Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives
Abstract
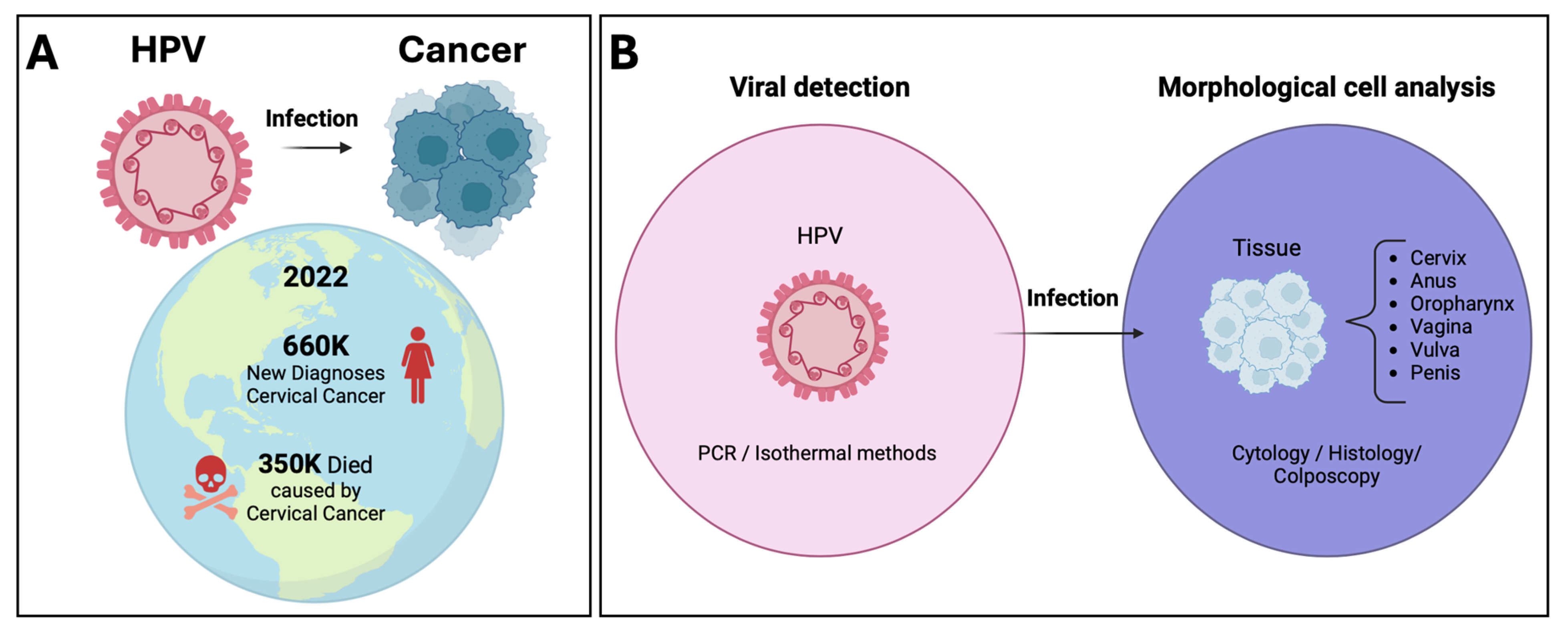
:1. Introduction
2. Molecular Testing Applied to the Global Strategy 90-70-90
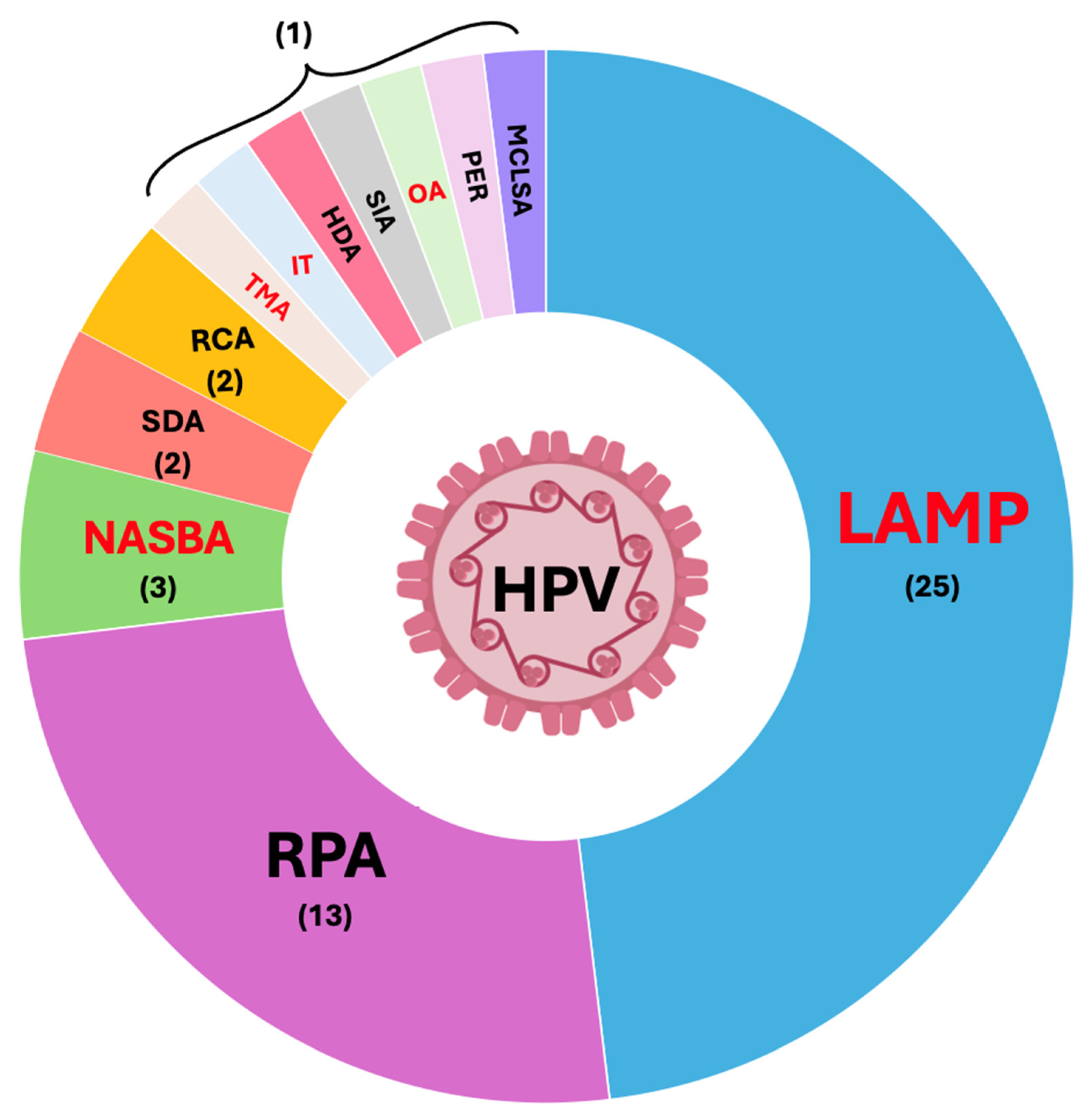
3. Isothermal Tests for HPV Detection
3.1. LAMP
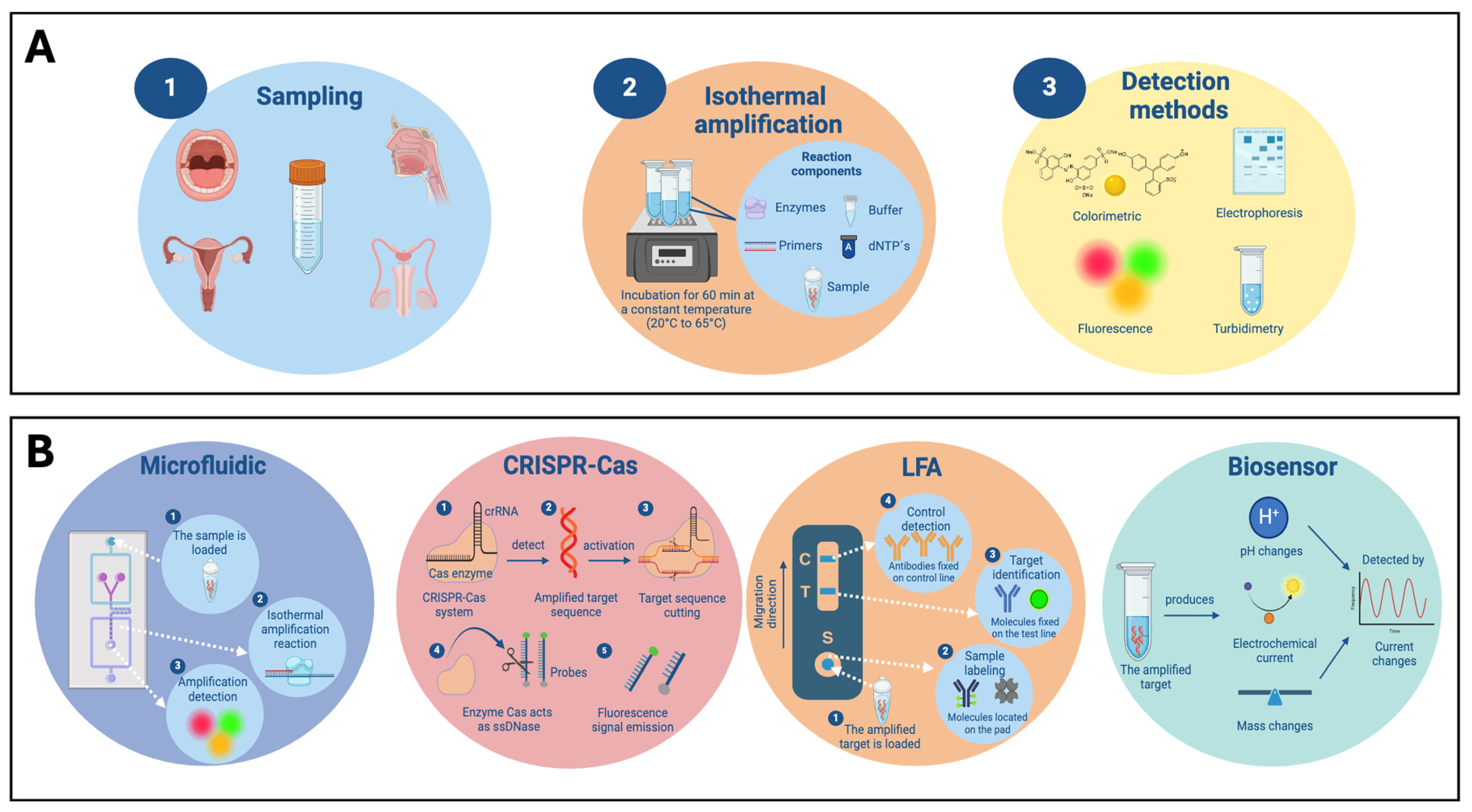
3.1.1. Conventional Detection Methods
3.1.2. Microfluidic-LAMP
3.1.3. LFA-LAMP
3.1.4. LAMP Integrated with Biosensors
| LAMP Test Type | Sample (Tissue) | Genotype | Target Gene | LOD | Detection Signal | Detection Time (min) | References |
|---|---|---|---|---|---|---|---|
| Conventional | Genital polypoid | 6-11-16-18 | E6-E7 | 103 copies/reaction | Turbidity | 59 | [55] |
| Oropharyngeal | 16-18-31-33-35 | - | - | Turbidity | 60 | [27] | |
| Cervical | 5-8-16-18-58 | L1-L2 | 10 copies/μL | Turbidity | 45 | [29] | |
| Cervical | 16-18-45-58 | - | 102 to 103 copies/reaction | Turbidity | 60 | [28] | |
| Cervical | 16-18 | E6 | 10 copies/reaction | Colorimetric (Phenol red) | <80 | [30] | |
| Oral rinses | 16-18 | E6 | 10 cells | Colorimetric (Phenol red) | 20 | [31] | |
| Cervical | 16-18-45-52-58 | E6-E7-L1 | 10 to 102 copies/reaction | Colorimetric (HNB) | 65 | [32] | |
| Cervical | 6-11-16-42-43-44 | E6-E7-L1 | 103 copies/reaction | Colorimetric (HNB) | 65 | [33] | |
| Cervical | 6 | - | 102 copies/mL | Colorimetric (HNB) | 60 | [34] | |
| Cervical | 16-18 | E7-L1 | 10 copies/reaction | Fluorescence (SYBR Green-I) | 60 | [40] | |
| Oral Saliva Blood | 16 | E7 | 46.8 copies/μL | Turbidity and Fluorescence (SYBR Green-I) | 23 | [41] | |
| Cervical | 16-18 | E7-L1 | 102 cells/mL | Fluorescence (Calcein) | 70 | [37] | |
| Cervical | 16-18-33-39-45-52-58 | - | 20 copies/μL to 205 copies/μL | Fluorescence (Calcein) | 19–75 | [39] | |
| Cervical | 16-18 | - | 10 copies | Colorimetric (Gold nanoparticles) | 25 | [36] | |
| Cervical | 16-18 | E7-L1 | 1 to 104 copies/reaction | --- | 45 | [51] | |
| Microfluidic | Cervical | 16-18 | E6-E7-L1 | 1 copy/μL | Fluorescence (EvaGreen) | 15 | [42] |
| Urine Cervical Vaginal | 16-18-31-33-35-39-45-51-52-56-58-59-66-68 | L1 | 10 to 103 copies/reaction | Fluorescence (Calcein) | 47 | [52] | |
| HUVEC and HeLa cells | 16-18-39-45-52 | - | 103 to 106 copies/μL | Fluorescence (EvaGreen) | 40 | [56] | |
