Optimizing Clubroot Management and the Role of Canola Cultivar Mixtures
Abstract
:1. Introduction
2. Life Cycle of P. brassicae
3. Management Strategies
3.1. Pathogen Avoidance
3.2. Clubroot Management in Infested Fields
3.2.1. Crop Rotation
3.2.2. Liming
3.2.3. Chemical Control
3.2.4. Host Resistance
4. Cultivar Mixtures as an Option for Disease Management
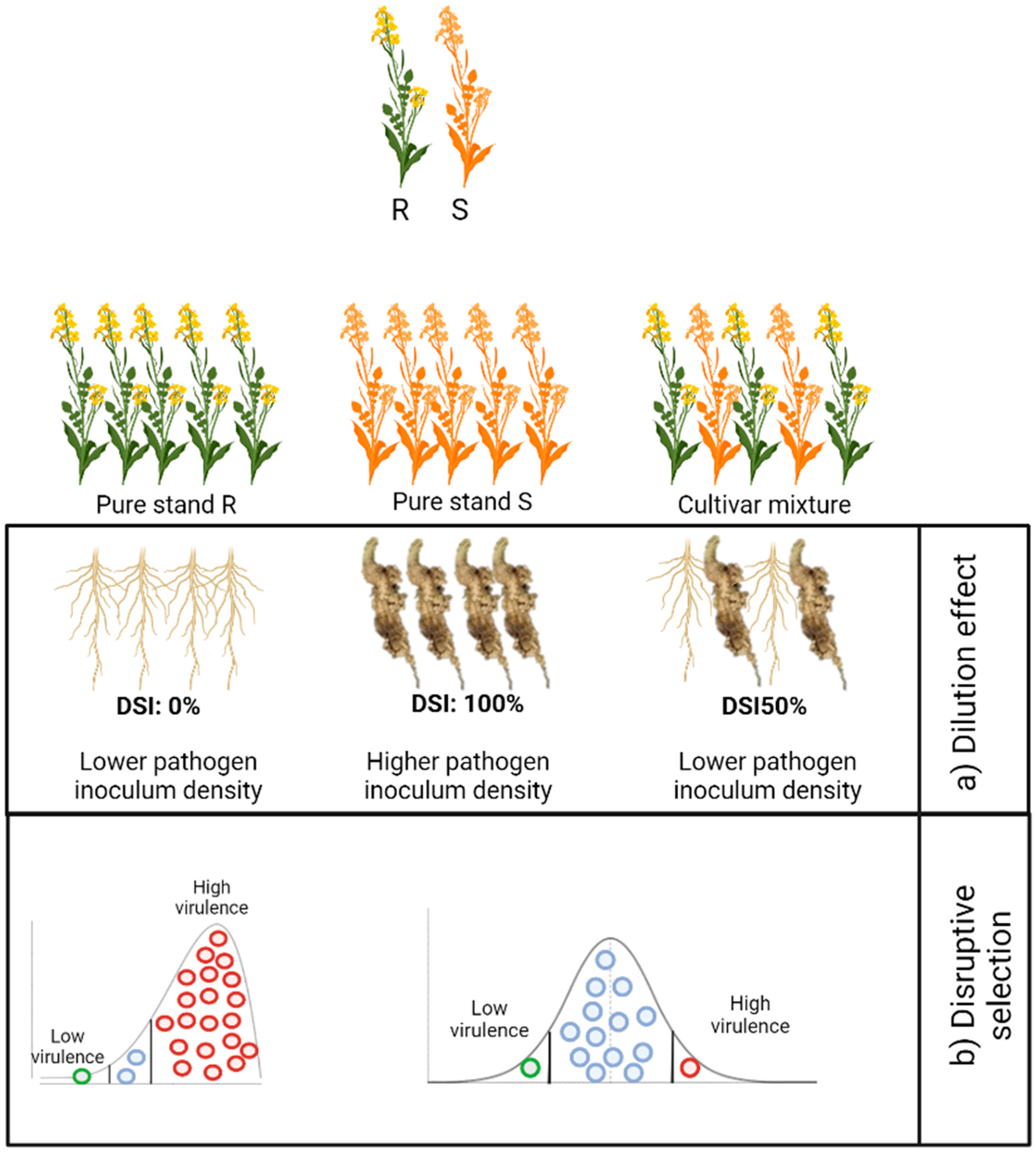
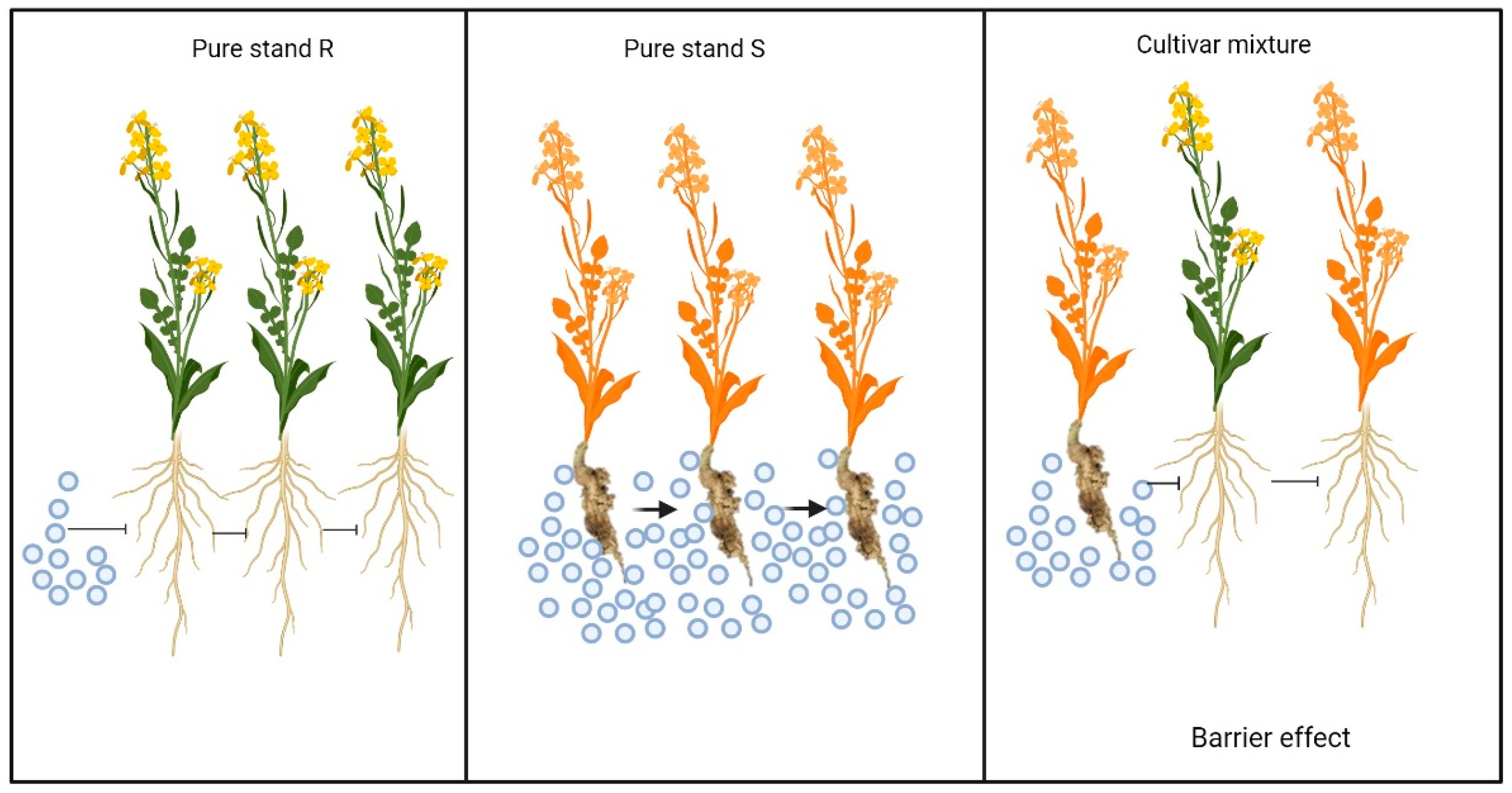
4.1. Mechanisms of Control
4.2. Designing Cultivar Mixtures
4.3. Benefits
4.4. Challenges
5. Potential for Clubroot Management
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Oilseeds: World Markets and Trade; Biodiesel Policies Suppress U.S. Soybean Oil Exports; USDA: Washington, DC, USA, 2023; p. 40. [Google Scholar]
- Javed, M.A.; Schwelm, A.; Zamani-Noor, N.; Salih, R.; Silvestre Vañó, M.; Wu, J.; González García, M.; Heick, T.M.; Luo, C.; Prakash, P.; et al. The Clubroot Pathogen Plasmodiophora brassicae: A Profile Update. Mol. Plant Pathol. 2023, 24, 89–106. [Google Scholar] [CrossRef]
- Digital Science. Dimensions (Software). 2018. Available online: https://app.dimensions.ai (accessed on 20 October 2023).
