Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media and Chemicals
2.2. Bacterial Strains
2.3. Groundwater Sampling Locations
2.4. Groundwater Sampling Procedure
2.5. Isolation of Free-Living Protozoa from Groundwater
2.6. Isolation of Amoebae from Groundwater Fauna
2.7. Feeding Experiments
2.8. DNA Extraction and PCR
2.9. DNA Sequencing and Bioinformatic Analyses
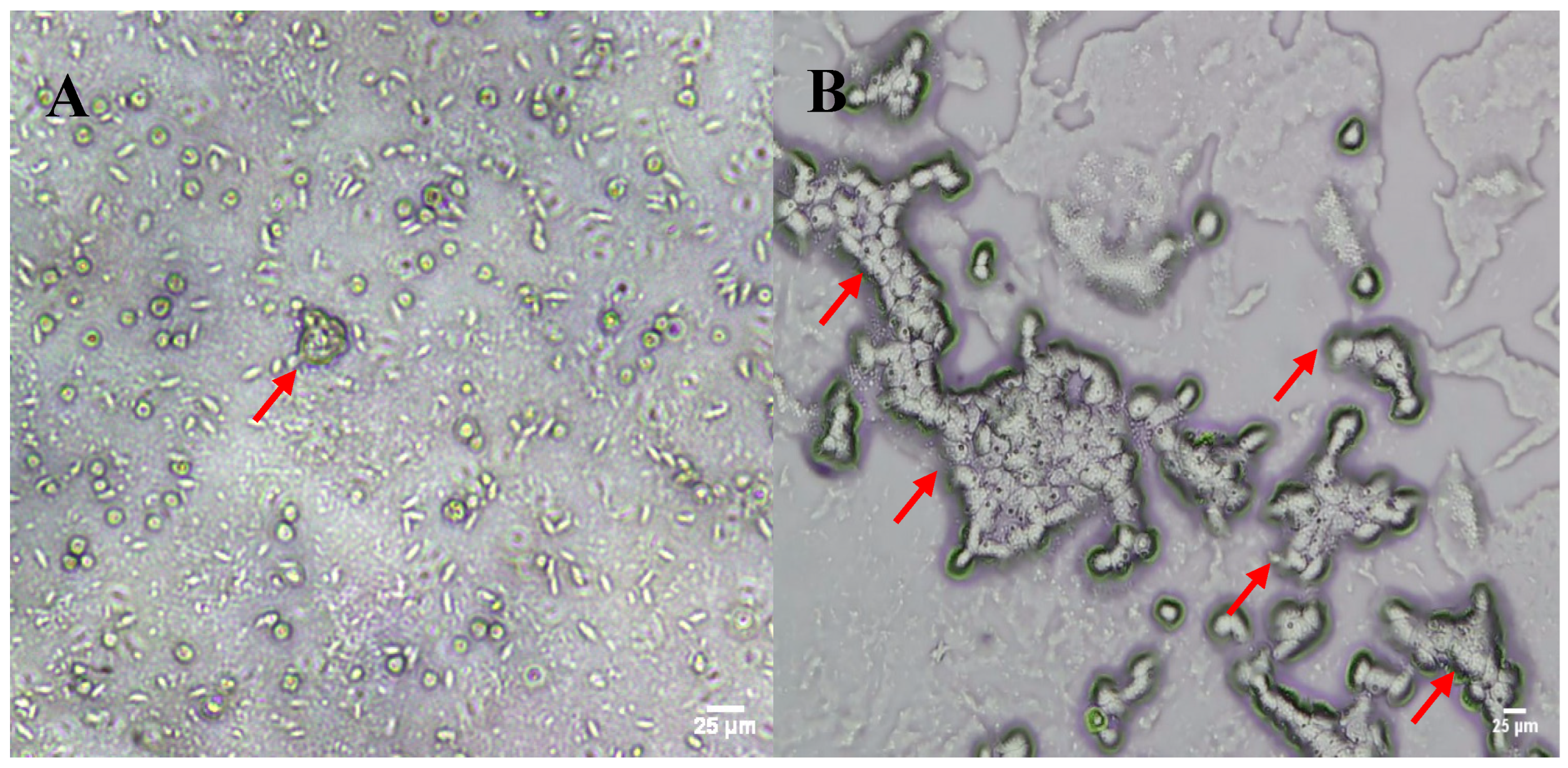
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, A.J. Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology, and Treatment of Disease; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Coşkun, K.A.; Özçelik, S.; Tutar, L.; Elaldı, N.; Tutar, Y. Isolation and identification of free-living amoebae from tap water in Sivas, Turkey. BioMed Res. Int. 2013, 2013, 675145. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, N.; Domingos, S.; Wylie, J.; Morgan, M.J.; Metcalfe, S.; Walsh, T.; Ahmed, W.; Kaksonen, A.H.; Puzon, G.J. Free-living amoeba and associated pathogenic bacteria in well-chlorinated drinking water storage tanks. ACS ES T Water 2022, 2, 1511–1520. [Google Scholar] [CrossRef]
- Logan-Jackson, A.; Rose, J.B. Cooccurrence of five pathogenic Legionella spp. and two free-living amoebae species in a complete drinking water system and cooling towers. Pathogens 2021, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Valciņa, O.; Pūle, D.; Mališevs, A.; Trofimova, J.; Makarova, S.; Konvisers, G.; Bērziņš, A.; Krūmiņa, A. Co-occurrence of free-living amoeba and Legionella in drinking water supply systems. Medicina 2019, 55, 492. [Google Scholar] [CrossRef] [PubMed]
- Rohr, U.; Weber, S.; Michel, R.; Selenka, F.; Wilhelm, M. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol. 1998, 64, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Valster, R.M.; Wullings, B.A.; Bakker, G.; Smidt, H.; van der Kooij, D. Free-living protozoa in two unchlorinated drinking water supplies, identified by phylogenic analysis of 18S rRNA gene sequences. Appl. Environ. Microbiol. 2009, 75, 4736–4746. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Raoult, D. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Snelling, W.J.; Moore, J.E.; McKenna, J.P.; Lecky, D.M.; Dooley, J.S. Bacterial–protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect. 2006, 8, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Bouchon, D.; Héchard, Y.; Moulin, L. Environmental factors shaping cultured free-living amoebae and their associated bacterial community within drinking water network. Water Res. 2016, 100, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Close, M.; Dann, R.; Ball, A.; Pirie, R.; Savill, M.; Smith, Z. Microbial groundwater quality and its health implications for a border-strip irrigated dairy farm catchment, South Island, New Zealand. J. Water Health 2008, 6, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Carrard, N.; Foster, T.; Willetts, J. Groundwater as a source of drinking water in southeast Asia and the Pacific: A multi-country review of current reliance and resource concerns. Water 2019, 11, 1605. [Google Scholar] [CrossRef]
- Lamoth, F.; Greub, G. Amoebal pathogens as emerging causal agents of pneumonia. FEMS Microbiol. Rev. 2010, 34, 260–280. [Google Scholar] [CrossRef] [PubMed]
