Systemic Factors Affecting Human Beta-Defensins in Oral Cavity
Abstract
1. Introduction
2. Periodontium
3. Periodontal Health and Diseases
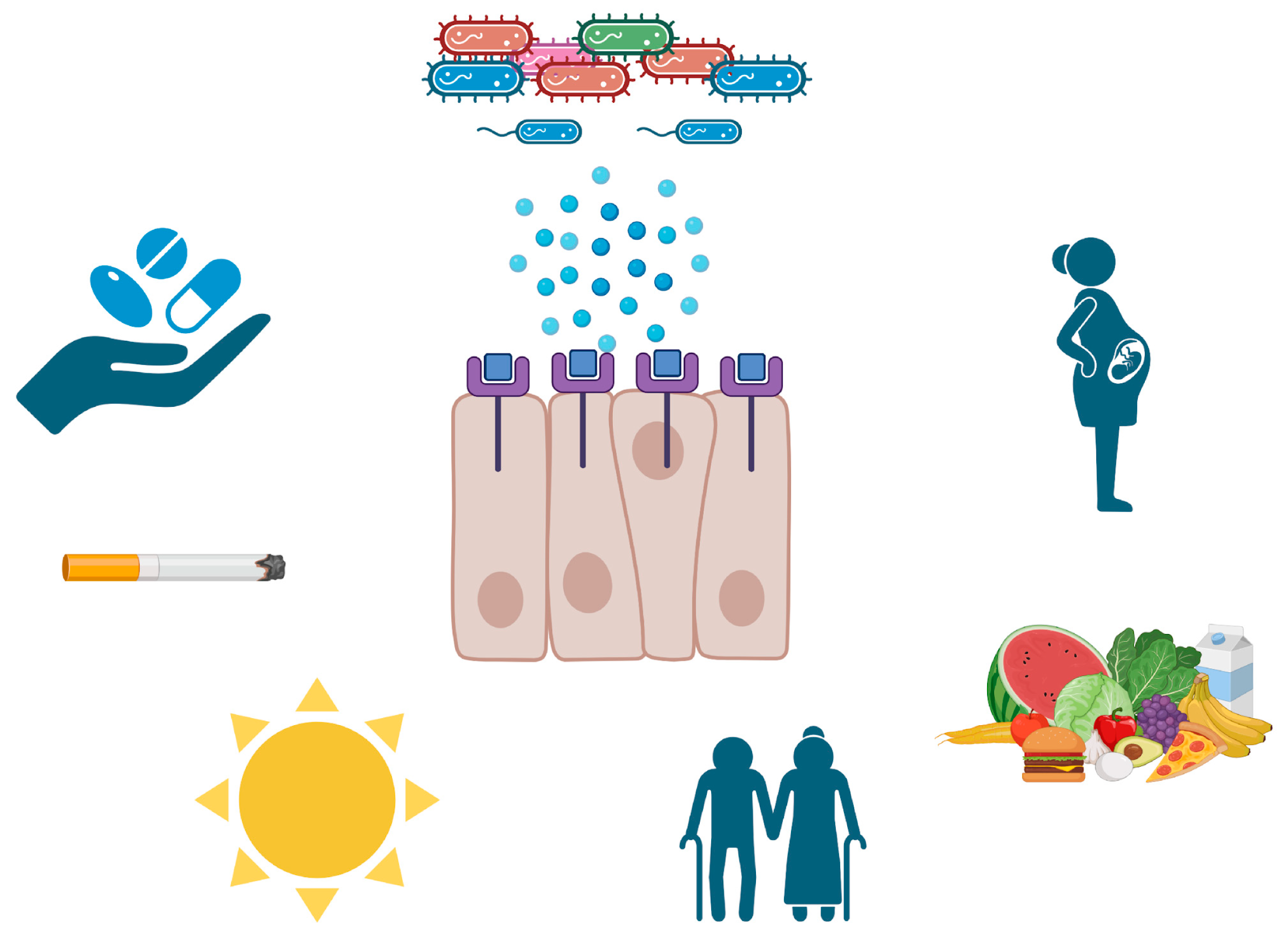
4. Human Beta-Defensins in Periodontal Tissues
5. Systemic Factors That Affect Both hBDs and Periodontal Tissues
5.1. Hyperglycemia
5.2. Retinoic Acid Use
5.3. Vitamin D Use
5.4. Pregnancy
5.5. Aging
6. Limitations and Future Prospects
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sulijaya, B.; Yamada-Hara, M.; Tsuzuno, T.; Tabeta, K.; Yamazaki, K. Gingival epithelial barrier: Regulation by beneficial and harmful microbes. Tissue Barriers 2019, 7, e1651158. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.; Zenobia, C.; Darveau, R.P. Defensins and LL-37: A review of function in the gingival epithelium. Periodontology 2000 2013, 63, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. 2006, 63, 1294–1313. [Google Scholar] [CrossRef]
- Diamond, G.; Ryan, L. Beta-defensins: What are they really doing in the oral cavity? Oral Dis. 2011, 17, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Takei, H.H. Newman and Carranza’s Clinical Periodontology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 181–305. [Google Scholar]
- Newman, M.G.; Takei, H.H. Carranza’s Clinical Periodontology, 12th ed.; Elsevier Saunders: St. Louis, MO, USA, 2015; pp. 9–40. [Google Scholar]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2000 2015, 69, 46–67. [Google Scholar] [CrossRef]
- Dabija-Wolter, G.; Bakken, V.; Cimpan, M.R.; Johannessen, A.C.; Costea, D.E. In vitro reconstruction of human junctional and sulcular epithelium. J. Oral Pathol. Med. 2013, 42, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000 2000, 24, 28–55. [Google Scholar] [CrossRef]
- Menicanin, D.; Hynes, K.; Han, J.; Gronthos, S.; Bartold, P.M. Cementum and Periodontal Ligament Regeneration. Adv. Exp. Med. Biol. 2015, 881, 207–236. [Google Scholar] [CrossRef]
- Lundgren, D.; Nyman, S.; Heijl, L.; Carlsson, G.E. Functional analysis of fixed bridges on abutment teeth with reduced periodontal support. J. Oral Rehabil. 1975, 2, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J. Host responses in periodontal diseases: Current concepts. J. Periodontol. 1992, 63 (Suppl. S4), 338–355. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Sula, A.; Peppoloni, S.; Meto, A.; Blasi, E. Leveraging Dental Stem Cells for Oral Health during Pregnancy: A Concise Review. Dent. J. 2024, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89 (Suppl. S1), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Theilade, E.; Jensen, S.B. Experimental Gingivitis in Man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Lu, S.; Lv, H.; Zhao, T.; Xie, G.; Du, Y.; Fan, Y.; Xu, L. Metabolic Disturbance and Th17/Treg Imbalance Are Associated with Progression of Gingivitis. Front. Immunol. 2021, 12, 670178. [Google Scholar] [CrossRef]
- Eksioglu-Demiralp, E.; Direskeneli, H.; Kibaroglu, A.; Yavuz, S.; Ergun, T.; Akoglu, T. Neutrophil activation in Behçet’s disease. Clin. Exp. Rheumatol. 2001, 19 (Suppl. S24), S19–S24. [Google Scholar]