- Tewari, J.P.; Strelkov, S.E.; Orchard, D.; Hartman, M.; Lange, R.M.; Turkington, T.K. Identification of Clubroot of Crucifers on Canola (Brassica napus) in Alberta. Can. J. Plant Pathol. 2005, 27, 143–144. [Google Scholar] [CrossRef]
- Pageau, D.; Lajeunesse, J.; Lafond, J. Impact de l’hernie des cruciferes [Plasmodiophora brassicae] sur la productivite et la qualite du canola. Can. J. Plant Pathol. Rev. Can. Phytopathol. 2006, 28, 137–143. [Google Scholar] [CrossRef]
- Dixon, G.R. The Occurrence and Economic Impact of Plasmodiophora brassicae and Clubroot Disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Myers, D.F.; Campell, R.N.; Greathead, A.S. Thermal Inactivation of Plasmodiophora brassicae Woron. and Its Attempted Control by Solarization in the Salinas Valley of California. Crop Prot. 1983, 2, 325–333. [Google Scholar] [CrossRef]
- Webster, M.A.; Dixon, G.R. Calcium, pH and Inoculum Concentration Influencing Colonization by Plasmodiophora brassicae. Mycol. Res. 1991, 95, 64–73. [Google Scholar] [CrossRef]
- Webster, M.A.; Dixon, G.R. Boron, pH and Inoculum Concentration Influencing Colonization by Plasmodiophora brassicae. Mycol. Res. 1991, 95, 74–79. [Google Scholar] [CrossRef]
- Donald, E.C.; Porter, I.J. A Sand—Solution Culture Technique Used to Observe the Effect of Calcium and pH on Root Hair and Cortical Stages of Infection by Plasmodiophora brassicae. Australas. Plant Pathol. 2004, 33, 585–589. [Google Scholar] [CrossRef]
- Samuel, G.; Garrett, S.D. The Infected Root-Hair Count for Estimating the Activity of Plasmodiophora brassicae Woron. in the Soil. Ann. Appl. Biol. 1945, 32, 96–101. [Google Scholar] [CrossRef]
- Hamilton, H.; Crête, R. Influence of Soil Moisture, Soil pH, and Liming Sources on the Incidence of Clubroot, Germination and Growth of Cabbage Produced in Mineral and Organic Soils under Controlled Conditions. Can. J. Plant Sci. 1978, 58, 45–53. [Google Scholar] [CrossRef]
- Dobson, R.; Gabrielson, R.L.; Baker, A.S. Soil Water Matric Potential Requirements for Root-Hair and Cortical Infection of Chinese Cabbage by Plasmodiophora brassicae. Phytopathology 1982, 72, 1598–1600. [Google Scholar] [CrossRef]
- Narisawa, K.; Shimura, M.; Usuki, F.; Fukuhara, S.; Hashiba, T. Effects of Pathogen Density, Soil Moisture, and Soil pH on Biological Control of Clubroot in Chinese Cabbage by Heteroconium Chaetospira. Plant Dis. 2005, 89, 285–290. [Google Scholar] [CrossRef]
- Ayers, G.W. Studies on the Life History of the Club Root Organism, Plasmodiophora brassicae. Can. J. Res. 1944, 22c, 143–149. [Google Scholar] [CrossRef]
- Thuma, B.A.; Rowe, R.C.; Madden, L.V. Relationships of Soil Temperature and Moisture to Clubroot (Plasmodiophora brassicae) Severity on Radish in Organic Soil. Plant Dis. 1983, 67, 758–762. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of Temperature on Primary Infection by Plasmodiophora brassicae and Initiation of Clubroot Symptoms. Plant Pathol. 2011, 60, 830–838. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of Temperature on Cortical Infection by Plasmodiophora brassicae and Clubroot Severity. Phytopathology 2011, 101, 1424–1432. [Google Scholar] [CrossRef]
- Gossen, B.D.; Adhikari, K.K.C.; McDonald, M.R. Effects of Temperature on Infection and Subsequent Development of Clubroot under Controlled Conditions. Plant Pathol. 2012, 61, 593–599. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.-F.; McDonald, M.R. Interaction of pH and Temperature Affect Infection and Symptom Development of Plasmodiophora brassicae in Canola. Can. J. Plant Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Luo, H.; Chen, G.; Liu, C.; Huang, Y.; Xiao, C. An Improved Culture Solution Technique for Plasmodiophora brassicae Infection and the Dynamic Infection in the Root Hair. Australas. Plant Pathol. 2014, 43, 53–60. [Google Scholar] [CrossRef]
- Botero-Ramírez, A.; Hwang, S.-F.; Strelkov, S.E. Effect of Clubroot (Plasmodiophora brassicae) on Yield of Canola (Brassica napus). Can. J. Plant Pathol. 2022, 44, 372–385. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Rashid, A.; Strelkov, S.E.; Gossen, B.D.; Peng, G.; Turnbull, G.D. Effect of Susceptible and Resistant Canola Plants on Plasmodiophora brassicae Resting Spore Populations in the Soil. Plant Pathol. 2013, 62, 404–412. [Google Scholar] [CrossRef]
