Utilization of In Vivo Imaging System to Study Staphylococcal Sepsis and Septic Arthritis Progression in Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice
2.3. Bacterial Strains
2.4. Mouse Models of S. aureus Sepsis and Hematogenous Septic Arthritis
2.5. Antibiotic Treatment in Mouse Model of S. aureus-Induced Sepsis
2.6. Mouse Model of Local S. aureus Septic Arthritis
2.7. Quantification of S. aureus Infection Using In Vivo Imaging System
2.8. Homogenate Preparation and Quantification of Bacterial Load in Kidneys and Joints
2.9. Quantification of Immunomodulators Using Enzyme-Linked Immunosorbent Assay
2.10. Bone Erosion Estimation Using μCT
3. Results
3.1. In Vivo Imaging Systems Are Able to Follow the Progression and Severity of Sepsis in Mice
3.2. Kidney IVIS Signals Correlate Positively with Kidney Abscess Severity and Kidney CFU Counts
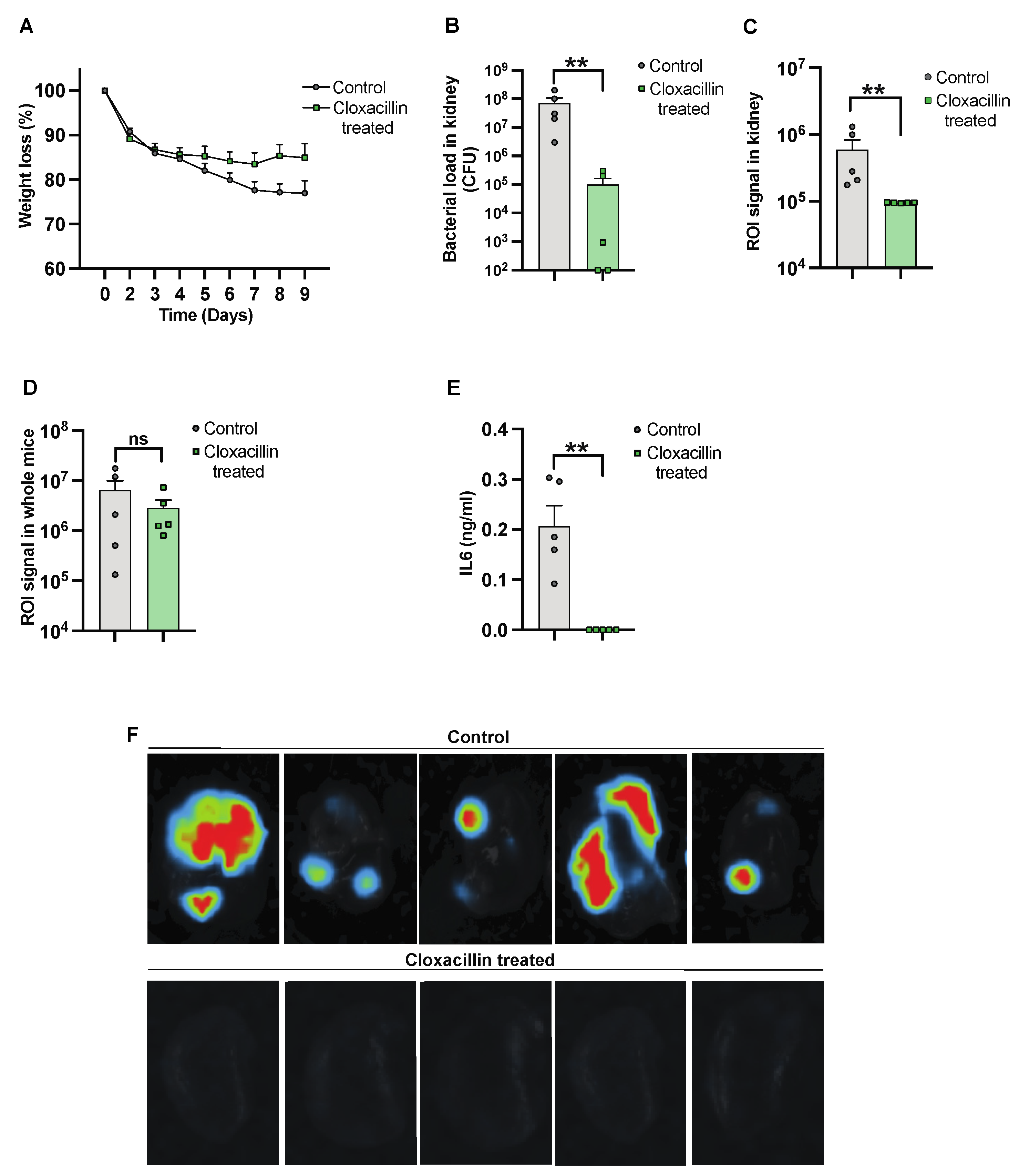
3.3. The Efficacy of Antibiotic Treatment against Infection Was Monitored Using the Imaging System
3.4. Assessing Clinical Arthritis Progression Using Imaging System
3.5. Imaging Technique Can Determine the Infectious Progression over Time in a Local Infection Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, T.; Mohammad, M.; Pullerits, R.; Ali, A. Bacteria and Host Interplay in Staphylococcus aureus Septic Arthritis and Sepsis. Pathogens 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock [Sepsis-3]. JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Naber, C.K. Staphylococcus aureus bacteremia: Epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. 2009, 48 (Suppl. 4), S231–S237. [Google Scholar] [CrossRef] [PubMed]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, C.J.; Dinant, H.J.; van de Laar, M.A.; Moens, H.J.; Prins, A.P.; Dijkmans, B.A. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann. Rheum. Dis. 1997, 56, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Weston, V.C.; Jones, A.C.; Bradbury, N.; Fawthrop, F.; Doherty, M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann. Rheum. Dis. 1999, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, A. Infection and musculoskeletal conditions: Infectious arthritis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Geirsson, A.J.; Statkevicius, S.; Víkingsson, A. Septic arthritis in Iceland 1990-2002: Increasing incidence due to iatrogenic infections. Ann. Rheum. Dis. 2008, 67, 638–643. [Google Scholar] [CrossRef]
- Gunnlaugsdóttir, S.L.; Erlendsdóttir, H.; Helgason, K.O.; Geirsson, Á.J.; Thors, V.; Guðmundsson, S.; Gottfreðsson, M. Native joint infections in Iceland 2003–2017: An increase in postarthroscopic infections. Ann. Rheum. Dis. 2022, 81, 132–139. [Google Scholar] [CrossRef]
- Kaandorp, C.J.; Van Schaardenburg, D.; Krijnen, P.; Habbema, J.D.; van de Laar, M.A. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1995, 38, 1819–1825. [Google Scholar] [CrossRef]