- Panagakos, F.; Scannapieco, F. Periodontal Inflammation: From Gingivitis to Systemic Disease? InTech: Rijeka, Croatia, 2011; pp. 155–169. [Google Scholar] [CrossRef]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and adaptive immunity of periodontal disease. From etiology to alveolar bone loss. Oral Dis. 2022, 28, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive periodontitis: The most promising salivary biomarkers for early diagnosis of periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Taubman, M.A.; Valverde, P.; Han, X.; Kawai, T. Immune response: The key to bone resorption in periodontal disease. J. Periodontol. 2005, 76 (Suppl. S11), 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.H.; Cho, E.R.; Thomas, R.J.; An, S.Y.; Ryu, J.J.; Kim, H.; Shin, C. The association between periodontitis and obstructive sleep apnea: A preliminary study. J. Periodontal. Res. 2013, 48, 500–506. [Google Scholar] [CrossRef]
- Stanko, P.; Izakovicova Holla, L. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed. Pap. 2014, 158, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lu, F.; Zhang, Z.; Yang, X.; Chen, Y. The role of psychologic stress-induced hypoxia-inducible factor-1α in rat experimental periodontitis. J. Periodontol. 2011, 82, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Yay, E.; Balci, N.; Toygar, H.; Kılıc, B.B.; Zirh, A.; Rivas, C.A.; Kantarci, A. Parkinson’s disease is positively associated with periodontal inflammation. J. Periodontol. 2023, 94, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Meade, K.G.; O’Farrelly, C. Β-Defensins: Farming the microbiome for homeostasis and health. Front. Immunol. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.M.; Wu, Z.F.; Pang, B.X.; Jin, L.Y.; Qin, L.Z.; Wang, S.L. From Nitrate to Nitric Oxide: The Role of Salivary Glands and Oral Bacteria. J. Dent. Res. 2016, 95, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, D.; Yang, J.; Wang, P.; Han, B.; Zhao, L.; Jepsen, S.; Dommisch, H.; Winter, J.; Xu, Y. The expression of human β-defensins (hBD-1, hBD-2, hBD-3, hBD-4) in gingival epithelia. Arch. Oral Biol. 2016, 66, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Könönen, E. Understanding the roles of gingival beta-defensins. J. Oral Microbiol. 2012, 4, 15127. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chertov, O.; Oppenheim, J.J. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell. Mol. Life Sci. 2001, 58, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.O.; Dommisch, H.; Yin, L.; Dale, B.A. Expression of defensins in gingiva and their role in periodontal health and disease. Curr. Pharm. Des. 2007, 13, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Könönen, E.; Luukkonen, N.; Uitto, V.-J. Human Neutrophil Defensins and Their Effect on Epithelial Cells. J. Periodontol. 2013, 84, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Aizawa, R. Maintaining a protective state for human periodontal tissue. Periodontology 2000 2021, 86, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Güncü, G.N.; Yilmaz, D.; Könönen, E.; Gürsoy, U.K. Salivary Antimicrobial Peptides in Early Detection of Periodontitis. Front. Cell. Infect. Microbiol. 2015, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Kuula, H.; Salo, T.; Pirilä, E.; Hagström, J.; Luomanen, M.; Gutierrez-Fernandez, A.; Romanos, G.E.; Sorsa, T. Human beta-defensin-1 and -2 and matrix metalloproteinase-25 and -26 expression in chronic and aggressive periodontitis and in peri-implantitis. Arch. Oral Biol. 2008, 53, 175–186. [Google Scholar] [CrossRef]