- Wallenhammar, A.-C. Prevalence of Plasmodiophora brassicae in a Spring Oilseed Rape Growing Area in Central Sweden and Factors Influencing Soil Infestation Levels. Plant Pathol. 1996, 45, 710–719. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Howard, R.J.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Management of Clubroot (Plasmodiophora brassicae) on Canola (Brassica napus) in Western Canada. Can. J. Plant Pathol. 2014, 36, 49–65. [Google Scholar] [CrossRef]
- Hennig, B.C.; Hwang, S.-F.; Manolii, V.P.; Turnbull, G.; Robinson, S.V.J.; Strelkov, S.E. Evaluation of Host Resistance, Hydrated Lime, and Weed Control to Manage Clubroot in Canola. Horticulturae 2022, 8, 215. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Feindel, D. Emergence of New Virulence Phenotypes of Plasmodiophora brassicae on Canola (Brassica napus) in Alberta, Canada. Eur. J. Plant Pathol. 2016, 145, 517–529. [Google Scholar] [CrossRef]
- Anand, S.C.; Koenning, S.R.; Sharma, S.B. Performance of Blends of Soybean Cyst Nematode Resistant and Susceptible Cultivars. Crop Sci. 1995, 35, 524–528. [Google Scholar] [CrossRef]
- Finckh, M.R.; Gacek, E.S.; Goyeau, H.; Lannou, C.; Merz, U.; Mundt, C.C.; Munk, L.; Nadziak, J.; Newton, A.C.; de Vallavieille-Pope, C.; et al. Cereal Variety and Species Mixtures in Practice, with Emphasis on Disease Resistance. Agronomie 2000, 20, 813–837. [Google Scholar] [CrossRef]
- Gigot, C.; Saint-Jean, S.; Huber, L.; Maumené, C.; Leconte, M.; Kerhornou, B.; de Vallavieille-Pope, C. Protective Effects of a Wheat Cultivar Mixture against Splash-Dispersed Septoria Tritici Blotch Epidemics. Plant Pathol. 2013, 62, 1011–1019. [Google Scholar] [CrossRef]
- Kristoffersen, R.; Eriksen, L.B.; Nielsen, G.C.; Jørgensen, J.R.; Jørgensen, L.N. Management of Septoria Tritici Blotch Using Cultivar Mixtures. Plant Dis. 2022, 106, 1341–1349. [Google Scholar] [CrossRef]
- Kumar, G.; Rashid, M.; Teli, B.; Bajpai, R.; Nanda, S.; Yadav, S. Cultivar Mixture: Old but Impactful Plant Disease Management Strategy. Int. J. Econ. Plants 2021, 8, 113–119. [Google Scholar] [CrossRef]
- Macfarlane, I. Germination of Resting Spores of Plasmodiophora brassicae. Trans. Br. Mycol. Soc. 1970, 55, 97–112. [Google Scholar] [CrossRef]
- Friberg, H.; Lagerlöf, J.; Rämert, B. Germination of Plasmodiophora brassicae Resting Spores Stimulated by a Non-Host Plant. Eur. J. Plant Pathol. 2005, 113, 275. [Google Scholar] [CrossRef]
- Rashid, A.; Ahmed, H.U.; Xiao, Q.; Hwang, S.F.; Strelkov, S.E. Effects of Root Exudates and pH on Plasmodiophora brassicae Resting Spore Germination and Infection of Canola (Brassica napus L.) Root Hairs. Crop Prot. 2013, 48, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Sarenqimuge, S.; von Tiedemann, A. The Soil Bacterial Community Regulates Germination of Plasmodiophora brassicae Resting Spores Rather than Root Exudates. PLOS Pathog. 2023, 19, e1011175. [Google Scholar] [CrossRef]
- Liu, L.; Qin, L.; Zhou, Z.; Hendriks, W.G.H.M.; Liu, S.; Wei, Y. Refining the Life Cycle of Plasmodiophora brassicae. Phytopathology 2020, 110, 1704–1712. [Google Scholar] [CrossRef]
- Aist, J.R.; Williams, P.H. The Cytology and Kinetics of Cabbage Root Hair Penetration by Plasmodiophora brassicae. Can. J. Bot. 1971, 49, 2023–2034. [Google Scholar] [CrossRef]
- Feng, J.; Hwang, S.-F.; Strelkov, S.E. Studies into Primary and Secondary Infection Processes by Plasmodiophora brassicae on Canola. Plant Pathol. 2013, 62, 177–183. [Google Scholar] [CrossRef]
- Ingram, D.S.; Tommerup, I.C. The Life History of Plasmodiophora brassicae Woron. Proc. R. Soc. Lond. B Biol. Sci. 1972, 180, 103–112. [Google Scholar]
- Mithen, R.; Magrath, R. A Contribution to the Life History of Plasmodiophora brassicae: Secondary Plasmodia Development in Root Galls of Arabidopsis Thaliana. Mycol. Res. 1992, 96, 877–885. [Google Scholar] [CrossRef]
- Bulman, S.; Braselton, J.P. 4 Rhizaria: Phytomyxea. In Systematics and Evolution: Part A; McLaughlin, D.J., Spatafora, J.W., Eds.; The Mycota; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–112. ISBN 978-3-642-55318-9. [Google Scholar]
- Hollman, K.B.; Hwang, S.F.; Manolii, V.P.; Strelkov, S.E. Pathotypes of Plasmodiophora brassicae Collected from Clubroot Resistant Canola (Brassica napus L.) Cultivars in Western Canada in 2017–2018. Can. J. Plant Pathol. 2021, 43, 622–630. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Fredua-Agyeman, R.; Hollman, K.B.; Storfie, E.; Manolii, V.P. New Clubroot Pathotypes and Second Generation Resistance. Available online: https://www.saskcanola.com/research-project-articles/new-clubroot-pathotypes-and-second-generation-resistance (accessed on 13 June 2024).