- Molmeret, M.; Horn, M.; Wagner, M.; Santic, M.; Abu Kwaik, Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Cirillo, S.L.; Yan, L.; Bermudez, L.E.; Falkow, S.; Tompkins, L.S. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 1999, 67, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Berk, S.G.; Ting, R.S.; Turner, G.W.; Ashburn, R.J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 1998, 64, 279–286. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Jones, S.; Pelaz, C.; Millar, R.D.; Abu Kwaik, Y. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 2007, 9, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.M.; Von Dwingelo, J.E.; Price, C.T.; Abu Kwaik, Y. Cellular microbiology and molecular ecology of Legionella–amoeba interaction. Virulence 2013, 4, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Ashbolt, N. The role of biofilms and protozoa in Legionella pathogenesis: Implications for drinking water. J. Appl. Microbiol. 2009, 107, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Legionella pneumophila and protozoan hosts: Implications for the control of hospital and potable water systems. Pathogens 2020, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Page, F.C. Illustrated Key to Freshwater and Soil Amoebae; Freshwater Biological Association: Cumbria, UK, 1976. [Google Scholar]
- Thomas, V.; Herrera-Rimann, K.; Blanc, D.S.; Greub, G. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 2006, 72, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Brouke, A.; Bouchon, D.; Moulin, L.; Héchard, Y. Microbiome of free-living amoebae isolated from drinking water. Water Res. 2013, 47, 6958–6965. [Google Scholar] [CrossRef] [PubMed]
- Ashutosh Panda, A.P.; Shehla Khalil, S.K.; Mirdha, B.; Yogita Singh, Y.S.; Samander Kaushik, S.K. Prevalence of Naegleria fowleri in environmental samples from northern part of India. PLoS ONE 2015, 10, e0137736. [Google Scholar]
- Hadziavdic, K.; Lekang, K.; Lanzen, A.; Jonassen, I.; Thompson, E.M.; Troedsson, C. Characterization of the 18S rRNA gene for designing universal eukaryote specific primers. PLoS ONE 2014, 9, e87624. [Google Scholar] [CrossRef] [PubMed]
- Leger, A.; Leonardi, T. pycoQC, interactive quality control for Oxford Nanopore Sequencing. J. Open Source Softw. 2019, 4, 1236. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2012, 41, D597–D604. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Rice, C.A.; Byrne, A.W.; Paré, P.E.; Beauvais, W. Modelling dynamics between free-living amoebae and bacteria. Environ. Microbiol. 2024, 26, e16623. [Google Scholar] [CrossRef] [PubMed]
- Weisse, T.; Scheffel, U.; Stadler, P. Temperature-dependent resistance to starvation of three contrasting freshwater ciliates. Eur. J. Protistol. 2023, 88, 125973. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ardiles, C.; Asserella-Rebollo, L.; Andrade, D.C. Free-living amoebas in extreme environments: The true survival in our planet. BioMed Res. Int. 2022, 2022, 2359883. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.J. Free-living amebas: Naegleria, acanthamoeba and balamuthia. In Medical Microbiology, 4th ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Rayamajhee, B.; Willcox, M.D.; Henriquez, F.L.; Petsoglou, C.; Subedi, D.; Carnt, N. Acanthamoeba, an environmental phagocyte enhancing survival and transmission of human pathogens. Trends Parasitol. 