- Gürsoy, M.; Könönen, E.; He, Q.; Liukkonen, A.; Huumonen, S.; Gürsoy, U.K. Toll-like receptor-1, -2, and -6 genotypes in relation to salivary human beta-defensin-1, -2, -3 and human neutrophilic peptide-1. J. Clin. Periodontol. 2022, 49, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Mehlotra, R.K.; Hall, N.B.; Willie, B.; Stein, C.M.; Weinberg, A.; Zimmerman, P.A.; Vernon, L.T. Associations of toll-like receptor and β-defensin polymorphisms with measures of periodontal disease (PD) in HIV+ North American adults: An exploratory study. PLoS ONE 2016, 11, e0164075. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Aisaiti, A.; Wu, Z.P.; Wang, T.T.; Zhao, J. Association of TLR-2 Gene Polymorphisms with the Risk of Periodontitis: A Meta-Analysis. Dis. Markers 2020, 2020, 9353958. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, Y.; Sugiyama, N.; Miura, J.; Yamashita, R.; Tominaga, S.; Izumi, Y.; Bamba, T.; Ishihama, Y.; Kashiwagi, Y.; Murakami, S. Chronic hyperglycemia reduces the expression of intercellular adhesion molecules and increases intercellular hyperpermeability in the periodontal epithelium. J. Periodontal. Res. 2023, 58, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Barnea, M.; Madar, Z.; Froy, O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem. Biophys. Res. Commun. 2008, 367, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Al-Kafaji, G. Glucose regulation of β-defensin-1 mRNA in human renal cells. Biochem. Biophys. Res. Commun. 2007, 353, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.E.; Wu, C.S.; Huang, S.M.; Kuo, H.Y.; Wu, I.H.; Wen, C.H.; Chai, C.Y.; Fang, A.H.; Chen, G.S. High-glucose environment inhibits p38MAPK signaling and reduces human β-3 expression in keratinocytes. Mol. Med. 2011, 17, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Filipe Rosa, L.; Rings, A.; Stolzer, I.; Koeninger, L.; Wehkamp, J.; Beisner, J.; Günther, C.; Nordkild, P.; Jensen, B.A.H.; Bischoff, S.C. Human α-Defensin 51–9 and Human β-Defensin 2 Improve Metabolic Parameters and Gut Barrier Function in Mice Fed a Western-Style Diet. Int. J. Mol. Sci. 2023, 24, 3878. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Güncü, G.N.; Könönen, E.; Bariş, E.; Çağlayan, F.; Gursoy, U.K. Overexpressions of hBD-2, hBD-3, and hCAP18/LL-37 in Gingiva of Diabetics with Periodontitis. Immunobiology 2015, 220, 1219–1226. [Google Scholar] [CrossRef]
- Jiménez-Escutia, R.; Vargas-Alcantar, D.; Flores-Espinosa, P.; Helguera-Repetto, A.C.; Villavicencio-Carrisoza, O.; Mancilla-Herrera, I.; Irles, C.; Torres-Ramos, Y.D.; Valdespino-Vazquez, M.Y.; Velázquez-Sánchez, P.; et al. High Glucose Promotes Inflammation and Weakens Placental Defenses against E. coli and S. agalactiae Infection: Protective Role of Insulin and Metformin. Int. J. Mol. Sci. 2023, 24, 5243. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, B.; Trujillo, V.; Montoya, A.; Gonzalez-Curiel, I.; Castañeda-Delgado, J.; Cardenas, A.; Rincon, K.; Hernandez, M.L.; Hernández-Pando, R. Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 2012, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; Ye, C.; Wu, W.; Liao, G.; Lu, Y.; Huang, P. Changes in advanced glycation end products, beta-defensin-3, and interleukin-17 during diabetic periodontitis development in rhesus monkeys. Exp. Biol. Med. 2018, 243, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Badr, G.; Badr, B.M.; Allam, A.; Ghamdi, A.A.; Al-Wadaan, M.A.; Al-Waili, N.S. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol. Immunol. 2018, 103, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Gkranias, N.; Mainas, G.; Di Pino, A. Periodontitis and implant complications in diabetes. Periodontology 2000 2022, 90, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Cruz Díaz, L.A.; Flores Miramontes, M.G.; Chávez Hurtado, P.; Allen, K.; Gonzalez Ávila, M.; Prado Montes De Oca, E. Ascorbic acid, ultraviolet c rays, and glucose but not hyperthermia are elicitors of human β-defensin 1 mRNA in normal keratinocytes. Biomed. Res. Int. 2015, 2015, 714580. [Google Scholar] [CrossRef] [PubMed]
