Google Scholar]
- Cavalera, M.A.; Zatelli, A.; Donghia, R.; Mendoza-Roldan, J.A.; Gernone, F.; Otranto, D.; Iatta, R. Conjunctival swab Real Time-PCR in Leishmania infantum seropositive dogs: Diagnostic and prognostic values. Biology 2022, 11, 184. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis emergence in Europe. Euro Surveill. 2010, 15, 19505. [Google Scholar] [CrossRef]
- Franco, A.O.; Davies, C.R.; Mylne, A.; Dedet, J.-P.; Gállego, M.; Ballart, C.; Gramiccia, M.; Gradoni, L.; Molina, R.; Gálvez, R.; et al. Predicting the distribution of canine leishmaniasis in western Europe based on environmental variables. Parasitology 2011, 138, 1878–1891. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Gernone, F.; Uva, A.; Donghia, R.; Zizzadoro, C.; Zatelli, A. Efficacy of domperidone plus renal diet in slowing the progression of chronic kidney disease in dogs with leishmaniosis. Parasit. Vectors 2022, 15, 397. [Google Scholar] [CrossRef]
- Maurelli, M.; Bosco, A.; Foglia Manzillo, V.; Vitale, F.; Giaquinto, D.; Ciuca, L.; Molinaro, G.; Cringoli, G.; Oliva, G.; Rinaldi, L.; et al. Clinical, molecular and serological diagnosis of canine leishmaniosis: An integrated approach. Vet. Sci. 2020, 7, 43. [Google Scholar] [CrossRef]
- Cabré, M.; Planellas, M.; Ordeix, L.; Solano-Gallego, L. Is signalment associated with clinicopathological findings in dogs with leishmaniosis? Vet. Rec. 2021, 189, e451. [Google Scholar] [CrossRef]
- Idrissi, H.; Hakkour, M.; Duchateau, L.; Zanatta, R.; Kachani, M.; Azrib, R.; Daminet, S.; Kichou, F.; El Asatey, S.; Tazi, N.; et al. Canine leishmaniasis in Morocco: A descriptive prospective clinical study. Vet. Med. Int. 2021, 2021, 6304127. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.A.; Latrofa, M.S.; Iatta, R.R.S.; Manoj, R.; Panarese, R.; Annoscia, G.; Pombi, M.; Zatelli, A.; Beugnet, F.; Otranto, D. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: Hindrances and opportunities. Parasit. Vectors 2021, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Prata, S.; Cardoso, L.; Pereira da Fonseca, I.; Leal, R.O. Dogs with leishmaniosis: How are we managing proteinuria in daily practice? A portuguese questionnaire-based study. Parasit. Vectors 2022, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Pabon-Rodriguez, F.M.; Brown, G.D.; Scorza, B.M.; Petersen, C.A. Bayesian multivariate longitudinal model for immune responses to Leishmania: A tick-borne co-infection study. Stat. Med. 2023, 42, 3860–3876. [Google Scholar] [CrossRef]
- Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New epidemiological aspects of animal leishmaniosis in Europe: The role of vertebrate hosts other than dogs. Pathogens 2021, 10, 307. [Google Scholar] [CrossRef]
- Ferreira, E.d.C.; de Lana, M.; Carneiro, M.; Reis, A.B.; Paes, D.V.; da Silva, E.S.; Schallig, H.; Gontijo, C.M. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet. Parasitol. 2007, 146, 235–241. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Villanueva-Saz, S.; Carbonell, M.; Trotta, M.; Furlanello, T.; Natale, A. Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan®, ID Screen® and Leishmania 96®), a rapid test (Speed Leish K®) and an in-house IFAT. Parasit. Vectors 2014, 7, 111. [Google Scholar] [CrossRef]
- Duarte, M.C.; Lage, D.P.; Martins, V.T.; Costa, L.E.; Salles, B.C.S.; Carvalho, A.M.R.S.; de Oliveira Santos, T.T.; Dias, D.S.; Ribeiro, P.A.F.; Chávez-Fumagalli, M.A.; et al. Performance of Leishmania braziliensis enolase protein for the serodiagnosis of canine and human visceral leishmaniosis. Vet. Parasitol. 2017, 238, 77–81. [Google Scholar] [CrossRef]