| Plasmid DNA | 18 | L1 | 150 copies/μL | Fluorescence (Calcein) | 80 | [57] | |
| Skin warts | 1-2-3-4-5-7-8-9-10-12-14-27-28-29-41-48-49-50-57-63-65-75-76-77-94-95-115-117-125-160 | L1 | 107 copies/μL | Colorimetric (HNB) | 60 | [58] | |
| Saliva Cervical | 16-18-31 | - | 50 copies/reaction | Colorimetric (HNB) | 60 | [53] | |
| Cervical | 16-18 | E7-L1 | - | Fluorescence (Calcein) | 60 | [54] | |
| Saliva Vaginal | 16 | - | 102 copies/reaction | Colorimetric (EBT) | 60 | [35] | |
| LFA | Cervical | 16 | E7 | - | Colorimetric (Gold nanoparticles) | 60 | [44] |
| Cervical | 16-18 | - | 1 to 105 copies/reaction | Colorimetric (Gold nanoparticles) | 35 | [45] | |
| Cervical | 16-18-45 | E6-L1-E7 | 10 to 102 copies/reaction | Colorimetric (Neutravidin-coated carbon nanoparticles) | 40 | [46] | |
| Biosensor | Cervical | 16-18 | E6-E7-L1 | 10 to 103 copies/reaction | Voltage | 25 | [47] |
| Cervical | 58 | - | 102 copies/mL | Mass changes | 30 | [49] | |
| Cervical | 16 | E6-E7 | 102 copies/mL | Electrochemical | <120 | [48] | |
| Cervical | 16-18 | E6-E7 | 1 ng/reaction | Electrochemical | 90 | [59] | |
| Cervical | 16-18 | E6-E7 | 10 cells/reaction | Electrochemical | 45 | [60] |
3.2. RPA
3.2.1. Conventional Detection Methods
3.2.2. RPA-CRISPR-Cas and Microfluidic
3.2.3. LFA-RPA
3.2.4. RPA Integrated with Biosensors
| RPA Test Type | Sample | Genotype | Target Gene | LOD | Detection Signal | Detection Time (min) | References |
|---|---|---|---|---|---|---|---|
| Conventional | Cervical | 6-11-16-18-26-31-32-33-34-35-39-40-42-43-44-45-51-52-53-54-56-58-59-61-66-68-69-70-72-73 -81-82-84-87 | E6-E7 | 102 to 103 copies/reaction | Electrophoresis | 40 | [64] |
| Cervical | 16-18 | E7-L1 | 50 to 103 copies/μL | Fluorescence (FAM and ROX) | 25 | [65] | |
| CRISPR-Cas | Cervical | 16-18-31-33-35-39-45-51-52-56-58-59-68 | L1 | 500 copies/reaction | Fluorescence | 35 | [66] |
| Cervical | 16-18-31-33-35-45 | L1-E6-E7 | 1 to 10 copies/μL | Fluorescence (FAM) | 40 | [67] | |
| Cervical | 16 | L1 | 1 pM/reaction | Electrochemical (Methylene Blue) | <120 | [74] | |
| Microfluidic | Cervical | 6-11-16-18-31-45-52-58 | L1 | 0.26 aM/reaction | Fluorescence (FAM) | 40 | [68] |
| Cervical | 16-18 | - | 10 to 102 copies/reaction | Fluorescence | 60 | [69] | |