- Kiselar, J.G.; Wang, X.; Dubyak, G.R.; El Sanadi, C.; Ghosh, S.K.; Lundberg, K.; Williams, W.M. Modification of β-defensin-2 by dicarbonyls methylglyoxal and glyoxal inhibits antibacterial and chemotactic function in vitro. PLoS ONE 2015, 10, e0130533. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Larange, A.; Cheroutre, H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu. Rev. Immunol. 2016, 34, 369–394. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Meyer-Hoffert, U.; Wehkamp, K.; Schwichtenberg, L.; Schröder, J.M. Differential gene induction of human β-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J. Investig. Dermatol. 2004, 123, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, J.S.; Kim, M.R.; Kim, M.Y.; Kim, S.C. Topical retinoids induce b-defensin 3 expression in mouse skin. Int. J. Dermatol. 2010, 49, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Isotretinoin: Dose, duration and relapse. What does 30 years of usage tell us? Australas. J. Dermatol. 2013, 54, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Borovaya, A.; Dombrowski, Y.; Zwicker, S.; Olisova, O.; Ruzicka, T.; Wolf, R.; Schauber, J.; Sárdy, M. Isotretinoin therapy changes the expression of antimicrobial peptides in acne vulgaris. Arch. Dermatol. Res. 2014, 306, 689–700. [Google Scholar] [CrossRef]
- Aksoy, G.; Adisen, E.; Erdem, Ö.; Aksakal, A.B. Comparison of Efficacy of Doxycycline and Isotretinoin on Cutaneous Human Beta-Defensin-1 and -2 Levels in Acne Vulgaris. Indian J. Dermatol. 2018, 63, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Atalay, N.; Balci, N.; Toygar, H.U.; Yardimci, G.; Gürsoy, U.K. Serum, saliva, and gingival tissue human β-defensin levels in relation to retinoic acid use. J. Periodontol. 2023, 94, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Klug-Micu, G.M.; Stenger, S.; Sommer, A.; Liu, P.T.; Krutzik, S.R.; Modlin, R.L.; Fabri, M. CD40 ligand and interferon-γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 2013, 139, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, Y.; Miao, L.; Yue, Z.; Xu, M.; Liu, K.; Hou, J. Human β-defensins are correlated with the immune infiltration and regulated by vitamin D3 in periodontitis. J. Periodontal Res. 2023, 58, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lan, Y.; Wang, W.; Zhang, J.; Shao, R.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Mai, K.; Ai, Q.; et al. Vitamin D influences gut microbiota and acetate production in zebrafish (Danio rerio) to promote intestinal immunity against invading pathogens. Gut Microbes 2023, 15, 2187575. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Hauger, H.; Ritz, C.; Mortensen, C.; Mølgaard, C.; Metzdorff, S.B.; Frøkiær, H.; Damsgaard, C.T. Winter cholecalciferol supplementation at 55°N has little effect on markers of innate immune defense in healthy children aged 4–8 years: A secondary analysis from a randomized controlled trial. Eur. J. Nutr. 2019, 58, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, H.M.J.; Pasch, M.C.; Van Erp, P.E.J.; Van Lingen, R.G.; Otero, M.E.; de Boer-van Huizen, R.T.; Van de Kerkhof, P.C. Treatment of plaque psoriasis with the two-compound product calcipotriol/betamethasone dipropionate versus both monotherapies: An immunohistochemical study. J. Dermatol. Treat. 