- Castellanos-Gonzalez, A.; Saldarriaga, O.A.; Tartaglino, L.; Gacek, R.; Temple, E.; Sparks, H.; Melby, P.C.; Travi, B.L. A novel molecular test to diagnose canine visceral leishmaniasis at the point of care. Am. J. Trop. Med. Hyg. 2015, 93, 970–975. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef]
- Machado, J.M.; Costa, L.E.; Dias, D.S.; Ribeiro, P.A.F.; Martins, V.T.; Lage, D.P.; Carvalho, G.B.; Franklin, M.L.; Tavares, G.S.V.; Oliveira-da-Silva, J.A.; et al. Diagnostic markers selected by immunoproteomics and phage display applied for the serodiagnosis of canine leishmaniosis. Res. Vet. Sci. 2019, 126, 4–8. [Google Scholar] [CrossRef]
- Aschar, M.; de Oliveira, E.T.B.; Laurenti, M.D.; Marcondes, M.; Tolezano, J.E.; Hiramoto, R.M.; Corbett, C.E.; da Matta, V.L. Value of the oral swab for the molecular diagnosis of dogs in different stages of infection with Leishmania infantum. Vet. Parasitol. 2016, 225, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Esteves, S.; Costa, I.; Brancal, H.; Lima, C.; Amorim, C.; Cardoso, L.; Santarém, N.; Cordeiro-da-Silva, A. Use of antigen combinations to address complex Leishmania-seropositivity patterns in dogs living in canine leishmaniosis endemic regions of Portugal. Microorganisms 2022, 10, 2018. [Google Scholar] [CrossRef]
- Curtin, J.M.; Aronson, N.E. Leishmaniasis in the United States: Emerging issues in a region of low endemicity. Microorganisms 2021, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Conceição, C.; Pereira, A.; Rocha, R.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; Pérez-Cutilllas, P.; Özbel, Y.; Töz, S.; et al. The estimated distribution of autochthonous leishmaniasis by Leishmania infantum in Europe in 2005–2020. PLoS Negl. Trop. Dis. 2023, 17, e0011497. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Acharya, K.P.; Acharya, N.; Phuyal, S.; Upadhyaya, M.; Lasee, S. One-Health approach: A best possible way to control rabies. One Health 2020, 10, 100161. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Behring, D.; Nissly, R.; Chothe, S.K.; Gontu, A.; Ravichandran, A.; Butler, T. Mitigating the impact of emerging animal infectious disease threats: First emerging animal infectious diseases conference (EAIDC) report. Viruses 2022, 14, 947. [Google Scholar] [CrossRef]
- Rocha, R.; Conceição, C.; Gonçalves, L.; Carvalho, A.C.; Maia, A.; Martins, A.; Carujo, A.; Maio, A.; Forra, C.; Melita, C.; et al. Epidemiological and clinical aspects of cutaneous and mucosal leishmaniases in Portugal: Retrospective analysis of cases diagnosed in public hospitals and reported in the literature between 2010 and 2020. Microorganisms 2024, 12, 819. [Google Scholar] [CrossRef]
- Rocha, R.; Pereira, A.; Maia, C. A global perspective on non-autochthonous canine and feline Leishmania infection and leishmaniosis in the 21st century. Acta Trop. 2023, 237, 106710. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Cheng, G.; Guo, X.; Zhou, X. Editorial: Needs and potential application of One Health approach in the control of vector-borne and zoonotic infectious disease. Front. Microbiol. 2022, 13, 1089174. [Google Scholar] [CrossRef]