- Parlevliet, J.E.; Zadoks, J.C. The Integrated Concept of Disease Resistance: A New View Including Horizontal and Vertical Resistance in Plants. Euphytica 1977, 26, 5–21. [Google Scholar] [CrossRef]
- Pink, D.; Puddephat, I. Deployment of Disease Resistance Genes by Plant Transformation—A ‘Mix and Match’ Approach. Trends Plant Sci. 1999, 4, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.J.; Zhan, J.; Barrett, L.G.; Papaïx, J.; Thrall, P.H. Addressing the Challenges of Pathogen Evolution on the World’s Arable Crops. Phytopathology 2016, 106, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.; Porter, I.; Porter, I. Integrated Control of Clubroot. J. Plant Growth Regul. 2009, 28, 289. [Google Scholar] [CrossRef]
- Howard, R.J.; Strelkov, S.E.; Harding, M.W. Clubroot of Cruciferous Crops—New Perspectives on an Old Disease. Can. J. Plant Pathol. 2010, 32, 43–57. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Ludwig-Müller, J. Clubroot Disease Management Challenges from a German Perspective. Can. J. Plant Pathol. 2014, 36, 85–98. [Google Scholar] [CrossRef]
- Hill, T.B.; Daniels, G.C.; Feng, J.; Harding, M.W. Hard to Kill: Inactivation of Plasmodiophora brassicae Resting Spores Using Chemical Disinfectants. Plant Dis. 2022, 106, 190–196. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Howard, R.J.; Hartman, M.; Turkington, T.K. Progress towards the Sustainable Risk Management of Clubroot (Plasmodiophora brassicae) of Canola on the Canadian Prairies. Prairie Soils Crop 2011, 4, 114–121. [Google Scholar] [CrossRef]
- Peng, G.; Lahlali, R.; Hwang, S.-F.; Pageau, D.; Hynes, R.K.; McDonald, M.R.; Gossen, B.D.; Strelkov, S.E. Crop Rotation, Cultivar Resistance, and Fungicides/Biofungicides for Managing Clubroot (Plasmodiophora brassicae) on Canola. Can. J. Plant Pathol. 2014, 36, 99–112. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Turnbull, G.D.; Strelkov, S.E.; Gossen, B.D.; Peng, G. Effect of Host and Non-Host Crops on Plasmodiophora brassicae Resting Spore Concentrations and Clubroot of Canola. Plant Pathol. 2015, 64, 1198–1206. [Google Scholar] [CrossRef]
- Anders, J.; Katarzyna, M.-S.; Gunnar, B.; Ann-Charlotte, W. Quantitative PCR Shows Propagation of Plasmodiophora brassicae in Swedish Long Term Field Trials. Eur. J. Plant Pathol. 2016, 145, 573–581. [Google Scholar] [CrossRef]
- Ernst, T.W.; Kher, S.; Stanton, D.; Rennie, D.C.; Hwang, S.F.; Strelkov, S.E. Plasmodiophora brassicae Resting Spore Dynamics in Clubroot Resistant Canola (Brassica napus) Cropping Systems. Plant Pathol. 2019, 68, 399–408. [Google Scholar] [CrossRef]
- Fox, N.M.; Hwang, S.-F.; Manolii, V.P.; Turnbull, G.; Strelkov, S.E. Evaluation of Lime Products for Clubroot (Plasmodiophora brassicae) Management in Canola (Brassica napus) Cropping Systems. Can. J. Plant Pathol. 2022, 44, 21–38. [Google Scholar] [CrossRef]
- Knox, O.G.G.; Oghoro, C.O.; Burnett, F.J.; Fountaine, J.M. Biochar Increases Soil pH, But Is as Ineffective as Liming at Controlling Clubroot. J. Plant Pathol. 2015, 97, 149–152. [Google Scholar]
- Murakami, H.; Tsushima, S.; Kuroyanagi, Y.; Shishido, Y. Reduction of Resting Spore Density of Plasmodiophora brassicae and Clubroot Disease Severity by Liming. Soil Sci. Plant Nutr. 2002, 48, 685–691. [Google Scholar] [CrossRef]
- Sharma, P.; Siddiqui, S.A.; Rai, P.K.; Meena, P.D.; Kumar, J.; Chauhan, J.S. Evaluation of Brassica Germplasm for Field Resistance against Clubroot (Plasmodiophora brassicae Woron). Arch. Phytopathol. Plant Prot. 2012, 45, 356–359. [Google Scholar] [CrossRef]
- Cao, T.; Rennie, D.C.; Manolii, V.P.; Hwang, S.F.; Falak, I.; Strelkov, S.E. Quantifying Resistance to Plasmodiophora brassicae in Brassica Hosts. Plant Pathol. 2014, 63, 715–726. [Google Scholar] [CrossRef]
- McGrann, G.R.D.; Gladders, P.; Smith, J.A.; Burnett, F. Control of Clubroot (Plasmodiophora brassicae) in Oilseed Rape Using Varietal Resistance and Soil Amendments. Field Crops Res. 2016, 186, 146–156. [Google Scholar] [CrossRef]
- Donald, E.; Porter, I. Clubroot (Plasmodiophora brassicae) an Imminent Threat to the Australian Canola Industry. In Proceedings of the Thirteenth Biennial Australian Research Assembly on Brassicas, Tamworth, NSW, Australia, 8–12 September 2003; pp. 114–118. [Google Scholar]
- Strelkov, S.E.; Dixon, G.R. Clubroot (Plasmodiophora brassicae) on Canola and Other Brassica Species—Disease Development, Epidemiology and Management. Can. J. Plant Pathol. 2014, 36, 1–4. [Google Scholar] [CrossRef]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia y Garcia, A.; Gaudin, A.C.M.; et al. Long-Term Evidence Shows That Crop-Rotation Diversification Increases Agricultural Resilience to Adverse Growing Conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Gossen, B.D.; Hwang, S.-F.; Lahlali, R. A >2-Year Crop Rotation Reduces Resting Spores of Plasmodiophora brassicae in Soil and the Impact of Clubroot on Canola. Eur. J. Agron. 2015, 70, 78–84. [Google Scholar] [CrossRef]
- Rauschert, E. Survivorship Curves. Nat. Educ. Knowl. 2010, 3, 18. [Google Scholar]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Lahlali, R.; Hynes, R.; Anderson, K.; Strelkov, S.; Mcdonald, M.R.; Gossen, B.; Turkington, T.; et al. Assessment of Crop Rotation in Combination with Cultivar Resistance or a Biofungicide Seed Treatment for Control of Clubroot on Canola. Acta Hortic. 2013, 1005, 595–598. [Google Scholar]
- Kim, J.-S.; Lee, J.-T.; Lee, G.-J. Effect of Crop Rotation on Control of Clubroot Disease of Chinese Cabbage Caused by Plasmodiophora brassicae. Res. Plant Dis. 2009, 15, 242–247. [Google Scholar] [CrossRef]
- Cao, T.; Manolii, V.P.; Zhou, Q.; Hwang, S.F.; Strelkov, S.E. Effect of Canola (Brassica napus) Cultivar Rotation on Plasmodiophora brassicae Pathotype Composition. Can. J. Plant Sci. 2020, 100, 218–225. [Google Scholar] [CrossRef]
- LeBoldus, J.M.; Manolii, V.P.; Turkington, T.K.; Strelkov, S.E. Adaptation to Brassica Host Genotypes by a Single-Spore Isolate and Population of Plasmodiophora brassicae (Clubroot). Plant Dis. 2011, 96, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, J. Observations on the Incidence of Club-Root Disease of Brassicae in Limed Soils in Relation to Temperature. Ann. Appl. Biol. 1953, 40, 639–644. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Hims, M.J.; Archer, F.C.; Brown, A. Effects of Adding Calcium and Sodium Salts to Field Soils on the Incidence of Clubroot. Ann. Appl. Biol. 1982, 100, 245–251. [Google Scholar] [CrossRef]