2010, 21, 13–22. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Fiorentino, M.; Guida, L.; Annunziata, M.; Nastri, L.; Rizzo, A. Vitamin D reduces the inflammatory response by Porphyromonas gingivalis infection by modulating human β-defensin-3 in human gingival epithelium and periodontal ligament cells. Int. Immunopharmacol. 2017, 47, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.M.; Lahiri, P.; Arrazuria, R.; De Buck, J.; Knight, C.G.; Orsel, K.; Barkema, H.W.; Cobo, E.R. Oxytetracycline reduces inflammation and treponeme burden whereas vitamin D3 promotes β-defensin expression in bovine infectious digital dermatitis. Cell Tissue Res. 2020, 379, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Alvarez, J.A.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Hao, L.; Brown, L.A.S.; Martin, G.S.; Ziegler, T.R. Impact of high-dose vitamin D3 on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition 2017, 38, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Merriman, K.E.; Kweh, M.F.; Powell, J.L.; Lippolis, J.D.; Nelson, C.D. Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J. Steroid Biochem. Mol. Biol. 2015, 154, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Frew, L.; Stock, S.J. Antimicrobial peptides and pregnancy. Reproduction 2011, 141, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.J.; Feng, Y.; Ma, X.; Ma, F. Defensins: Defenders of human reproductive health. Hum. Reprod. Update 2023, 29, 126–154. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, I.A.; Jabr, M.; Buhimschi, C.S.; Petkova, A.P.; Weiner, C.P.; Saed, G.M. The novel antimicrobial peptide β3-defensin is produced by the amnion: A possible role of the fetal membranes in innate immunity of the amniotic cavity. Am. J. Obstet. Gynecol. 2004, 191, 1678–1687. [Google Scholar] [CrossRef]
- King, A.E.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R.G. Innate Immune Defences in the Human Uterus during Pregnancy. Placenta 2007, 28, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Kelly, R.W.; Riley, S.C.; Calder, A.A. Natural antimicrobial production by the amnion. Am. J. Obstet. Gynecol. 2007, 196, e1–e255. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R.G. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Akinbi, H.T.; Narendran, V.; Pass, A.K.; Markart, P.; Hoath, S.B. Host defense proteins in vernix caseosa and amniotic fluid. Am. J. Obstet. Gynecol. 2004, 191, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vince, G.S.; Lewis-Jones, I.; Bates, M.D.; Gazvani, R. The expression of human alpha and beta defensin in the endometrium and their effect on implantation. J. Assist. Reprod. Genet. 2007, 24, 533–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varrey, A.; Romero, R.; Panaitescu, B.; Miller, D.; Chaiworapongsa, T.; Patwardhan, M.; Faro, J.; Pacora, P.; Hassan, S.S.; Hsu, C.D.; et al. Human β-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am. J. Reprod. Immunol. 2018, 80, e13031. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Weiner, C.P.; Buhimschi, I.A. Proteomics, part II: The emerging role of proteomics over genomics in spontaneous preterm labor/birth. Obstet. Gynecol. Surv. 2006, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Tassis, K.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Hadjithomas, A.; Botsis, D.; Malamitsi-Puchner, A. The role of human beta defensins 2 and 3 in the second trimester amniotic fluid in predicting preterm labor and premature rupture of membranes. Arch. Gynecol. Obstet. 2010, 281, 793–799. [Google Scholar] [CrossRef]