- Jia, Z. Insight on infectious diseases from the perspective of One Health. China CDC Wkly. 2022, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.M.; de Lima, V.M.F.; de Melo, G.D.; de Paula, H.B.; Pereira, M.E.G.; Tronco, C.d.M.T.; Hiramoto, R.M.; Laurenti, M.D.; Burattini, M.N. Serological, parasitological and molecular tests for canine visceral leishmaniosis diagnosis in a longitudinal study. Rev. Bras. Parasitol. Vet. 2015, 24, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Failing, K.; Taubert, A.; Pantchev, N. Serological diagnosis of canine leishmaniosis: Comparison of three commercially available tests. Parasitol. Res. 2014, 113, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, D.; Berriatua, E.; Thomas, M.C.; Bernal, L.J.; Ortuño, M.; Benitez, C.; Egui, A.; Papasouliotis, K.; Tennant, B.; Chambers, J.; et al. Performance of Leishmania PFR1 recombinant antigen in serological diagnosis of asymptomatic canine leishmaniosis by ELISA. BMC Vet. Res. 2017, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Fraga, D.B.M.; Cristóvão, J.; Borja, L.S.; da Silva Solcà, M.; Campino, L.; Veras, P.S.T.; Gonçalves, L. Leishmania exposure in dogs from two endemic countries from New and Old Worlds (Brazil and Portugal): Evaluation of three serological tests using Bayesian Latent Class models. Parasit. Vectors 2022, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Riffenburgh, R.H.; Gillen, D.L. Statistics in Medicine; Elsevier: London, UK, 2020. [Google Scholar]
- Patrie, A.; Watson, P. Statistics for Veterinary and Animal Science, 3rd ed.; Wiley-Blackwell: Chichester, UK, 2013. [Google Scholar]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, P.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Veterinary Epidemiology; Wiley-Blackwell: Chichester, UK, 2018. [Google Scholar]
- Cortes, S.; Vaz, Y.; Neves, R.; Maia, C.; Cardoso, L.; Campino, L. Risk factors for canine leishmaniasis in an endemic mediterranean region. Vet. Parasitol. 2012, 189, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Gonçalves, L.; Conceição, C.; Andrade, P.; Cristóvão, J.M.; Condeço, J.; Delgado, B.; Caeiro, C.; Kuzmenko, T.; Vasconcelos, E.; et al. Prevalence of asymptomatic Leishmania infection and knowledge, perceptions, and practices in blood donors in mainland Portugal. Parasit. Vectors 2023, 16, 357. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Altet, L.; Serrano, L.; Cristóvão, J.M.; Tabar, M.D.; Francino, O.; Cardoso, L.; Campino, L.; Roura, X. Molecular detection of Leishmania infantum, Filariae and Wolbachia spp. in dogs from southern Portugal. Parasit. Vectors 2016, 9, 170. [Google Scholar] [CrossRef]
- Schallig, H.D.; Cardoso, L.; Semião-Santos, S.J. Seroepidemiology of canine leishmaniosis in Évora (southern Portugal): 20-year trends. Parasit. Vectors 2013, 6, 100. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine leishmaniasis: Update on epidemiology, diagnosis, treatment, and prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Pereira, S.; Pita-Pereira, D.; Araujo-Pereira, T.; Britto, C.; Costa-Rego, T.; Ferrolho, J.; Vilhena, M.; Rangel, E.F.; Vilela, M.L.; Afonso, M.O. First molecular detection of Leishmania infantum in Sergentomyia minuta (Diptera, Psychodidae) in Alentejo, southern Portugal. Acta Trop. 2017, 174, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Pires, H.; Martins, M.; Matos, A.C.; Cardoso, L.; Monteiro, F.; Roque, N.; Nunes, T.; Gottstein, B.; Cortes, H. Geospatial analysis applied to seroepidemiological survey of canine leishmaniosis in east-central Portugal. Vet. Parasitol. 2019, 274, 108930. [Google Scholar] [CrossRef]