- Abram, S.G.F.; Alvand, A.; Judge, A.; Beard, D.J.; Price, A.J. Mortality and adverse joint outcomes following septic arthritis of the native knee: A longitudinal cohort study of patients receiving arthroscopic washout. Lancet Infect. Dis. 2020, 20, 341–349. [Google Scholar] [CrossRef]
- Mathews, C.J.; Weston, V.C.; Jones, A.; Field, M.; Coakley, G. Bacterial septic arthritis in adults. Lancet 2010, 375, 846–855. [Google Scholar] [CrossRef]
- Sakiniene, E.; Bremell, T.; Tarkowski, A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996, 39, 1596–1605. [Google Scholar] [CrossRef]
- Fei, Y.; Wang, W.; Kwiecinski, J.; Josefsson, E.; Pullerits, R.; Jonsson, I.M.; Magnusson, M.; Jin, T. The combination of a tumor necrosis factor inhibitor and antibiotic alleviates staphylococcal arthritis and sepsis in mice. J. Infect. Dis. 2011, 204, 348–357. [Google Scholar] [CrossRef]
- Ali, A.; Zhu, X.; Kwiecinski, J.; Gjertsson, I.; Lindholm, C.; Iwakura, Y.; Wang, X.; Lycke, N.; Josefsson, E.; Pullerits, R.; et al. Antibiotic-killed Staphylococcus aureus induces destructive arthritis in mice. Arthritis Rheumatol. 2015, 67, 107–116. [Google Scholar] [CrossRef]
- Jin, T. Exploring the role of bacterial virulence factors and host elements in septic arthritis: Insights from animal models for innovative therapies. Front. Microbiol. 2024, 15, 1356982. [Google Scholar] [CrossRef]
- Lim, E.; Modi, K.; Christensen, A.; Meganck, J.; Oldfield, S.; Zhang, N. Monitoring tumor metastases and osteolytic lesions with bioluminescence and micro CT imaging. J. Vis. Exp. 2011, 2775. [Google Scholar] [CrossRef]
- Cosette, J.; Ben Abdelwahed, R.; Donnou-Triffault, S.; Sautès-Fridman, C.; Flaud, P.; Fisson, S. Bioluminescence-Based Tumor Quantification Method for Monitoring Tumor Progression and Treatment Effects in Mouse Lymphoma Models. J. Vis. Exp. 2016, 53609. [Google Scholar] [CrossRef]
- Lim, E.; Modi, K.D.; Kim, J. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J. Vis. Exp. 2009, 1210. [Google Scholar] [CrossRef]
- Tu, S.H.; Hsieh, Y.C.; Huang, L.C.; Lin, C.Y.; Hsu, K.W.; Hsieh, W.S.; Chi, W.M.; Lee, C.H. A rapid and quantitative method to detect human circulating tumor cells in a preclinical animal model. BMC Cancer 2017, 17, 440. [Google Scholar] [CrossRef]
- Vuong, C.; Kocianova, S.; Yu, J.; Kadurugamuwa, J.L.; Otto, M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J. Infect. Dis. 2008, 198, 258–261. [Google Scholar] [CrossRef]
- Konjufca, V.; Miller, M.J. Imaging Listeria monocytogenes infection in vivo. Curr. Top. Microbiol. Immunol. 2009, 334, 199–226. [Google Scholar] [CrossRef]
- Archer, N.K.; Wang, Y.; Ortines, R.V.; Liu, H.; Nolan, S.J.; Liu, Q.; Alphonse, M.P.; Dikeman, D.A.; Mazhar, M.; Miller, R.J.; et al. Preclinical Models and Methodologies for Monitoring Staphylococcus aureus Infections Using Noninvasive Optical Imaging. Methods Mol. Biol. 2020, 2069, 197–228. [Google Scholar] [CrossRef]
- Hardy, J.W.; Levashova, Z.; Schmidt, T.L.; Contag, C.H.; Blankenberg, F.G. [99mTc]Annexin V-128 SPECT Monitoring of Splenic and Disseminated Listeriosis in Mice: A Model of Imaging Sepsis. Mol. Imaging Biol. 2015, 17, 345–354. [Google Scholar] [CrossRef]