| Cervical | 16 | L1 | 10 to 102 copies/μL | Fluorescence (FAM) | 30 | [70] | |
| Cervical | 16 | L1 | 0.24 pg/μL | Fluorescence (SYBR Green I) | 30 | [71] | |
| LFA | Cell lines (C33A, SiHa, HeLa, and MS751) | 16-18-45 | E7 | 5000 to 50,000 cells/mL | Colorimetric (Gold nanoshells) | 35 | [75] |
| Cervical | 16-18 | L1 | 10 copies/reaction | Colorimetric (Crimson red) | 60 | [72] | |
| Cervical | 16-18 | E6-E7 | 5 to 10 copies/reaction | Colorimetric (Gold nanoparticles) | 30 | [76] | |
| Cervical | 6-11-16-18-26-31- 33-35-39-40-42-43-44-45-51-52-53-56-58-59-68-73-81-83 | L1 | 0.1 to 1 pg/reaction | Colorimetric (Gold nanoparticles) | 60 | [73] | |
| Biosensor | Cervical | 16 | E6-E7 | 0.23 copies/μL | Electrochemical (Methylene Blue) | 75 | [61] |
3.3. NASBA and TMA
3.4. SDA
3.5. RCA
3.6. IT
3.7. HDA
3.8. SIA
3.9. OA
3.10. PER
3.11. MCLSA
| Technique | Enzymes Required | # Primers | Temperature | Time (min) | Developed | Commercial (HPV) | Pros | Cons |
|---|---|---|---|---|---|---|---|---|
| NASBA |
| 2 | 41 °C | 90–120 | 1991 | No |
|
|
| SDA |
| 2–4 | 37 °C | 120 | 1992 | No |
|
|
| RCA |
| 1 | 30 °C | 60–90 | 1995 | No |
|
|
| TMA |
| 2 | 41 °C | 90–120 | 1995 | Yes |
|
|
| LAMP |
| 4 or 6 | 60–65 °C | <60 | 2000 | Yes |
|
|
| IT |
| 3 | 63 °C | 240 | 2002 | Yes |
|
|
| HDA |
| 2 | 65 °C | 90 | 2004 | No |
|
|
| RPA |
| 2 | 37–42 °C | 20–40 | 2006 | Yes |
|
|
| SIA |
| 1 | 37 °C | 30 | 2016 | No |
|
|
| OA |
| 6 | 60 °C | <60 | 2017 | Yes |
|
|
| PER |
| 2 | 37 °C | 60 | 2017 | No |
|
|
| MCLSA |
| 5 | 62 °C | 45 | 2021 | No |
|
|
4. Commercial and FDA-Approved Molecular Test for HPV Detection
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; Desalle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989. [Google Scholar] [CrossRef]
- Shah, U.J.; Nasiruddin, M.; Dar, S.A.; Khan, M.K.A.; Akhter, M.R.; Singh, N.; Rabaan, A.A.; Haque, S. Emerging biomarkers and clinical significance of HPV genotyping in prevention and management of cervical cancer. Microb. Pathog. 2020, 143, 104131. [Google Scholar] [CrossRef] [PubMed]
- De Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Quinlan, J.D. Human Papillomavirus: Screening, Testing, and Prevention. Am. Fam. Physician 2021, 104, 152–159. [Google Scholar]
- Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 11 July 2024).