- Myers, D.F.; Campbell, R.N. Lime and the Control of Clubroot of Crucifers: Effects of pH, Calcium, Magnesium, and Their Interactions. Phytopathology 1985, 75, 670. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Hwang, S.-F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A Review of an Emerging Pathogen of the Canadian Canola (Brassica napus) Crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Bélec, C.; Coulombe, J.; Godin, C. Evaluation of Calcium Cyanamide and Liming for Control of Clubroot Disease in Cauliflower. Crop Prot. 2005, 24, 798–803. [Google Scholar] [CrossRef]
- Dobson, R.L. Effects of Lime Particle Size and Distribution and Fertilizer Formulation on Clubroot Disease Caused by Plasmodiophora brassicae. Plant Dis. 1983, 67, 50. [Google Scholar] [CrossRef]
- Donald, E.C.; Lawrence, J.M.; Porter, I.J. Influence of Particle Size and Application Method on the Efficacy of Calcium Cyanamide for Control of Clubroot of Vegetable Brassicas. Crop Prot. 2004, 23, 297–303. [Google Scholar] [CrossRef]
- Donald, E.C.; Porter, I.J.; Lancaster, R.A. Band Incorporation of Fluazinam (Shirlan) into Soil to Control Clubroot of Vegetable Brassica Crops. Aust. J. Exp. Agric. 2001, 41, 1223–1226. [Google Scholar] [CrossRef]
- Mitani, S.; Sugimoto, K.; Hayashi, H.; Takii, Y.; Ohshima, T.; Matsuo, N. Effects of Cyazofamid against Plasmodiophora brassicae Woronin on Chinese Cabbage. Pest Manag. Sci. 2003, 59, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.F.; Strelkov, S.E.; Gossen, B.D.; Turnbull, G.D.; Ahmed, H.U.; Manolii, V.P. Soil Treatments and Amendments for Amelioration of Clubroot of Canola. Can. J. Plant Sci. 2011, 91, 999–1010. [Google Scholar] [CrossRef]
- Kowata-Dresch, L.S.; May-De Mio, L.L. Clubroot Management of Highly Infested Soils. Crop Prot. 2012, 35, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Huang, Y.; Xu, L.; Yin, Y. Improved Control of Clubroot (Plasmodiophora brassicae) by a Mixture of a Fungicide and a Plant Defense Inducer. J. Plant Dis. Prot. 2017, 124, 67–71. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; He, P.; Munir, S.; He, P.; Wu, Y.; Ho, H.; He, Y. Fluazinam Positively Affected the Microbial Communities in Clubroot Cabbage Rhizosphere. Sci. Hortic. 2019, 256, 108519. [Google Scholar] [CrossRef]
- Hwang, S.F.; Cao, T.; Xiao, Q.; Ahmed, H.U.; Manolii, V.P.; Turnbull, G.D.; Gossen, B.D.; Peng, G.; Strelkov, S.E. Effects of Fungicide, Seeding Date and Seedling Age on Clubroot Severity, Seedling Emergence and Yield of Canola. Can. J. Plant Sci. 2012, 92, 1175–1186. [Google Scholar] [CrossRef]
- White, J.G.; Buczacki, S.T. Observations on Suppression of Clubroot by Artificial or Natural Heating of Soil. Trans. Br. Mycol. Soc. 1979, 73, 271–275. [Google Scholar] [CrossRef]
- Porter, I.J.; Merriman, P.R.; Keane, P.J. Soil Solarisation Combined with Low Rates of Soil Fumigants Controls Clubroot of Cauliflowers, Caused by Plasmodiophora brassicae Woron. Aust. J. Exp. Agric. 1991, 31, 843–851. [Google Scholar] [CrossRef]
- McDonald, M.R.; Gossen, B.D.; Robson, J.; Al-Daoud, F. Interaction of solarization, fumigation, and totally impermeable film for the management of clubroot (Plasmodiophora brassicae) on brassica crops. In Proceedings of the IX International Symposium on Soil and Substrate Disinfestation, Heraklion, Greece, 27 February 2020; pp. 153–160. [Google Scholar] [CrossRef]
- Gossen, B.D.; Mcdonald, M.R.; Hwang, S.-F.; Strelkov, S.E.; Peng, G. A Comparison of Clubroot Development and Management on Canola and Brassica Vegetables. Can. J. Plant Pathol. 2013, 35, 175–191. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Strelkov, S.E.; Gossen, B.D.; Peng, G.; Turnbull, G.D. Efficacy of Vapam Fumigant against Clubroot (Plasmodiophora brassicae) of Canola. Plant Pathol. 2014, 63, 1374–1383. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Zhou, Q.; Gossen, B.D.; McDonald, M.R.; Peng, G.; Turnbull, G.D. Suppression of Clubroot by Dazomet Fumigant. Can. J. Plant Sci. 2017, 98, 172–182. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Zhou, Q.; Gossen, B.D.; Peng, G.; Turnbull, G.D.; Fu, H. Effects of Rate and Application Method on the Efficacy of Metam Sodium to Reduce Clubroot (Plasmodiophora brassicae) of Canola. Eur. J. Plant Pathol. 2018, 150, 341–349. [Google Scholar] [CrossRef]
- Zuzak, K.A.; Strelkov, S.E.; Turnbull, G.D.; Manolii, V.P.; Hwang, S.-F. Soil Fumigation with Vapam (Metam Sodium) to Control Clubroot (Plasmodiophora brassicae) of Canola (Brassica napus). Can. J. Plant Sci. 2023, 103, 29–38. [Google Scholar] [CrossRef]
- Al-Mughrabi, K.I.; Vikram, A.; Poirier, R.; Jayasuriya, K.; Moreau, G. Management of Common Scab of Potato in the Field Using Biopesticides, Fungicides, Soil Additives, or Soil Fumigants. Biocontrol Sci. Technol. 2016, 26, 125–135. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Hwang, S.-F.; Strelkov, S.E.; Falak, I.; Huang, X.; Sun, R. Mapping of Clubroot (Plasmodiophora brassicae) Resistance in Canola (Brassica napus). Plant Pathol. 2016, 65, 435–440. [Google Scholar] [CrossRef]
- Piao, Z.; Ramchiary, N.; Lim, Y.P. Genetics of Clubroot Resistance in Brassica Species. J. Plant Growth Regul. 2009, 28, 252–264. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Chen, B.; Xu, K.; Zhang, F.; Li, H.; Huang, Q.; Xiao, X.; Zhang, T.; Hu, J.; et al. A Genome-Wide Association Study Reveals New Loci for Resistance to Clubroot Disease in Brassica napus. Front. Plant Sci. 2016, 7, 1418. [Google Scholar] [CrossRef]
- Jiang, X.; Su, Y.; Wang, M. Mapping of a Novel Clubroot Disease Resistance Locus in Brassica napus and Related Functional Identification. Front. Plant Sci. 2022, 13, 1014376. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.J.; Shaikh, R.; Basu, U.; Rahman, H. Mapping Clubroot Resistance of Brassica rapa Introgressed into Brassica napus and Development of Molecular Markers for the Resistance. Crop Sci. 2021, 61, 4112–4127. [Google Scholar] [CrossRef]
- Rahman, H.; Peng, G.; Yu, F.; Falk, K.C.; Kulkarni, M.; Selvaraj, G. Genetics and Breeding for Clubroot Resistance in Canadian Spring Canola (Brassica napus L.). Can. J. Plant Pathol. 2014, 36, 122–134. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Suwabe, K.; Tomita, R.N.; Kato, T.; Nunome, T.; Fukuoka, H.; Matsumoto, S. Identification and Characterization of Crr1a, a Gene for Resistance to Clubroot Disease (Plasmodiophora brassicae Woronin) in Brassica rapa L. PLoS ONE 2013, 8, e54745. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, K.; Niwa, T.; Kato, T.; Ohara, T.; Kakizaki, T.; Matsumoto, S. The Tandem Repeated Organization of NB-LRR Genes in the Clubroot-Resistant CRb Locus in Brassica rapa L. Mol. Genet. Genom. 2017, 292, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Harada, T.; Kubo, N.; Tsukada, M.; Suwabe, K.; Matsumoto, S. A Novel Locus for Clubroot Resistance in Brassica rapa and Its Linkage Markers. Theor. Appl. Genet. 2004, 108, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Peng, G.; Liu, X.; Deora, A.; Falk, K.C.; Gossen, B.D.; McDonald, M.R.; Yu, F. Fine Mapping of a Clubroot Resistance Gene in Chinese Cabbage Using SNP Markers Identified from Bulked Segregant RNA Sequencing. Front. Plant Sci. 2017, 8, 1448. [Google Scholar] [CrossRef]