- Gürsoy, M.; Gürsoy, U.K.; Liukkonen, A.; Kauko, T.; Penkkala, S.; Könönen, E. Salivary antimicrobial defensins in pregnancy. J. Clin. Periodontol. 2016, 43, 807–815. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Abdus-salam, R.A. Pregnancy-induced periodontal inflammation: Influence of salivary cytokines and antimicrobial proteins. Saudi Dent. J. 2018, 30, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Nomellini, V.; Faunce, D.E.; Kovacs, E.J. Innate immunity and aging. Exp. Gerontol. 2008, 43, 718–728. [Google Scholar] [CrossRef]
- Hajishengallis, G. Too old to fight? Aging and its toll on innate immunity. Mol. Oral Microbiol. 2010, 25, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Zavala, W.D.; Cavicchia, J.C. Deterioration of the Langerhans cell network of the human gingival epithelium with aging. Arch. Oral Biol. 2006, 51, 1150–1155. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Gürsoy, M.; Liukkonen, A.; Suominen, A.L.; Könönen, E. Salivary Human β-Defensin 1-3 and Human α-Defensin-1 Levels in Relation to the Extent of Periodontal Disease and Tooth Loss in the Elderly. J. Clin. Med. 2023, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Hanaoka, Y.; Akama, T.; Kono, I. Ageing and free-living daily physical activity effects on salivary beta-defensin 2 secretion. J. Sports Sci. 2017, 35, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Lefeuvre, C.; Preisser, L.; Pivert, A.; Soleti, R.; Blanchard, S.; Delneste, Y.; Ducancelle, A.; Couez, D.; Jeannin, P. Age-Related Expression of IFN-λ1 Versus IFN-I and Beta-Defensins in the Nasopharynx of SARS-CoV-2-Infected Individuals. Front. Immunol. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Delgado, J.E.; Miranda-Castro, N.Y.; González-Amaro, R.; González-Curiel, I.; Montoya-Rosales, A.; Rivas-Calderon, B.; Rivas-Santiago, B. Production of antimicrobial peptides is preserved in aging. Clin. Immunol. 2013, 148, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, K.; Sato, D.; Ishihara, K.; Hashimoto, S.; Yoshinari, M.; Katakura, A.; Inoue, T. Age-related differences in localization of beta-defensin-2 in human gingival epithelia. Bull. Tokyo Dent. Coll. 2006, 47, 167–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Author, Year | Population | Condition | Defensin Type | Detection Method | Findings |
|---|---|---|---|---|---|
| Malik et al. 2006 [54] | Human mesangial cells. | Hyperglycemia | HBD-2 | Quantitative real-time PCR. | hBD-1 expression increases to correct increased oxidative stress resulting from hyperglycemia. |
| Barnea et al. 2008 [53] | Human embryonic kidney and colon adenocarcinoma cells. | Hyperglycemia | HBD-1 | Quantitative real-time PCR. | High concentrations of glucose enhanced hBD-1 expression, and these levels were further elevated after insulin treatment. |
| Lan et al. 2011 [55] | Keratinocyte cultures. | Hyperglycemia | HBD-2 | Real-time quantitative PCR, and small interfering RNA, Western blot. | A high-glucose environment reduces hBD2 expression of keratinocytes via downregulation of STAT-1 signaling. |