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human Papilloma Virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islam. Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef]
- Human Papillomavirus (HPV) Vaccine—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/human-papillomavirus-hpv-vaccine (accessed on 6 November 2023).
- Gupta, S.; Kumar, P.; Das, B.C. HPV: Molecular pathways and targets. Curr. Probl. Cancer 2018, 42, 161–174. [Google Scholar] [CrossRef]
- De Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of Human Papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Majerciak, V.; Zheng, Z.M. HPV16 and HPV18 Genome Structure, Expression, and Post-Transcriptional Regulation. Int. J. Mol. Sci. 2022, 23, 4943. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Nelson, C.W.; Mirabello, L. Human papillomavirus genomics: Understanding carcinogenicity. Tumour Virus Res. 2023, 15, 200258. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, L.; He, Y.; Qi, X. Human Papillomavirus associated cervical lesion: Pathogenesis and therapeutic interventions. MedComm 2023, 4, e368. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.A.; Kornør, H.; Klemp, M.; Lauvrak, V.; Kristiansen, I.S. HPV mRNA tests for the detection of cervical intraepithelial neoplasia: A systematic review. Gynecol. Oncol. 2011, 120, 430–438. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M. Cross-roads in the classification of Papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, E.; Rollo, F.; Donà, M.G.; Garbuglia, A.R. Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens 2021, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.M.; Talpe-Nunes, V.; Sichero, L. Epidemiology and biology of cutaneous human papillomavirus. Clinics 2018, 73, e489s. [Google Scholar] [CrossRef] [PubMed]
- Flores-Miramontes, M.G.; Olszewski, D.; Artaza-Irigaray, C.; Willemsen, A.; Bravo, I.G.; Vallejo-Ruiz, V.; Leal-Herrera, Y.A.; Piña-Sánchez, P.; Molina-Pineda, A.; Cantón-Romero, J.C.; et al. Detection of Alpha, Beta, Gamma, and Unclassified Human Papillomaviruses in Cervical Cancer Samples from Mexican Women. Front. Cell. Infect. Microbiol. 2020, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664. [Google Scholar] [CrossRef] [PubMed]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, M.; Moranova, L.; Izadi, N.; Strmiskova, J.; Sebuyoya, R.; Holcakova, J.; Hrstka, R. Advanced technologies towards improved HPV diagnostics. J. Med. Virol. 2024, 96, e29409. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; Carrasco-González, J.A.; Linhares, D.C.L.; Corzo, C.A.; Campos-Villalobos, J.I.; Henao-Díaz, A.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; González-González, R.B.; Parra-Saldívar, R.; et al. Emergent Molecular Techniques Applied to the Detection of Porcine Viruses. Vet. Sci. 2023, 10, 609. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; pp. 1–56. [Google Scholar]
- Otoo, J.A.; Schlappi, T.S. Reassured Multiplex Diagnostics: A Critical Review and Forecast. Biosensors 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- González-González, E.; Lara-Mayorga, I.M.; Rodríguez-Sánchez, I.P.; Zhang, Y.S.; Martínez-Chapa, S.O.; De Santiago, G.T.; Alvarez, M.M. Colorimetric loop-mediated isothermal amplification (LAMP) for cost-effective and quantitative detection of SARS-CoV-2: The change in color in LAMP-based assays quantitatively correlates with viral copy number. Anal. Methods 2021, 13, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Naderi Ghale-noie, Z.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.M.; Rohatensky, M.; Mintchev, P.; Nakoneshny, S.C.; Demetrick, D.J.; van Marle, G.; Dort, J.C. Loop mediated isothermal amplification (LAMP) for the detection and subtyping of Human Papillomaviruses (HPV) in oropharyngeal squamous cell carcinoma (OPSCC). J. Clin. Virol. 2016, 75, 37–41. [Google Scholar] [CrossRef]
- Saetiew, C.; Limpaiboon, T.; Jearanaikoon, P.; Daduang, S.; Pientong, C.; Kerdsin, A.; Daduang, J. Rapid detection of the most common high-risk human papillomaviruses by loop-mediated isothermal amplification. J. Virol. Methods 2011, 178, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-F.; Zhao, C.-Z.; Lu, K.-X. Development and application of a rapid detection system for Human Papillomavirus and Herpes simplex virus-2 by loop-mediated isothermal amplification assay. Microb. Pathog. 2016, 97, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Daskou, M.; Tsakogiannis, D.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D.; Kottaridi, C.; Gartzonika, C.; Markoulatos, P. WarmStart colorimetric LAMP for the specific and rapid detection of HPV16 and HPV18 DNA. J. Virol. Methods 2019, 270, 87–94. [Google Scholar] [CrossRef]
- Vo, D.T.; Story, M.D. Facile and direct detection of Human Papillomavirus (HPV) DNA in cells using loop-mediated isothermal amplification (LAMP). Mol. Cell. Probes 2021, 59, 101760. [Google Scholar] [CrossRef]
- Luo, L.; Nie, K.; Yang, M.-J.; Wang, M.; Li, J.; Zhang, C.; Liu, H.-T.; Ma, X.-J. Visual Detection of High-Risk Human Papillomavirus Genotypes 16, 18, 45, 52, and 58 by Loop-Mediated Isothermal Amplification with Hydroxynaphthol Blue Dye. J. Clin. Microbiol. 2011, 49, 3545–3550. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, K.; Chen, D.; Wang, H.; Lin, Q.; Liu, W. Rapid detection and subtyping of human papillomaviruses in condyloma acuminatum using loop-mediated isothermal amplification with hydroxynaphthol blue dye. Br. J. Biomed. Sci. 2018, 75, 110–115. [Google Scholar] [CrossRef]