- Laila, R.; Park, J.-I.; Robin, A.H.K.; Natarajan, S.; Vijayakumar, H.; Shirasawa, K.; Isobe, S.; Kim, H.-T.; Nou, I.-S. Mapping of a Novel Clubroot Resistance QTL Using ddRAD-Seq in Chinese Cabbage (Brassica rapa L.). BMC Plant Biol. 2019, 19, 13. [Google Scholar] [CrossRef]
- Saito, M.; Kubo, N.; Matsumoto, S.; Suwabe, K.; Tsukada, M.; Hirai, M. Fine Mapping of the Clubroot Resistance Gene, Crr3, in Brassica rapa. Theor. Appl. Genet. 2006, 114, 81. [Google Scholar] [CrossRef]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Matsumoto, S.; Hirai, M. Identification of Two Loci for Resistance to Clubroot (Plasmodiophora brassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 2003, 107, 997–1002. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Peng, G.; Falk, K.C.; Strelkov, S.E.; Gossen, B.D. Genotyping-by-Sequencing Reveals Three QTL for Clubroot Resistance to Six Pathotypes of Plasmodiophora brassicae in Brassica rapa. Sci. Rep. 2017, 7, 4516. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, E.; Beckmann, J.; Schondelmeier, J.; Dreyer, F. Genetics of Clubroot Resistance in Brassica napus “Mendel”. Acta Hortic. 2006, 706, 307–311. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Z.; Zhang, C.; Pang, W.; Choi, S.R.; Lim, Y.P.; Piao, Z. Fine Genetic and Physical Mapping of the CRb Gene Conferring Resistance to Clubroot Disease in Brassica rapa. Mol. Breed. 2014, 34, 1173–1183. [Google Scholar] [CrossRef]
- Chu, M.; Song, T.; Falk, K.C.; Zhang, X.; Liu, X.; Chang, A.; Lahlali, R.; McGregor, L.; Gossen, B.D.; Yu, F.; et al. Fine Mapping of Rcr1 and Analyses of Its Effect on Transcriptome Patterns during Infection by Plasmodiophora brassicae. BMC Genom. 2014, 15, 1166. [Google Scholar] [CrossRef]
- Karim, M.M.; Dakouri, A.; Zhang, Y.; Chen, Q.; Peng, G.; Strelkov, S.E.; Gossen, B.D.; Yu, F. Two Clubroot-Resistance Genes, Rcr3 and Rcr9wa, Mapped in Brassica rapa Using Bulk Segregant RNA Sequencing. Int. J. Mol. Sci. 2020, 21, 5033. [Google Scholar] [CrossRef]
- Neik, T.X.; Barbetti, M.J.; Batley, J. Current Status and Challenges in Identifying Disease Resistance Genes in Brassica napus. Front. Plant Sci. 2017, 8, 1788. [Google Scholar] [CrossRef] [PubMed]
- Rocherieux, J.; Glory, P.; Giboulot, A.; Boury, S.; Barbeyron, G.; Thomas, G.; Manzanares-Dauleux, M.J. Isolate-Specific and Broad-Spectrum QTLs Are Involved in the Control of Clubroot in Brassica Oleracea. Theor. Appl. Genet. 2004, 108, 1555–1563. [Google Scholar] [CrossRef]
- Voorrips, R.E.; Jongerius, M.C.; Kanne, H.J. Mapping of Two Genes for Resistance to Clubroot (Plasmodiophora brassicae) in a Population of Doubled Haploid Lines of Brassica Oleracea by Means of RFLP and AFLP Markers. Theor. Appl. Genet. 1997, 94, 75–82. [Google Scholar] [CrossRef]
- Landry, B.S.; Hubert, N.; Crete, R.; Chang, M.S.; Lincoln, S.E.; Etoh, T. A Genetic Map for Brassica Oleracea Based on RFLP Markers Detected with Expressed DNA Sequences and Mapping of Resistance Genes to Race 2 of Plasmodiophora brassicae (Woronin). Genome 1992, 35, 409–420. [Google Scholar] [CrossRef]
- Nagaoka, T.; Doullah, M.A.U.; Matsumoto, S.; Kawasaki, S.; Ishikawa, T.; Hori, H.; Okazaki, K. Identification of QTLs That Control Clubroot Resistance in Brassica Oleracea and Comparative Analysis of Clubroot Resistance Genes between B. rapa and B. oleracea. Theor. Appl. Genet. 2010, 120, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Ce, F.; Mei, J.; He, H.; Zhao, Y.; Hu, W.; Yu, F.; Li, Q.; Ren, X.; Si, J.; Song, H.; et al. Identification of Candidate Genes for Clubroot-Resistance in Brassica Oleracea Using Quantitative Trait Loci-Sequencing. Front. Plant Sci. 2021, 12, 703520. [Google Scholar] [CrossRef] [PubMed]
- Dakouri, A.; Zhang, X.; Peng, G.; Falk, K.C.; Gossen, B.D.; Strelkov, S.E.; Yu, F. Analysis of Genome-Wide Variants through Bulked Segregant RNA Sequencing Reveals a Major Gene for Resistance to Plasmodiophora brassicae in Brassica Oleracea. Sci. Rep. 2018, 8, 17657. [Google Scholar] [CrossRef] [PubMed]
- Frauen, M. A New Clubroot Resistant Variety in Winter Oilseed Rape. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999. [Google Scholar]
- Hasan, M.J.; Rahman, H. Genetics and Molecular Mapping of Resistance to Plasmodiophora brassicae Pathotypes 2, 3, 5, 6, and 8 in Rutabaga (Brassica napus Var. Napobrassica). Genome 2016, 59, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, R.; Rahman, H. Mapping of the Clubroot Disease Resistance in Spring Brassica napus Canola Introgressed from European Winter Canola Cv. ‘Mendel’. Euphytica 2016, 211, 201–213. [Google Scholar] [CrossRef]
- Diederichsen, E.; Sacristan, M.D. Disease Response of Resynthesized Brassica napus L. Lines Carrying Different Combinations of Resistance to Plasmodiophora brassicae Wor. Plant Breed. 1996, 115, 5–10. [Google Scholar] [CrossRef]
- Rahman, H.; Shakir, A.; Jakir Hasan, M. Breeding for Clubroot Resistant Spring Canola (Brassica napus L.) for the Canadian Prairies: Can the European Winter Canola Cv. Mendel Be Used as a Source of Resistance? Can. J. Plant Sci. 2011, 91, 447–458. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Linders, E.G.A.; Hatakeyama, K.; Hirai, M. Status and Perspectives of Clubroot Resistance Breeding in Crucifer Crops. J. Plant Growth Regul. 2009, 28, 265–281. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Turnbull, G.; Fredua-Agyeman, R.; Hollman, K.; Kaus, S. Characterization of Clubroot (Plasmodiophora brassicae) from Canola (Brassica napus) in the Peace Country of Alberta, Canada. Can. J. Plant Pathol. 2021, 43, 155–161. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Peng, G.; Gossen, B.D.; McDonald, M.R.; Feindel, D. Virulence and Pathotype Classification of Plasmodiophora brassicae Populations Collected from Clubroot Resistant Canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Canola Council of Canada. Canola Cultivar Traits. Available online: https://www.canolacouncil.org/canola-encyclopedia/history-of-canola-seed-development/canola-seed-traits/ (accessed on 30 October 2023).