| Diaz et al. 2014 [63] | Human neonate keratinocytes. | Hyperglycemia | DEFB-1 | Q-PCR and cell culture. | At 24 h of glucose exposure (45.5 mM), DEFB1 expression was significantly upregulated. |
| Kiselar et al. 2015 [64] | Recombinant hBD-2. | Hyperglycemia | HBD-2 | Proteolysis and MS analysis, radial diffusion assay, and chemotaxis assay. | HBD-2 is susceptible to altering modifications of Arg and Gly residues by dicarbonyl molecular species. |
| Rosa et al. 2023 [56] | 84 mice that received HD51–9 (n = 28), hBD2 (n = 28), and BSA (n = 28). | Hyperglycemia | HBD-2 | Oral glucose tolerance test, immunohistochemical analysis, organoid cell culture, and real-time PCR. | Administration of either HD51–9- or hBD2-attenuated glucose intolerance, resulting in a lowering of blood glucose. |
| Author, Year | Population | Condition | Defensin Type | Detection Method | Findings |
|---|---|---|---|---|---|
| Harder et al. 2004 [68] | Keratinocytes. | Retinoic acid use | HBD-1, -2, -3, and -4 | Real-time RT-PCR, Luciferase gene reporter assay, and Western blot. | Treatment of keratinocytes with ATRA downregulated P. aeruginosa-mediated hBD-2, -3, and -4 gene expressions. |
| Lee et al. 2010 [69] | Hairless mice (no additive data). | Retinoic acid use | mBD3 | Immunostaining and RT-PCR. | Topical retinoids upregulate mBD3 RNA expression in mouse skin. |
| Aksoy G. et al. 2018 [72] | Acne vulgaris patients (n = 44) and a control (n = 20). | Retinoic acid use | HBD-2 | Immunohistochemical staining. | Systemic use of isotretinoin decreased cutaneous hBD-2 levels, with no difference in hBD-1 levels. |
| Atalay et al. 2022 [73] | Systemic retinoic acid users (n = 34) and a control (n = 35). | Retinoic acid use | HBD-1, -2, and -3 | ELISA and immunohistochemical staining. | Systemic retinoic acid use was associated with a suppressed salivary hBD-2 level, which was independent of gingival inflammation. |
| Author, Year | Population | Condition | Defensin Type | Detection Method | Findings |
|---|---|---|---|---|---|
| Van Der Velden et al. 2009 [82] | Psoriasis patients (n = 6). | Vitamin D use | HBD-2 | Immunohistochemical staining. | HBD2 expressions decrease in psoriasis lesions after topical vitamin D treatment. |
| Wang et al. 2010 [79] | Primary human monocytic and epithelial cells. | Vitamin D use | HBD-2 and -3 | Western blotting and RT/qPCR. | Regulation of NOD2 expression by vitamin D affects the downregulation of HBD release. |
| Klug-Micu et al. 2013 [76] | Monocytes. | Vitamin D use | DEFB4 | Radioimmunoassay, PCR, flow cytometry, PBMC assays, and T-cell clone assays. | CD40L and IFN-γ can activate the vitamin D-dependent antimicrobial pathway by inducing CYP27B1, VDR, cathelicidin, and DEFB4. |
| Merriman et al. 2015 [86] | Lactating cows’ monocytes, neutrophils, and epithelial cells. | Vitamin D use | Bovine β-defensin-3, -4, -6, -7, and -10 | Q-PCR. | Multiple β-defensin genes are upregulated by 1,25-dihydroxyvitamin D3 in cattle. |
| Han et al. 2017 [85] | Patients with respiratory failure (n = 30). | Vitamin D use | HBD-2 | ELISA, IDS-İsys, and RT-qPCR. | There were no correlations between changes in total and free 25 (OH)D and hBD-2 concentrations. |