- Zhu, H.H.; Li, Y.; Wu, L.X.; Wang, K.S.; Zhang, Y.; Fan, Q.Y.; Ming, Z.Z.; Chen, W.Q.; Liu, W.W. Internal heating method of loop-mediated isothermal amplification for detection of HPV-6 DNA. Microchim. Acta 2022, 189, 212. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Pandian, V.; Kadimisetty, K.; Zhang, X.; Ruiz, C.; Cooper, K.; Liu, C. Real-time Colorimetric Quantitative Molecular Detection of Infectious Diseases on Smartphone-based Diagnostic Platform. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumvongpin, R.; Jearanaikool, P.; Wilailuckana, C.; Sae-ung, N.; Prasongdee, P.; Daduang, S.; Wongsena, M.; Boonsiri, P.; Kiatpathomchai, W.; Swangvaree, S.S.; et al. High sensitivity, loop-mediated isothermal amplification combined with colorimetric gold-nanoparticle probes for visual detection of high risk Human Papillomavirus genotypes 16 and 18. J. Virol. Methods 2016, 234, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Feng, X.; Zhang, W.; Li, N.; Zhang, X.; Lin, J.M. Visual detection of high-risk HPV16 and HPV18 based on loop-mediated isothermal amplification. Talanta 2020, 217, 121015. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ma, B.; Fang, J.; Wang, Y.; He, H.; Lin, W.; Su, W.; Zhang, M. Colorimetric Detection of 23 Human Papillomavirus Genotypes by Loop-Mediated Isothermal Amplification. Clin. Lab. 2017, 63, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Cao, W.L.; Yao, X.; Chen, J.; Huang, D.; Yang, T.; Liu, Z.; Xie, W.; Xia, Y.; Zhong, T. Rapid diagnosis of seven high-risk human papillomavirus subtypes by a novel loop-mediated isothermal amplification method. Mol. Cell. Probes 2022, 61, 101787. [Google Scholar] [CrossRef] [PubMed]
- Mudhigeti, N.; Kalawat, U.; Hulikal, N.; Kante, M. Evaluation of Loop-Mediated Isothermal Amplification Assay for Detection and Typing of Human Papilloma Virus 16 and 18 from Endocervical Samples. Indian J. Med. Microbiol. 2019, 37, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hamzan, N.I.; Rahman, N.A.; Suraiya, S.; Mohamad, I.; Kalarakkal, T.G.; Mohamad, S. Real-time loop-mediated isothermal amplification assay for rapid detection of Human Papillomavirus 16 in oral squamous cell carcinoma. Arch. Oral Biol. 2021, 124, 105051. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Liu, Y.; Gao, L.; Wang, T.; Zhang, X.; Hu, J.; Ding, L.; Zhang, Y.; Wang, Q.; Wang, L.; et al. A portable all-in-one microfluidic device with real-time colorimetric LAMP for HPV16 and HPV18 DNA point-of-care testing. Biosens. Bioelectron. 2024, 248, 115968. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, R.B.; Rodríguez-Sánchez, I.P.; León, J.F.Y.-D.; Iqbal, H.M.N.; González-González, E. Microfluidics-Based Biosensing Platforms: Emerging Frontiers in Point-of-Care Testing SARS-CoV-2 and Seroprevalence. Biosensors 2022, 12, 179. [Google Scholar] [CrossRef]
- Landaverde, L.; Wong, W.; Hernandez, G.; Fan, A.; Klapperich, C. Method for the elucidation of LAMP products captured on lateral flow strips in a point of care test for HPV 16. Anal. Bioanal. Chem. 2020, 412, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Kumvongpin, R.; Jearanaikoon, P.; Wilailuckana, C.; Sae-Ung, N.; Prasongdee, P.; Daduang, S.; Wongsena, M.; Boonsiri, P.; Kiatpathomchai, W.; Swangvaree, S.S.; et al. Detection assay for HPV16 and HPV18 by loop-mediated isothermal amplification with lateral flow dipstick tests. Mol. Med. Rep. 2017, 15, 3203–3209. [Google Scholar] [CrossRef]
- Barra, M.; Chang, M.; Salcedo, M.P.; Schmeler, K.; Scheurer, M.; Maza, M.; Lopez, L.; Alfaro, K.; Richards-Kortum, R. Single-tube four-target lateral flow assay detects human papillomavirus types associated with majority of cervical cancers. Anal. Biochem. 2024, 688, 115480. [Google Scholar] [CrossRef]
- Wormald, B.W.; Moser, N.; deSouza, N.M.; Mantikas, K.T.; Malpartida-Cardenas, K.; Pennisi, I.; Ind, T.E.J.; Vroobel, K.; Kalofonou, M.; Rodriguez-Manzano, J.; et al. Lab-on-chip assay of tumour markers and Human Papillomavirus for cervical cancer detection at the point-of-care. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Yang, N.; Liu, P.; Cai, C.; Zhang, R.; Sang, K.; Shen, P.; Huang, Y.; Lu, Y. Triple signal amplification strategy for the ultrasensitive electrochemical detection of Human Papillomavirus 16 E6/E7 mRNA. Enzym. Microb. Technol. 2021, 149, 109855. [Google Scholar] [CrossRef]
- Prakrankamanant, P.; Leelayuwat, C.; Promptmas, C.; Limpaiboon, T.; Wanram, S.; Prasongdee, P.; Pientong, C.; Daduang, J.; Jearanaikoon, P. The development of DNA-based quartz crystal microbalance integrated with isothermal DNA amplification system for Human Papillomavirus type 58 detection. Biosens. Bioelectron. 2013, 40, 252–257. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Wormald, B.; Rodriguez-Manzano, J.; Moser, N.; Pennisi, I.; Ind, T.E.J.; Vroobel, K.; Attygalle, A.; Georgiou, P.; de Souza, N.M. Loop-Mediated Isothermal Amplification Assay for Detecting Tumor Markers and Human Papillomavirus: Accuracy and Supplemental Diagnostic Value to Endovaginal MRI in Cervical Cancer. Front. Oncol. 2021, 11, 747614. [Google Scholar] [CrossRef]
- Wang, J.; Staheli, J.P.; Wu, A.; Kreutz, J.E.; Hu, Q.; Wang, J.; Schneider, T.; Fujimoto, B.S.; Qin, Y.; Yen, G.S.; et al. Detection of 14 High-risk Human Papillomaviruses Using Digital LAMP Assays on a Self-digitization Chip. Anal. Chem. 2021, 93, 3266–3272. [Google Scholar] [CrossRef]
- Yin, K.; Pandian, V.; Kadimisetty, K.; Ruiz, C.; Cooper, K.; You, J.; Liu, C. Synergistically enhanced colorimetric molecular detection using smart cup: A case for instrument-free HPV-associated cancer screening. Theranostics 2019, 9, 2637–2645. [Google Scholar] [CrossRef]