- Ostermann, E.; Macquin, C.; Bahram, S.; Georgel, P. Use of in vivo imaging to monitor the progression of experimental mouse cytomegalovirus infection in neonates. J. Vis. Exp. 2013, e50409. [Google Scholar] [CrossRef]
- Caine, E.A.; Osorio, J.E. In Vivo Imaging with Bioluminescent Enterovirus 71 Allows for Real-Time Visualization of Tissue Tropism and Viral Spread. J. Virol. 2017, 91, e01759-16. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Taylor, B.N.; Meganck, J.A.; Wang, Y.; Shahbazian, J.H.; Niska, J.A.; Francis, K.P.; Miller, L.S. Combined in vivo optical and µCT imaging to monitor infection, inflammation, and bone anatomy in an orthopaedic implant infection in mice. J. Vis. Exp. 2014, e51612. [Google Scholar] [CrossRef]
- Miller, R.J.; Crosby, H.A.; Schilcher, K.; Wang, Y.; Ortines, R.V.; Mazhar, M.; Dikeman, D.A.; Pinsker, B.L.; Brown, I.D.; Joyce, D.P.; et al. Development of a Staphylococcus aureus reporter strain with click beetle red luciferase for enhanced in vivo imaging of experimental bacteremia and mixed infections. Sci. Rep. 2019, 9, 16663. [Google Scholar] [CrossRef]
- Mohammad, M.; Hu, Z.; Ali, A.; Kopparapu, P.K.; Na, M.; Jarneborn, A.; Stroparo, M.d.N.; Nguyen, M.-T.; Karlsson, A.; Götz, F.; et al. The role of Staphylococcus aureus lipoproteins in hematogenous septic arthritis. Sci. Rep. 2020, 10, 7936. [Google Scholar] [CrossRef]
- Fatima, F.; Fei, Y.; Ali, A.; Mohammad, M.; Erlandsson, M.C.; Bokarewa, M.I.; Nawaz, M.; Valadi, H.; Na, M.; Jin, T. Radiological features of experimental staphylococcal septic arthritis by micro computed tomography scan. PLoS ONE 2017, 12, e0171222. [Google Scholar] [CrossRef]
- Ali, A.; Na, M.; Svensson, M.N.; Magnusson, M.; Welin, A.; Schwarze, J.C.; Mohammad, M.; Josefsson, E.; Pullerits, R.; Jin, T. IL-1 Receptor Antagonist Treatment Aggravates Staphylococcal Septic Arthritis and Sepsis in Mice. PLoS ONE 2015, 10, e0131645. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Welin, A.; Schwarze, J.C.; Svensson, M.N.; Na, M.; Jarneborn, A.; Magnusson, M.; Mohammad, M.; Kwiecinski, J.; Josefsson, E.; et al. CTLA4 Immunoglobulin but Not Anti-Tumor Necrosis Factor Therapy Promotes Staphylococcal Septic Arthritis in Mice. J. Infect. Dis. 2015, 212, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Nguyen, M.T.; Engdahl, C.; Na, M.; Jarneborn, A.; Hu, Z.; Karlsson, A.; Pullerits, R.; Ali, A.; Götz, F.; et al. The YIN and YANG of lipoproteins in developing and preventing infectious arthritis by Staphylococcus aureus. PLoS Pathog. 2019, 15, e1007877. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Subhash, S.; Hu, Z.; Mohammad, M.; Jarneborn, A.; Pullerits, R.; Jin, T.; Kopparapu, P.K. Gene expression of S100a8/a9 predicts Staphylococcus aureus-induced septic arthritis in mice. Front. Microbiol. 2023, 14, 1146694. [Google Scholar] [CrossRef] [PubMed]