| Watts et al. 2019 [84] | Cows with digital dermatitis (n = 15). | Vitamin D use | β-defensin tracheal antimicrobial peptide (TAP) | Q-PCR. | Vitamin D3 can boost endogenous β-defensins in cow skin. |
| Liao et al. 2023 [78] | Cyp2r1 mutant zebrafish. | Vitamin D use | zfBD-1, -2, and -3 | qRT-PCR, in vivo luciferase assay, and Western blotting. | The expressions of zfBD-1, -2, and -3 were reduced in VD-deficient zebrafish. VD induced zfBD expression in zebrafish intestines by activating IL-22 signaling. |
| Zhang et al. 2023 [77] | Human gingival fibroblasts. | Vitamin D use | HBD-2 and -3 | RT-PCR. | Vitamin D3 upregulated the expression of HBD-2 and HBD-3 through CYP27A1. |
| Author, Year | Population | Condition | Defensin Type | Detection Method | Findings |
|---|---|---|---|---|---|
| King et al. 2007 [95] | Placental and chorion trophoblasts. | Pregnancy | HBD-1, -2, and -3 | RT-PCR and immunohistochemical analysis. | Fetal membranes and placenta are key sources of HBDs in the uterus at term. The expression of selective HBDs can be upregulated in these tissues in response to inflammatory cytokines. |
| Gürsoy et al. 2016 [101] | Pregnant (n = 30) and non-pregnant women (n = 24). | Pregnancy | HBD-1, -2, and -3 | ELISA. | Pregnancy has suppressive effects of salivary concentrations of hBD-1 and hBD-2, while that of hBD-3 remains unaffected. |
| Lasisi et al. 2018 [102] | Pregnant women (n = 47). | Pregnancy | HBD-1 | ELISA. | Salivary HBD-1 did not show a significant difference when comparing levels during pregnancy and postpartum. |
| Author, Year | Population | Condition | Defensin Type | Detection Method | Findings |
|---|---|---|---|---|---|
| Matsuzaka et al. 2006 [110] | Young group (n = 6) and elderly group (n = 7). | Aging | HBD-2 | Immunohistochemistry staining. | The results reveal HBD-2-positive cells in spinous cells in the elderly group and in the parakeratinized layer in the young group. |
| Delgado et al. 2013 [109] | Mononuclear cells of participants from 25 to 34 years old (n = 23) and participants from 65 to 84 years old (n = 21). | Aging | HBD-2 | ELISA and RT-qPCR. | HBD-2 production in serum is not affected by aging. |
| Shimizu et al. 2016 [107] | Participants from 65 to 85 years old (n = 168) and from 22 to 30 years old (n = 26). | Aging | HBD-2 | ELISA. | Elderly participants had lower salivary hBD-2 secretion levels than young participants. |
| Gilbert et al. 2021 [108] | Human epithelial cells of SARS-CoV-2-negative, asymptomatic SARS-CoV-2-positive, and symptomatic SARS-CoV-2-positive patients with different age groups. | Aging | HBD-1, -2, and -3 | RT-qPCR. | hBD-1–3 mRNA transcript levels and age were correlated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atalay, N.; Balci, N.; Gürsoy, M.; Gürsoy, U.K. Systemic Factors Affecting Human Beta-Defensins in Oral Cavity. Pathogens 2024, 13, 654. https://doi.org/10.3390/pathogens13080654
Atalay N, Balci N, Gürsoy M, Gürsoy UK. Systemic Factors Affecting Human Beta-Defensins in Oral Cavity. Pathogens. 2024; 13(8):654. https://doi.org/10.3390/pathogens13080654
Chicago/Turabian StyleAtalay, Nur, Nur Balci, Mervi Gürsoy, and Ulvi Kahraman Gürsoy. 2024. "Systemic Factors Affecting Human Beta-Defensins in Oral Cavity" Pathogens 13, no. 8: 654. https://doi.org/10.3390/pathogens13080654
APA StyleAtalay, N., Balci, N., Gürsoy, M., & Gürsoy, U. K. (2024). Systemic Factors Affecting Human Beta-Defensins in Oral Cavity. Pathogens, 13(8), 654. https://doi.org/10.3390/pathogens13080654