- Yu, Z.; Lyu, W.; Yu, M.; Wang, Q.; Qu, H.; Ismagilov, R.F.; Han, X.; Lai, D.; Shen, F. Self-partitioning SlipChip for slip-induced droplet formation and human papillomavirus viral load quantification with digital LAMP. Biosens. Bioelectron. 2020, 155, 112107. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Sasaki, H.; Matsuo, K.; Honda, M.; Kawase, M.; Nakagawa, H. Loop-Mediated Isothermal Amplification Method for Detection of Human Papillomavirus Type 6, 11, 16, and 18. J. Med. Virol. 2007, 79, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Yang, W.; Peng, J.; Huang, J.; Mi, S. An integrated microfluidic detection system for the automated and rapid diagnosis of high-risk Human Papillomavirus. Analyst 2021, 146, 5102–5114. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Kreutz, J.E.; Schneider, T.; Yen, G.S.; Shah, E.S.; Wu, L.; Chiu, D.T. A Reinforced PDMS Mold for Hot Embossing of Cyclic Olefin Polymer in the Fabrication of Microfluidic Chips. Lab Chip 2022, 22, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, G.; Mao, R.; Wang, Z.; Sun, Y.Z.; Du, Y.G.; Gao, X.H.; Qi, R.Q.; Chen, H.D. Genotyping of 30 kinds of cutaneous Human Papillomaviruses by a multiplex microfluidic loop-mediated isothermal amplification and visual detection method. Virol. J. 2020, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, M.; Jirakova, L.; Anton, M.; Vojtesek, B.; Hrstka, R. Genomagnetic LAMP-based electrochemical test for determination of high-risk HPV16 and HPV18 in clinical samples. Anal. Chim. Acta 2018, 1042, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Sebuyoya, R.; Moranova, L.; Hrstka, R.; Anton, M.; Bartosik, M. Electrochemical bioassay coupled to LAMP reaction for determination of high-risk HPV infection in crude lysates. Anal. Chim. Acta 2021, 1187, 339145. [Google Scholar] [CrossRef] [PubMed]
- Nakowong, P.; Chatchawal, P.; Chaibun, T.; Boonapatcharoen, N.; Promptmas, C.; Buajeeb, W.; Lee, S.Y.; Jearanaikoon, P.; Lertanantawong, B. Detection of high-risk HPV 16 genotypes in cervical cancers using isothermal DNA amplification with electrochemical genosensor. Talanta 2024, 269, 125495. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, 1115–1121. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; Von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144, 31–67. [Google Scholar] [CrossRef]
- Wongsamart, R.; Bhattarakasol, P.; Chaiwongkot, A.; Wongsawaeng, D.; Okada, P.A.; Palaga, T.; Leelahavanichkul, A.; Khovidhunkit, W.; Dean, D.; Somboonna, N. Multiplex recombinase polymerase amplification for high-risk and low-risk type HPV detection, as potential local use in single tube. Sci. Rep. 2023, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Mao, L.; Tang, Y.; Fassatoui, M.; Song, W.; Xu, X.; Tang, X.; Li, J.; Liu, H.; Jian, F.; et al. Development and validation of real-time recombinase polymerase amplification-based assays for detecting HPV16 and HPV18 DNA. Microbiol. Spectr. 2023, 11, e0120723. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, G.; Wang, W.; Liang, L.; Li, Q.; Liu, M.; Xue, L.; Tang, G. A simple and rapid diagnostic method for 13 types of high-risk human papillomavirus (HR-HPV) detection using CRISPR-Cas12a technology. Sci. Rep. 2021, 11, 12800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chao, Z.; Ding, W.; Fang, T.; Gu, X.; Xue, M.; Wang, W.; Han, R.; Sun, W. A multiplex RPA-CRISPR/Cas12a-based POCT technique and its application in Human Papillomavirus (HPV) typing assay. Cell. Mol. Biol. Lett. 2024, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, D.; Li, T.; Yan, J.; Zhu, J.; He, T.; Hu, R.; Li, Y.; Yang, Y.; Liu, M. Microfluidic space coding for multiplexed nucleic acid detection via CRISPR-Cas12a and recombinase polymerase amplification. Nat. Commun. 2022, 13, 6480. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Ding, X.; Li, Z.; Zhao, H.; Cooper, K.; Liu, C. Dynamic aqueous multiphase reaction system for one-pot CRISPR-Cas12a based ultrasensitive and quantitative molecular diagnosis. Anal. Chem. 2020, 92, 8561–8568. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Q.; Liu, F.X.; Park, H.; Chan, K.W.; Leung, T.; Tang, B.Z.; Yao, S. Droplet digital recombinase polymerase amplification (ddRPA) reaction unlocking via picoinjection. Biosens. Bioelectron. 2022, 202, 114019. [Google Scholar] [CrossRef] [PubMed]
- Khamcharoen, W.; Siangproh, W.; Henry, C.S.; Sreamsukcharoenchai, N.; Ratthawongjirakul, P.; Chailapakul, O. Capillary-driven microfluidic device integrating recombinase polymerase amplification for human papillomavirus detection. Sens. Actuators B Chem. 2024, 401, 135016. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, W.-W.; Wang, Y.; Wang, Y.-C.; Shen, C.; Qi, H.; Shen, A.-D. Graphene oxide and self-avoiding molecular recognition systems-assisted recombinase polymerase amplification coupled with lateral flow bioassay for nucleic acid det Graphene oxide and self-avoiding molecular recognition systems-assisted recombinase polymerase amplification coupled with lateral flow bioassay for nucleic acid detection. Mikrochim. Acta 2020, 187, 667. [Google Scholar] [CrossRef]
- Ma, B.; Fang, J.; Lin, W.; Yu, X.; Sun, C.; Zhang, M. A simple and efficient method for potential point-of-care diagnosis of Human Papillomavirus genotypes: Combination of isothermal recombinase polymerase amplification with lateral flow dipstick and reverse dot blot. Anal. Bioanal. Chem. 2019, 411, 7451–7460. [Google Scholar] [CrossRef]
- Li, Z.; Ding, X.; Yin, K.; Xu, Z.; Cooper, K.; Liu, C. Electric field-enhanced electrochemical CRISPR biosensor for DNA detection. Biosens. Bioelectron. 2021, 192, 113498. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.; Ma, A.; Novak, E.N.; Barra, M.; Kundrod, K.A.; Montealegre, J.R.; Scheurer, M.E.; Castle, P.E.; Schmeler, K.; Richards-Kortum, R. A novel tailed primer nucleic acid test for detection of HPV 16, 18 and 45 DNA at the point of care. Sci. Rep. 2023, 13, 20397. [Google Scholar] [CrossRef] [PubMed]