- Holtz, M.D.; Hwang, S.-F.; Strelkov, S.E. Genotyping of Plasmodiophora brassicae Reveals the Presence of Distinct Populations. BMC Genom. 2018, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatkish, A.; Gossen, B.D.; Yu, F.; Torkamaneh, D.; McDonald, M.R. Whole-Genome DNA Similarity and Population Structure of Plasmodiophora brassicae Strains from Canada. BMC Genom. 2019, 20, 744. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, Y.; Mishra, V.; Zhou, Q.; Zuzak, K.; Feindel, D.; Harding, M.W.; Feng, J. Most Plasmodiophora brassicae Populations in Single Canola Root Galls from Alberta Fields Are Mixtures of Multiple Strains. Plant Dis. 2020, 104, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Kuginuki, Y.; Yoshikawa, H.; Hirai, M. Variation in Virulence of Plasmodiophora brassicae in Japan Tested with Clubroot-Resistant Cultivars of Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Eur. J. Plant Pathol. 1999, 105, 327–332. [Google Scholar] [CrossRef]
- Orgeur, G.; Jestin, C.; Delaunay, A.; Lebourg, D.; Bagot, P.; Corbel, A.; Perrot, S.; Manzanares-Dauleux, M.; Grimault, V. Caractérisation Des Pathotypes de Hernie Des Crucifères En France et Mise Au Point d’un Test Pour l’évaluation de La Résistance Des Variétés de Colza. Innov. Agron. 2016, 50, 145–155. [Google Scholar]
- Mundt, C.C. Pyramiding for Resistance Durability: Theory and Practice. Phytopathology 2018, 108, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Sapoukhina, N.; Durel, C.-E.; Le Cam, B. Spatial Deployment of Gene-for-Gene Resistance Governs Evolution and Spread of Pathogen Populations. Theor. Ecol. 2009, 2, 229–238. [Google Scholar] [CrossRef]
- Ye, G.; Smith, K.F. Marker-Assisted Gene Pyramiding for Inbred Line Development: Basic Principles and Practical Guidelines. Int. J. Plant Breed. 2008, 2, 11–22. [Google Scholar]
- Djian-Caporalino, C.; Palloix, A.; Fazari, A.; Marteu, N.; Barbary, A.; Abad, P.; Sage-Palloix, A.-M.; Mateille, T.; Risso, S.; Lanza, R.; et al. Pyramiding, Alternating or Mixing: Comparative Performances of Deployment Strategies of Nematode Resistance Genes to Promote Plant Resistance Efficiency and Durability. BMC Plant Biol. 2014, 14, 53. [Google Scholar] [CrossRef]
- Clin, P.; Grognard, F.; Andrivon, D.; Mailleret, L.; Hamelin, F.M. Host Mixtures for Plant Disease Control: Benefits from Pathogen Selection and Immune Priming. Evol. Appl. 2022, 15, 967–975. [Google Scholar] [CrossRef]
- Duan, X.; Pan, S.; Fan, M.; Chu, B.; Ma, Z.; Gao, F.; Zhao, Z. Cultivar Mixture Enhances Crop Yield by Decreasing Aphids. Agronomy 2022, 12, 335. [Google Scholar] [CrossRef]
- Mundt, C.C. Use of Multiline Cultivars and Cultivar Mixtures for Disease Management. Annu. Rev. Phytopathol. 2002, 40, 381. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.E.; Peter, R.; Niklaus, P.A. Ecological and Evolutionary Approaches to Improving Crop Variety Mixtures. Nat. Ecol. Evol. 2021, 5, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Barot, S.; Allard, V.; Cantarel, A.; Enjalbert, J.; Gauffreteau, A.; Goldringer, I.; Lata, J.-C.; Le Roux, X.; Niboyet, A.; Porcher, E. Designing Mixtures of Varieties for Multifunctional Agriculture with the Help of Ecology. A Review. Agron. Sustain. Dev. 2017, 37, 13. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant Diversity and Productivity Experiments in European Grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.; Kiær, L.P.; Lecarpentier, C.; Goldringer, I.; Gauffreteau, A.; Saint-Jean, S.; Barot, S.; Enjalbert, J. Unfolding the Potential of Wheat Cultivar Mixtures: A Meta-Analysis Perspective and Identification of Knowledge Gaps. Field Crops Res. 2018, 221, 298–313. [Google Scholar] [CrossRef]
- Vidal, T.; Boixel, A.-L.; Durand, B.; de Vallavieille-Pope, C.; Huber, L.; Saint-Jean, S. Reduction of Fungal Disease Spread in Cultivar Mixtures: Impact of Canopy Architecture on Rain-Splash Dispersal and on Crop Microclimate. Agric. For. Meteorol. 2017, 246, 154–161. [Google Scholar] [CrossRef]
- BioRender. Available online: https://app.biorender.com/illustrations/6646781e155a47d2a2a0bf32?slideId=75942763-66e9-4cc1-acc7-3eff0c46340c (accessed on 13 June 2024).