2022, 38, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Novarino, G.; Warren, A.; Butler, H.; Lambourne, G.; Boxshall, A.; Bateman, J.; Kinner, N.E.; Harvey, R.W.; Mosse, R.A.; Teltsch, B. Protistan communities in aquifers: A review. FEMS Microbiol. Rev. 1997, 20, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Karaman, U.; Koloren, Z.; Karanis, P. Survey and first report of Acanthamoeba T4 genotype in natural spring water resources in the Black Sea, Turkey. J. Water Health 2022, 20, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Baquero, R.A.; Reyes-Batlle, M.; Nicola, G.G.; Martin-Navarro, C.M.; Lopez-Arencibia, A.; Guillermo Esteban, J.; Valladares, B.; Martinez-Carretero, E.; Pinero, J.E.; Lorenzo-Morales, J. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog. Glob. Health 2014, 108, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Montalbano Di Filippo, M.; Santoro, M.; Lovreglio, P.; Monno, R.; Capolongo, C.; Calia, C.; Fumarola, L.; D’Alfonso, R.; Berrilli, F.; Di Cave, D. Isolation and molecular characterization of free-living amoebae from different water sources in Italy. Int. J. Environ. Res. Public Health 2015, 12, 3417–3427. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Ashbolt, N.J. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol. 2011, 45, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.S.; Vaccaro, L.; Magnet, A.; Izquierdo, F.; Ollero, D.; Martínez-Fernández, C.; Mayo, L.; Moran, M.; Pozuelo, M.J.; Fenoy, S. Presence and interaction of free-living amoebae and amoeba-resisting bacteria in water from drinking water treatment plants. Sci. Total Environ. 2020, 719, 137080. [Google Scholar] [CrossRef] [PubMed]
- Chaúque, B.J.M.; Dos Santos, D.L.; Anvari, D.; Rott, M.B. Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol. Res. 2022, 121, 3033–3050. [Google Scholar] [CrossRef] [PubMed]
- Fouque, E.; Trouilhé, M.-C.; Thomas, V.; Humeau, P.; Héchard, Y. Encystment of Vermamoeba (Hartmannella) vermiformis: Effects of environmental conditions and cell concentration. Exp. Parasitol. 2014, 145, S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, M.W.; Valster, R.M.; Wullings, B.A.; Boonstra, H.; Smidt, H.; van der Kooij, D. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl. Environ. Microbiol. 2006, 72, 5750–5756. [Google Scholar] [CrossRef] [PubMed]
- Pagnier, I.; Valles, C.; Raoult, D.; La Scola, B. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb. Pathog. 2015, 80, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dobrowsky, P.H.; Khan, S.; Cloete, T.E.; Khan, W. Molecular detection of Acanthamoeba spp., Naegleria fowleri and Vermamoeba (Hartmannella) vermiformis as vectors for Legionella spp. in untreated and solar pasteurized harvested rainwater. Parasites Vectors 2016, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Samba-Louaka, A.; Delafont, V.; Rodier, M.-H.; Cateau, E.; Héchard, Y. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol. Rev. 2019, 43, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Feng, W.; Jiping, L.; Nan, L.; Quan, L. Isolation and identification of Naegleria species from environmental water in Changchun, Northeastern China. Iran. J. Parasitol. 2014, 9, 254. [Google Scholar]