- Rungkamoltip, P.; Temisak, S.; Piboonprai, K.; Japrung, D.; Thangsunan, P.; Chanpanitkitchot, S.; Chaowawanit, W.; Chandeying, N.; Tangjitgamol, S.; Iempridee, T. Rapid and ultrasensitive detection of circulating Human Papillomavirus E7 cell-free DNA as a cervical cancer biomarker. Exp. Biol. Med. 2021, 246, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.L.; Van Gemen, B.; Schukkink, R.; Van Der Velden, J.; Tjong-A-Hung, S.P.; Jebbink, M.F.; Ter Schegget, J. Application of the NASBA nucleic acid amplification method for the detection of Human Papillomavirus type 16 E6-E7 transcripts. J. Virol. Methods 1995, 54, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Stratton, C.W. Advanced Techniques in Diagnostic Microbiology; Springer Science + Business Media: New York, NY, USA, 2006; pp. 1–540. [Google Scholar] [CrossRef]
- Kraus, I.; Molden, T.; E Ernø, L.; Skomedal, H.; Karlsen, F.; Hagmar, B. Human papillomavirus oncogenic expression in the dysplastic portio; an investigation of biopsies from 190 cervical cones. Br. J. Cancer 2004, 90, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Boulet, G.A.V.; Micalessi, I.M.; Horvath, C.A.J.; Benoy, I.H.; Depuydt, C.E.; Bogers, J.J. Nucleic acid sequence-based amplification assay for human papillomavirus mRNA detection and typing: Evidence for DNA amplification. J. Clin. Microbiol. 2010, 48, 2524–2529. [Google Scholar] [CrossRef]
- Munkhdelger, J.; Choi, Y.; Lee, D.; Kim, S.; Kim, G.; Park, S.; Choi, E.; Jin, H.; Jeon, B.Y.; Lee, H.; et al. Comparison of the performance of the NucliSENS EasyQ HPV E6/E7 mRNA assay and HPV DNA chip for testing squamous cell lesions of the uterine cervix. Diagn. Microbiol. Infect. Dis. 2014, 79, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Chrelias, C.; Pappas, A.; Makridima, S.; Kountouris, E.; Alepaki, M.; Spathis, A.; Stathopoulou, V.; Panayiotides, I.; Panagopoulos, P.; et al. The diagnostic accuracy of two methods for E6&7 mRNA detection in women with minor cytological abnormalities. Acta Obstet. Gynecol. Scand. 2012, 91, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Haedicke, J.; Iftner, T. A review of the clinical performance of the Aptima HPV assay. J. Clin. Virol. 2016, 76, S40–S48. [Google Scholar] [CrossRef]
- Han, M.; Bernadt, C.T.; Murray, B.; Johnson, S.M.; Jalaly, J.B.; Garcia, T.; Adhikari, L.J. Aptima HR-HPV testing from Diff-Quick-stained fine-needle aspiration smears of oropharyngeal squamous cell carcinoma. J. Am. Soc. Cytopathol. 2016, 5, 221–226. [Google Scholar] [CrossRef]
- Getman, D.; Aiyer, A.; Dockter, J.; Giachetti, C.; Zhang, F.; Ginocchio, C.C. Efficiency of the APTIMA HPV Assay for detection of HPV RNA and DNA targets. J. Clin. Virol. 2009, 45, S49–S54. [Google Scholar] [CrossRef]
- Munson, E.; Kroeger, L.; Balzer, S.; Amrhein, R.; Munson, K.L.; Napierala, M.; Hudspeth, R.; Dillon, P.J. Comparison of commercial hybridization and automated transcription-mediated amplification modalities for detection of high-risk human papillomavirus nucleic acid. J. Clin. Microbiol. 2014, 52, 331–334. [Google Scholar] [CrossRef]
- Obande, G.A.; Singh, K.K.B. Current and Future Perspectives on Isothermal Nucleic Acid Amplification Technologies for Diagnosing Infections. Infect. Drug Resist. 2020, 13, 455–483. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.T.; Little, M.C.; Nadeau, J.G.; Shank, D.D. Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc. Natl. Acad. Sci. USA 1992, 89, 392–396. [Google Scholar] [CrossRef]
- Nuovo, G.J. In situ strand displacement amplification: An improved technique for the detection of low copy nucleic acids. Diagn. Mol. Pathol. 2000, 9, 195–202. [Google Scholar] [CrossRef]
- Yan, B.; Li, M.; Luo, F.; Jin, X.Y.; Qiu, B.; Lin, Z. Photothermal biosensor for HPV16 based on strand-displacement amplification and gold nanoparticles using a thermometer as readout. Microchim. Acta 2022, 189, 437. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 1995, 92, 4641–4645. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zheng, L.; Wei, K.; Li, M.; Jiang, M.; Qiu, J.; Zhou, Y.; Ke, R.; Lin, C. Novel in Situ Hybridization Assay for Chromogenic Single-Molecule Detection of Human Papillomavirus E6/E7 mRNA. Microbiol. Spectr. 2023, 11, e0389622. [Google Scholar] [CrossRef]
- De Arruda, M.; Lyamichev, V.I.; Eis, P.S.; Iszczyszyn, W.; Kwiatkowski, R.W.; Law, S.M.; Olson, M.C.; Rasmussen, E.B. Invader technology for DNA and RNA analysis: Principles and applications. Expert Rev. Mol. Diagn. 2002, 2, 487–496. [Google Scholar] [CrossRef]
- Day, S.P.; Hudson, A.; Mast, A.; Sander, T.; Curtis, M.; Olson, S.; Chehak, L.; Quigley, N.; Ledford, J.; Yen-Lieberman, B.; et al. Analytical performance of the Investigational Use Only Cervista HPV HR test as determined by a multi-center study. J. Clin. Virol. 2009, 45, S63–S72. [Google Scholar] [CrossRef]
- Alameda, F.; Garrote, L.; Mojal, S.; Sousa, C.; Muset, M.; LLoveras, B.; Bellosillo, B.; Saldanha, C.; Carreras, R.; Serrano, S. Cervista HPV HR test for cervical cancer screening: A comparative study in the Catalonian population. Arch. Pathol. Lab. Med. 2015, 139, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Youens, K.E.; Hosler, G.A.; Washington, P.J.; Jenevein, E.P.; Murphy, K.M. Clinical Experience with the Cervista HPV HR Assay: Correlation of Cytology and HPV Status from 56,501 Specimens. J. Mol. Diagn. 2011, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Boers, A.; Slagter-Menkema, L.; Van Hemel, B.M.; Belinson, J.L.; Ruitenbeek, T.; Buikema, H.J.; Klip, H.; Ghyssaert, H.; Van Der Zee, A.G.J.; De Bock, G.H.; et al. Comparing the Cervista HPV HR Test and Hybrid Capture 2 Assay in a Dutch Screening Population: Improved Specificity of the Cervista HPV HR Test by Changing the Cut-Off. PLoS ONE 2014, 9, e101930. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Venturoli, S.; Rösl, F.; Rincon-Orozco, B. Detection of high-risk human papillomavirus type 16 and 18 using isothermal helicase-dependent amplification. Diagn. Microbiol. Infect. Dis. 2014, 79, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yuan, Q.; Yang, Z.; Yao, B. Self-primed isothermal amplification for genomic DNA detection of human papillomavirus. Biosens. Bioelectron. 