- Thomaz-Soccol, V.; Gonçalves, A.L.; Piechnik, C.A.; Baggio, R.A.; Boeger, W.A.; Buchman, T.L.; Rodrigues Dos Santos, D.; Celestino, A.; Aquino, J., Jr.; Leandro, A.S.; et al. Hidden danger: Unexpected scenario in the vector-parasite dynamics of leishmaniases in the Brazil side of triple border (Argentina, Brazil and Paraguay). PLoS Negl. Trop. Dis. 2018, 12, e0006336. [Google Scholar] [CrossRef]
- Miranda, S.; Roura, X.; Picado, A.; Ferrer, L.; Ramis, A. Characterization of sex, age, and breed for a population of canine leishmaniosis diseased dogs. Res. Vet. Sci. 2008, 85, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Acosta, L.; Gutierrez, M.N.; Cruz, I.; Nieto, J.; Deschutter, E.J.; Bornay-Llinares, F.J. A novel sampling model to study the epidemiology of canine leishmaniasis in an urban environment. Front. Vet. Sci. 2021, 8, 642287. [Google Scholar] [CrossRef] [PubMed]
- Edo, M.; Marín-García, P.J.; Llobat, L. Is the prevalence of Leishmania infantum linked to breeds in dogs? Characterization of seropositive dogs in Ibiza. Animals 2021, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Macià, M.; Marín-García, P.-J.; Ahuir-Baraja, A.-E.; Llobat, L. Immunological profile of two canine breeds in an endemic region of Leishmania infantum. Vet. Parasitol. Reg. Stud. Rep. 2023, 40, 100861. [Google Scholar] [CrossRef]
- Varjão, B.M.; de Pinho, F.A.; Solcà, M.d.S.; Silvestre, R.; Fujimori, M.; Goto, H.; Varjão, N.M.; Dias, R.C.; Barrouin-Mello, S.M. Spatial distribution of canine Leishmania infantum infection in a municipality with endemic human leishmaniasis in eastern Bahia, Brazil. Rev. Bras Parasitol. Vet. 2021, 30, e022620. [Google Scholar] [CrossRef] [PubMed]
- Nicolato, R.d.C.; de Abreu, R.T.; Roatt, B.M.; Aguiar-Soares, R.D.d.O.; Reis, L.E.S.; Carvalho, M.d.G.; Carneiro, C.M.; Giunchetti, R.C.; Bouillet, L.E.M.; Lemos, D.S.; et al. Clinical forms of canine visceral leishmaniasis in naturally Leishmania infantum–infected dogs and related myelogram and hemogram changes. PLoS ONE 2013, 8, e82947. [Google Scholar] [CrossRef]
- Maia, C.; Campino, L. Biomarkers associated with Leishmania infantum exposure, infection, and disease in dogs. Front. Cell Infect. Microbiol. 2018, 8, 302. [Google Scholar] [CrossRef]
- Di Pietro, S.; Crinò, C.; Falcone, A.; Crupi, R.; Francaviglia, F.; Vitale, F.; Giudice, E. Parasitemia and its daily variation in canine leishmaniasis. Parasitol. Res. 2020, 119, 3541–3548. [Google Scholar] [CrossRef]
- Baxarias, M.; Jornet-Rius, O.; Donato, G.; Mateu, C.; Alcover, M.M.; Pennisi, M.G.; Solano-Gallego, L. Signalment, immunological and parasitological status and clinicopathological findings of Leishmania-seropositive apparently healthy dogs. Animals 2023, 13, 1649. [Google Scholar] [CrossRef]
- Cavalera, M.A.; Iatta, R.; Laricchiuta, P.; Passantino, G.; Abramo, F.; Mendoza-Roldan, J.A.; Otranto, D.; Zatelli, A. Clinical, haematological and biochemical findings in tigers infected by Leishmania infantum. BMC Vet. Res. 2020, 16, 214. [Google Scholar] [CrossRef]
- Geisweid, K.; Mueller, R.; Sauter-Louis, C.; Hartmann, K. Prognostic analytes in dogs with Leishmania infantum infection living in a non-endemic area. Vet. Rec. 2012, 171, 399. [Google Scholar] [CrossRef]
- Molina, C.C.; Dias, M.J.; Domingues, T.D.; Englar, R.E.; Leal, R.O. Clinical findings and prognostic factors for mortality in hospitalized dogs with leishmaniosis: A retrospective study. Comp. Immunol. Microbiol. Infect Dis. 2023, 101, 102041. [Google Scholar] [CrossRef]