- Marinho, B.T.S.; Santos, D.L.d.; Santos, D.L.d.; Rott, M.B. First report of free-living amoebae in watercourses in southern Brazil: Molecular diagnosis and phylogenetic analysis of Vermamoeba vermiformis, Naegleria gruberi, and Acanthamoeba spp. J. Water Health 2023, 21, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, A.M.; Power, J.F.; Washburne, A.; Cary, S.C.; Stott, M.B.; Fierer, N. The ecology and diversity of microbial eukaryotes in geothermal springs. ISME J. 2018, 12, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Scheikl, U.; Sommer, R.; Kirschner, A.; Rameder, A.; Schrammel, B.; Zweimüller, I.; Wesner, W.; Hinker, M.; Walochnik, J. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur. J. Protistol. 2014, 50, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Pohl, N.; Solbach, M.D.; Dumack, K. The wastewater protist Rhogostoma minus (Thecofilosea, Rhizaria) is abundant, widespread, and hosts Legionellales. Water Res. 2021, 203, 117566. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed]
- Boamah, D.K.; Zhou, G.; Ensminger, A.W.; O’Connor, T.J. From many hosts, one accidental pathogen: The diverse protozoan hosts of Legionella. Front. Cell. Infect. Microbiol. 2017, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Buse, H.; Ashbolt, N. Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Lett. Appl. Microbiol. 2011, 53, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Weber, S.; Hilbi, H. Formation of the Legionella-containing vacuole: Phosphoinositide conversion, GTPase modulation and ER dynamics. Int. J. Med. Microbiol. 2018, 308, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, R.; Bliton, J.; Goodman, A.; Ali, I.K.M.; Yoder, J.; Cope, J.R. Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by Naegleria fowleri: A global review. Clin. Infect. Dis. 2021, 73, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Kathol, M.; Norf, H.; Arndt, H.; Weitere, M. Effects of temperature increase on the grazing of planktonic bacteria by biofilm-dwelling consumers. Aquat. Microb. Ecol. 2009, 55, 65–79. [Google Scholar] [CrossRef]
- Dey, R.; Dlusskaya, E.; Oloroso, M.; Ashbolt, N.J. First evidence of free-living Naegleria species in recreational lakes of Alberta, Canada. J. Water Health 2023, 21, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, A.; Rueda, Z.; Alexander, D.; Laupland, K.B.; Keynan, Y. Impact of climate change on amoeba and the bacteria they host. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2024, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyadasa, S.; van Hamelsveld, S.; Taylor, W.; Lin, S.; Sitthirit, P.; Pang, L.; Billington, C.; Weaver, L. Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila. Pathogens 2024, 13, 665. https://doi.org/10.3390/pathogens13080665
Ariyadasa S, van Hamelsveld S, Taylor W, Lin S, Sitthirit P, Pang L, Billington C, Weaver L. Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila. Pathogens. 2024; 13(8):665. https://doi.org/10.3390/pathogens13080665
Chicago/Turabian StyleAriyadasa, Sujani, Sophie van Hamelsveld, William Taylor, Susan Lin, Panan Sitthirit, Liping Pang, Craig Billington, and Louise Weaver. 2024. "Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila" Pathogens 13, no. 8: 665. https://doi.org/10.3390/pathogens13080665