2017, 90, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Chan, K.G.; Yean, C.Y.; Ang, G.Y. Nucleic Acid-Based Diagnostic Tests for the Detection SARS-CoV-2: An Update. Diagnostics 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Lozano, L.; Chen, X.; Querec, T.D.; Katabi, N.; Moreno-Docón, A.; Wang, H.; Fix, D.; De Brot, L.; McMillen, T.A.; et al. An Isothermal, Multiplex Amplification Assay for Detection and Genotyping of Human Papillomaviruses in Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2020, 22, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A.; Abbott, S.; Jair, K.; Daniels, J.; Lintner, M.; Klein, D.; Wimpleberg, A.; Jordan, J.A. Cross Comparison of AmpFire HPV Genotyping Assay and Roche Human Papillomavirus (HPV) Linear Array for HPV Genotyping of Anal Swab Samples. J. Virol. Methods 2021, 292, 114113. [Google Scholar] [CrossRef]
- Hou, J.; Belinson, J.L.; Du, H.; Li, C.; Zhang, W.; Zhang, L.; Zhang, Y.; Qu, X.; Wu, R. AmpFire HPV and ScreenFire RS HPV validation trial. Am. J. Clin. Pathol. 2024, 161, 535–542. [Google Scholar] [CrossRef]
- Kishi, J.Y.; Schaus, T.E.; Gopalkrishnan, N.; Xuan, F.; Yin, P. Programmable autonomous synthesis of single-stranded DNA. Nat. Chem. 2018, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Su, W.; Zhong, G.; Zhang, X.; Wu, Y.; Situ, B.; Xiao, Y.; Yan, X.; Zheng, L. A highly sensitive and versatile fluorescent biosensor for pathogen nucleic acid detection based on toehold-mediated strand displacement initiated primer exchange reaction. Anal. Chim. Acta. 2022, 15, 340125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, D.; Liu, B.; Ma, X. Development of a Novel Multiple Cross-Linking Spiral Amplification for Rapid and Sensitive Detection of HPV16 DNA. J. Microbiol. Biotechnol. 2021, 31, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Oštrbenk Valenčak, A.; Cuschieri, K.; Bohinc, K.B.; Arbyn, M. 2023 global inventory of commercial molecular tests for Human Papillomaviruses (HPV). J. Clin. Virol. 2024, 172, 105671. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Glökler, J.; Lim, T.S.; Ida, J.; Frohme, M. Isothermal amplifications—A comprehensive review on current methods. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 543–586. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Song, J.; Liu, C.; Bau, H.H. Simple Approaches to Minimally-Instrumented, Microfluidic-Based Point-of-Care Nucleic Acid Amplification Tests. Biosensors 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef]
- Peeling, R.; McNerney, R. Increasing Access to Diagnostics through Technology Transfer and Local Production; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- TwistAmp® Exo—TwistDx Limited. Available online: https://www.twistdx.co.uk/product/twistamp-exo/ (accessed on 13 June 2024).

| Product | Technology | Company | Molecule (Target) | Gene (Target) | Genotype (Detection) | Year Approved |
|---|---|---|---|---|---|---|
| Digene Hybrid Capture 2 High-Risk HPV DNA Test | Hybrid capture technology | Digene Corporation (Qiagen) | DNA | - | 16,18,31,33,35,39,45,51, 52,56,58,59, 68 | 2003 |
| Cervista HPV 16/18 | Invader Technology | Hologic, Inc. | DNA | L1 | 16,18 | 2009 |
| Cervista HPV HR | Invader Technology | Hologic, Inc. | DNA | L1 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2009 |
| COBAS HPV Test | PCR | Roche Molecular Systems, Inc. | DNA | L1 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2011 |
| APTIMA HPV Assay | TMA | Gen-Probe, Inc. | RNA | E6/E7 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2011 |
| APTIMA HPV 16 18/45 Genotype Assay | TMA | Gen-Probe, Inc. | RNA | E6/E7 | 16, 18, 45 | 2012 |
| BD ONCLARITY HPV ASSAY | PCR | Becton, Dickinson, and company | DNA | E6/E7 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2018 |
| Cobas HPV for use on the Cobas 6800/8800 Systems | PCR | Roche Molecular Systems, Inc. | DNA | L1 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2020 |
| Alinity m HR HPV | PCR | Abbott Molecular, Inc. | DNA | L1 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 | 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Contreras, E.A.; González-González, E.; Trujillo-Rodríguez, G.d.J.; Rodríguez-Sánchez, I.P.; Ancer-Rodríguez, J.; Pérez-Maya, A.A.; Alvarez-Cuevas, S.; Martinez-Fierro, M.L.; Marino-Martínez, I.A.; Garza-Veloz, I. Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives. Pathogens 2024, 13, 653. https://doi.org/10.3390/pathogens13080653
Flores-Contreras EA, González-González E, Trujillo-Rodríguez GdJ, Rodríguez-Sánchez IP, Ancer-Rodríguez J, Pérez-Maya AA, Alvarez-Cuevas S, Martinez-Fierro ML, Marino-Martínez IA, Garza-Veloz I. Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives. Pathogens. 2024; 13(8):653. https://doi.org/10.3390/pathogens13080653
Chicago/Turabian StyleFlores-Contreras, Elda A., Everardo González-González, Gerardo de Jesús Trujillo-Rodríguez, Iram P. Rodríguez-Sánchez, Jesús Ancer-Rodríguez, Antonio Alí Pérez-Maya, Salomon Alvarez-Cuevas, Margarita L. Martinez-Fierro, Iván A. Marino-Martínez, and Idalia Garza-Veloz. 2024. "Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives" Pathogens 13, no. 8: 653. https://doi.org/10.3390/pathogens13080653
APA StyleFlores-Contreras, E. A., González-González, E., Trujillo-Rodríguez, G. d. J., Rodríguez-Sánchez, I. P., Ancer-Rodríguez, J., Pérez-Maya, A. A., Alvarez-Cuevas, S., Martinez-Fierro, M. L., Marino-Martínez, I. A., & Garza-Veloz, I. (2024). Isothermal Technologies for HPV Detection: Current Trends and Future Perspectives. Pathogens, 13(8), 653. https://doi.org/10.3390/pathogens13080653