- Planellas, M.; Roura, X.; Lloret, A. Presence of renal disease in dogs with patent leishmaniasis. Parassitologia 2009, 51, 65–68. [Google Scholar]
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; Zatelli, A.; Canine leishmaniosis working group. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J. Small Anim. Pract. 2021, 62, 3. [Google Scholar]
- Pereira, M.; Santos, R.; Oliveira, R.; Costa, L.; Prata, A.; Gonçalves, V.; Roquette, M.; Vala, H.; Santos-Gomes, G. Prognostic factors and life expectancy in canine leishmaniosis. Vet. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; da Silva, S.M.; Fulgêncio, G.d.O.; Michalick, M.S.M.; Frézard, F.J.G. Relationship between clinical and pathological signs and severity of canine leishmaniasis. Rev. Bras. Parasitol. Vet. 2013, 22, 373–378. [Google Scholar] [CrossRef]
- Amusategui, I.; Sainz, A.; Rodríguez, F.; Tesouro, M.A. Distribution and relationships between clinical and biopathological parameters in canine leishmaniasis. Eur. J. Epidemiol. 2002, 18, 147–156. [Google Scholar] [CrossRef]
- Dias, A.F.d.L.R.; Ayres, E.d.C.B.S.; Maruyama, F.H.; Monteiro, B.R.G.; de Freitas, M.S.; de Almeida, A.d.B.P.F.; Mendonça, A.J.; Sousa, V.R.F. Monitoring of serum and urinary biomarkers during treatment of canine visceral leishmaniasis. Vet. World 2020, 13, 1620–1626. [Google Scholar] [CrossRef]
- Abbehusen, M.M.C.; Almeida, V.d.A.; Solcà, M.d.S.; Pereira, L.d.S.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci. Rep. 2017, 7, 15914. [Google Scholar] [CrossRef]
- Pereira, M.A.; Santos, R.; Nóbrega, C.; Mega, C.; Cruz, R.; Esteves, F.; Santos, C.; Coelho, C.; Mesquita, J.R.; Vala, H.; et al. A questionnaire-based survey on the long-term management of canine leishmaniosis by veterinary practitioners. Animals 2022, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Barrera-Chacón, R.; Peña, F.J.; Belinchón-Lorenzo, S.; Robles, N.R.; Pérez-Merino, E.M.; Martín-Cano, F.E.; Duque, F.J. Proteomic research on new urinary biomarkers of renal disease in canine leishmaniosis: Survival and monitoring response to treatment. Res. Vet. Sci. 2023, 161, 180–190. [Google Scholar]
- Coyne, M.J.; Schultze, A.E.; McCrann, D.J., 3rd; Murphy, R.E.; Cross, J.; Strong-Townsend, M.; Drake, C.; Mack, R. Evaluation of renal injury and function biomarkers, including symmetric dimethylarginine (SDMA), in the rat passive Heymann nephritis (PHN) model. PLoS ONE 2022, 17, e0269085. [Google Scholar] [CrossRef]
- Giapitzoglou, S.; Saridomichelakis, M.N.; Leontides, L.S.; Kasabalis, D.; Chatzis, M.; Apostolidis, K.; Theodorou, K.; Roumpeas, E.; Mylonakis, M.E. Evaluation of serum symmetric dimethylarginine as a biomarker of kidney disease in canine leishmaniosis due to Leishmania infantum. Vet. Parasitol. 2020, 277, 109015. [Google Scholar] [CrossRef]
- De Castañeda, R.R.; Villers, J.; Guzmán, C.A.F.; Eslanloo, T.; de Paula, N.; Machalaba, C.; Zinsstag, J.; Utzinger, J.; Flahault, A.; Bolon, I. One Health and Planetary Health research: Leveraging differences to grow together. Lancet Planet Health 2023, 7, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Abdullahi, A.A.; Yusuf, R.O.; Abimbola, S.O.; Adebayo, A.O.; Ikebuaso, J.G.; et al. Preventing the next pandemic through a Planetary Health approach: A focus on key drivers of zoonosis. Challenges 2022, 13, 50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).