- Grettenberger, I.M.; Tooker, J.F. Moving beyond Resistance Management toward an Expanded Role for Seed Mixtures in Agriculture. Agric. Ecosyst. Environ. 2015, 208, 29–36. [Google Scholar] [CrossRef]
- Kristoffersen, R.; Heick, T.M.; Müller, G.M.; Eriksen, L.B.; Nielsen, G.C.; Jørgensen, L.N. The Potential of Cultivar Mixtures to Reduce Fungicide Input and Mitigate Fungicide Resistance Development. Agron. Sustain. Dev. 2020, 40, 36. [Google Scholar] [CrossRef]
- Mikaberidze, A.; McDonald, B.A.; Bonhoeffer, S. Developing Smarter Host Mixtures to Control Plant Disease. Plant Pathol. 2015, 64, 996–1004. [Google Scholar] [CrossRef]
- Rimbaud, L.; Papaïx, J.; Barrett, L.G.; Burdon, J.J.; Thrall, P.H. Mosaics, Mixtures, Rotations or Pyramiding: What Is the Optimal Strategy to Deploy Major Gene Resistance? Evol. Appl. 2018, 11, 1791–1810. [Google Scholar] [CrossRef] [PubMed]
- Stam, R.; McDonald, B.A. When Resistance Gene Pyramids Are Not Durable—The Role of Pathogen Diversity. Mol. Plant Pathol. 2018, 19, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Fabre, F.; Rousseau, E.; Mailleret, L.; Moury, B. Epidemiological and Evolutionary Management of Plant Resistance: Optimizing the Deployment of Cultivar Mixtures in Time and Space in Agricultural Landscapes. Evol. Appl. 2015, 8, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, C.; Xia, J.; Wu, L.; Xu, G.; Wu, W.; Li, D.; Qin, W.; Han, X.; Chen, Q.; et al. Teosinte Ligule Allele Narrows Plant Architecture and Enhances High-Density Maize Yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef]
- Abakumova, M.; Zobel, K.; Lepik, A.; Semchenko, M. Plasticity in Plant Functional Traits Is Shaped by Variability in Neighbourhood Species Composition. New Phytol. 2016, 211, 455–463. [Google Scholar] [CrossRef] [PubMed]
- De Vallavieille-Pope, C.; Giosue, S.; Munk, L.; Newton, A.; Niks, R.; Østergård, H.; Pons-Kühnemann, J.; Rossi, V.; Sache, I. Assessment of Epidemiological Parameters and Their Use in Epidemiological and Forecasting Models of Cereal Airborne Diseases. Agronomie 2000, 20, 715–727. [Google Scholar] [CrossRef]
- Cox, C.M.; Garrett, K.A.; Bowden, R.L.; Fritz, A.K.; Dendy, S.P.; Heer, W.F. Cultivar Mixtures for the Simultaneous Management of Multiple Diseases: Tan Spot and Leaf Rust of Wheat. Phytopathology 2004, 94, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Reiss, E.R.; Drinkwater, L.E. Cultivar Mixtures: A Meta-Analysis of the Effect of Intraspecific Diversity on Crop Yield. Ecol. Appl. 2018, 28, 62–77. [Google Scholar] [CrossRef]
- Sapoukhina, N.; Paillard, S.; Dedryver, F.; Vallavieille-Pope, C. de Quantitative Plant Resistance in Cultivar Mixtures: Wheat Yellow Rust as a Modeling Case Study. New Phytol. 2013, 200, 888–897. [Google Scholar] [CrossRef]
- Montazeaud, G.; Flutre, T.; Ballini, E.; Morel, J.-B.; David, J.; Girodolle, J.; Rocher, A.; Ducasse, A.; Violle, C.; Fort, F.; et al. From Cultivar Mixtures to Allelic Mixtures: Opposite Effects of Allelic Richness between Genotypes and Genotype Richness in Wheat. New Phytol. 2022, 233, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Kaut, A.H.; Mason, H.E.; Navabi, A.; O’Donovan, J.T.; Spaner, D. Performance and Stability of Performance of Spring Wheat Variety Mixtures in Organic and Conventional Management Systems in Western Canada. J. Agric. Sci. 2009, 147, 141–153. [Google Scholar] [CrossRef]
- Finckh, M.R. Stripe Rust, Yield, and Plant Competition in Wheat Cultivar Mixtures. Phytopathology 1992, 82, 905. [Google Scholar] [CrossRef]
- Vidal, T.; Saint-Jean, S.; Lusley, P.; Leconte, M.; Ben Krima, S.; Boixel, A.-L.; Consortium, W.; de Vallavieille-Pope, C. Cultivar Mixture Effects on Disease and Yield Remain despite Diversity in Wheat Height and Earliness. Plant Pathol. 2020, 69, 1148–1160. [Google Scholar] [CrossRef]
- Crété, R.; Pires, R.N.; Barbetti, M.J.; Renton, M. Rotating and Stacking Genes Can Improve Crop Resistance Durability While Potentially Selecting Highly Virulent Pathogen Strains. Sci. Rep. 2020, 10, 19752. [Google Scholar] [CrossRef] [PubMed]
- Hariri, D.; Fouchard, M.; Prud’homme, H. Incidence of Soil-Borne Wheat Mosaic Virus in Mixtures of Susceptible and Resistant Wheat Cultivars. Eur. J. Plant Pathol. 2001, 107, 625–631. [Google Scholar] [CrossRef]
- Bonhoeffer, S.; Nowak, M.A. Mutation and the Evolution of Virulence. Proc. Biol. Sci. 1994, 258, 133–140. [Google Scholar]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of Spontaneous Mutation. Genetics 1998, 148, 1667–1686. [Google Scholar] [CrossRef]
- Canola Council of Canada Clubroot Disease|Canola Encyclopedia. 2023. Available online: https://www.canolacouncil.org/canola-encyclopedia/diseases/clubroot/ (accessed on 13 June 2024).
- Canola Council of Canada. Canola Performance Trials. Available online: https://canolaperformancetrials.ca/ (accessed on 13 June 2024).
- Askarian, H.; Akhavan, A.; Manolii, V.P.; Cao, T.; Hwang, S.-F.; Strelkov, S.E. Virulence Spectrum of Single-Spore and Field Isolates of Plasmodiophora brassicae Able to Overcome Resistance in Canola (Brassica napus). Plant Dis. 2021, 105, 43–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botero-Ramirez, A.; Kirk, B.; Strelkov, S.E. Optimizing Clubroot Management and the Role of Canola Cultivar Mixtures. Pathogens 2024, 13, 640. https://doi.org/10.3390/pathogens13080640
Botero-Ramirez A, Kirk B, Strelkov SE. Optimizing Clubroot Management and the Role of Canola Cultivar Mixtures. Pathogens. 2024; 13(8):640. https://doi.org/10.3390/pathogens13080640
Chicago/Turabian StyleBotero-Ramirez, Andrea, Brennon Kirk, and Stephen E. Strelkov. 2024. "Optimizing Clubroot Management and the Role of Canola Cultivar Mixtures" Pathogens 13, no. 8: 640. https://doi.org/10.3390/pathogens13080640
APA StyleBotero-Ramirez, A., Kirk, B., & Strelkov, S. E. (2024). Optimizing Clubroot Management and the Role of Canola Cultivar Mixtures. Pathogens, 13(8), 640. https://doi.org/10.3390/pathogens13080640