- Kopparapu, P.K.; Deshmukh, M.; Hu, Z.; Mohammad, M.; Maugeri, M.; Götz, F.; Valadi, H.; Jin, T. Lipoproteins Are Responsible for the Pro-Inflammatory Property of Staphylococcus aureus Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 7099. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Jarneborn, A.; Thorn, K.; Eriksson, K.; Jin, T. Tofacitinib Treatment in Primary Herpes Simplex Encephalitis Interferes With Antiviral Response. J. Infect. Dis. 2022, 225, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Wang, W.; Fei, Y.; Josefsson, E.; Ali, A.; Jin, T. Both anti-TNF and CTLA4 Ig treatments attenuate the disease severity of staphylococcal dermatitis in mice. PLoS ONE 2017, 12, e0173492. [Google Scholar] [CrossRef] [PubMed]
- Niska, J.A.; Meganck, J.A.; Pribaz, J.R.; Shahbazian, J.H.; Lim, E.; Zhang, N.; Rice, B.W.; Akin, A.; Ramos, R.I.; Bernthal, N.M.; et al. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and μCT imaging in an orthopaedic implant infection in mice. PLoS ONE 2012, 7, e47397. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.M.; Bremell, T.; Rydén, C.; Cheung, A.L.; Tarkowski, A. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect. Immun. 1996, 64, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Németh, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef]
- Hu, Z.; Kopparapu, P.K.; Ebner, P.; Mohammad, M.; Lind, S.; Jarneborn, A.; Dahlgren, C.; Schultz, M.; Deshmukh, M.; Pullerits, R.; et al. Phenol-soluble modulin α and β display divergent roles in mice with staphylococcal septic arthritis. Commun. Biol. 2022, 5, 910. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Sin, L.V.; Yu, J.; Francis, K.P.; Purchio, T.F.; Contag, P.R. Noninvasive optical imaging method to evaluate postantibiotic effects on biofilm infection in vivo. Antimicrob. Agents Chemother. 2004, 48, 2283–2287. [Google Scholar] [CrossRef]




| Parameters | NMRI | C57BL/6 | ||||||
|---|---|---|---|---|---|---|---|---|
| Kidney | Whole Mice | Kidney | Whole Mice | |||||
| ROI | ROI | ROI | ROI | |||||
| p | r | p | r | p | r | p | r | |
| Weight loss | ns | - | 0.01 | 0.3 | ns | - | ns | - |
| Abscess score | 0.03 | 0.18 | ns | - | 0.0001 | 0.6 | ns | - |
| KidneyCount | 0.008 | 0.2 | ns | - | 0.0001 | 0.6 | ns | - |
| IL6 | ns | - | ns | - | ns | - | ns | - |
| S100A8/A9 | 0.06 | 0.2 | 0.08 | 0.2 | ns | - | ns | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, M.; Hu, Z.; Mohammad, M.; Jin, T. Utilization of In Vivo Imaging System to Study Staphylococcal Sepsis and Septic Arthritis Progression in Mouse Model. Pathogens 2024, 13, 652. https://doi.org/10.3390/pathogens13080652
Deshmukh M, Hu Z, Mohammad M, Jin T. Utilization of In Vivo Imaging System to Study Staphylococcal Sepsis and Septic Arthritis Progression in Mouse Model. Pathogens. 2024; 13(8):652. https://doi.org/10.3390/pathogens13080652
Chicago/Turabian StyleDeshmukh, Meghshree, Zhicheng Hu, Majd Mohammad, and Tao Jin. 2024. "Utilization of In Vivo Imaging System to Study Staphylococcal Sepsis and Septic Arthritis Progression in Mouse Model" Pathogens 13, no. 8: 652. https://doi.org/10.3390/pathogens13080652





