Investigation of a Serine Protease Inhibitor Active in the Infectious Stage of the Human Liver Fluke Opisthorchis viverrini
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites
2.2. Molecular Cloning of Opisthorchis viverrini SIS cDNA
2.3. Expression and Purification of Recombinant OvSIS
2.4. Reverse Transcriptase PCR Analysis
2.5. Phylogenetic Analysis of Serpins in the Genera Opisthorchis/Clonorchis and Schistosoma
2.6. Indirect ELISA of Human Opisthorchiasis Sera with Recombinant OvSIS
2.7. Inhibition of Human Serine Proteases by rOvSIS
3. Results
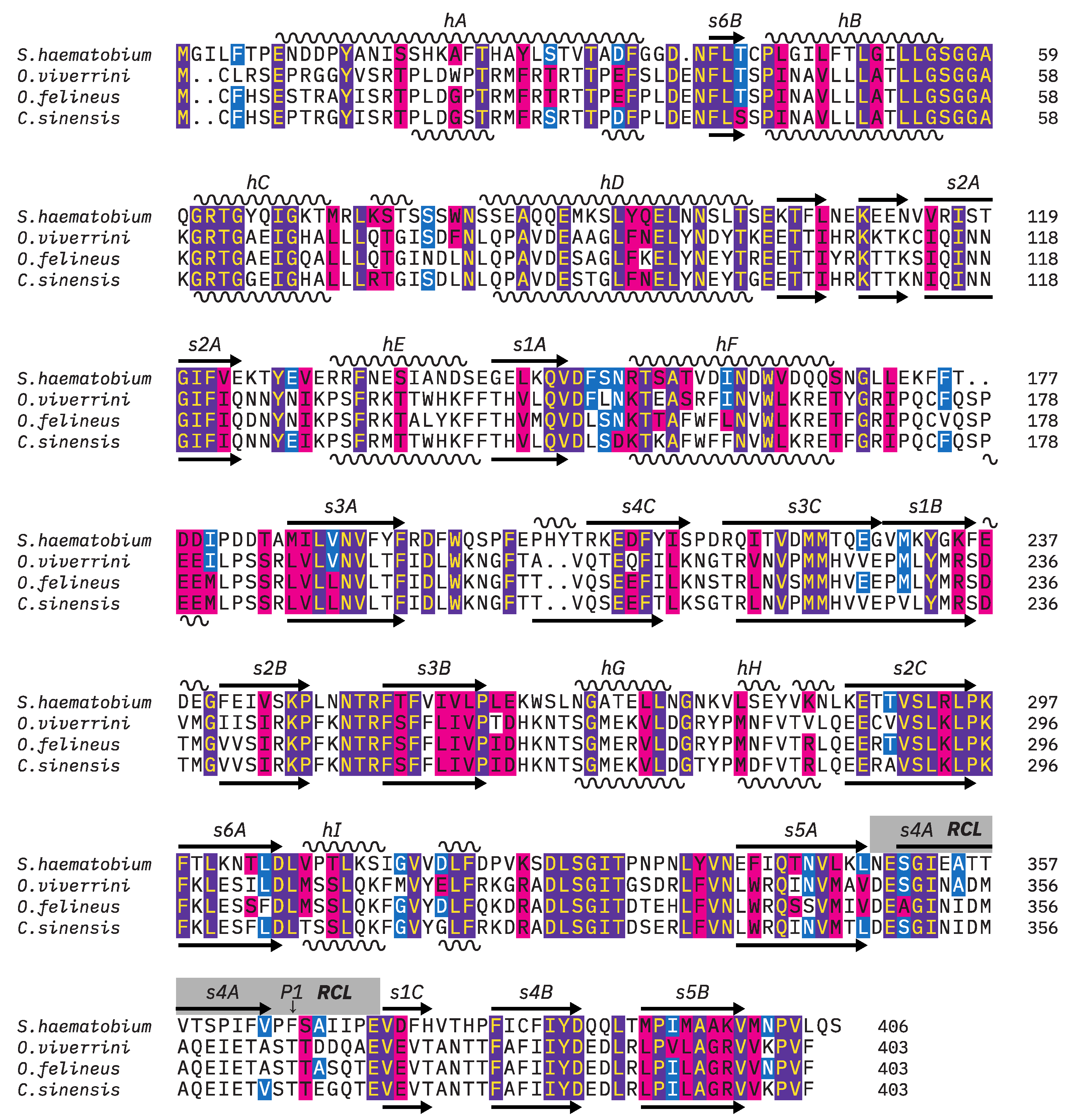
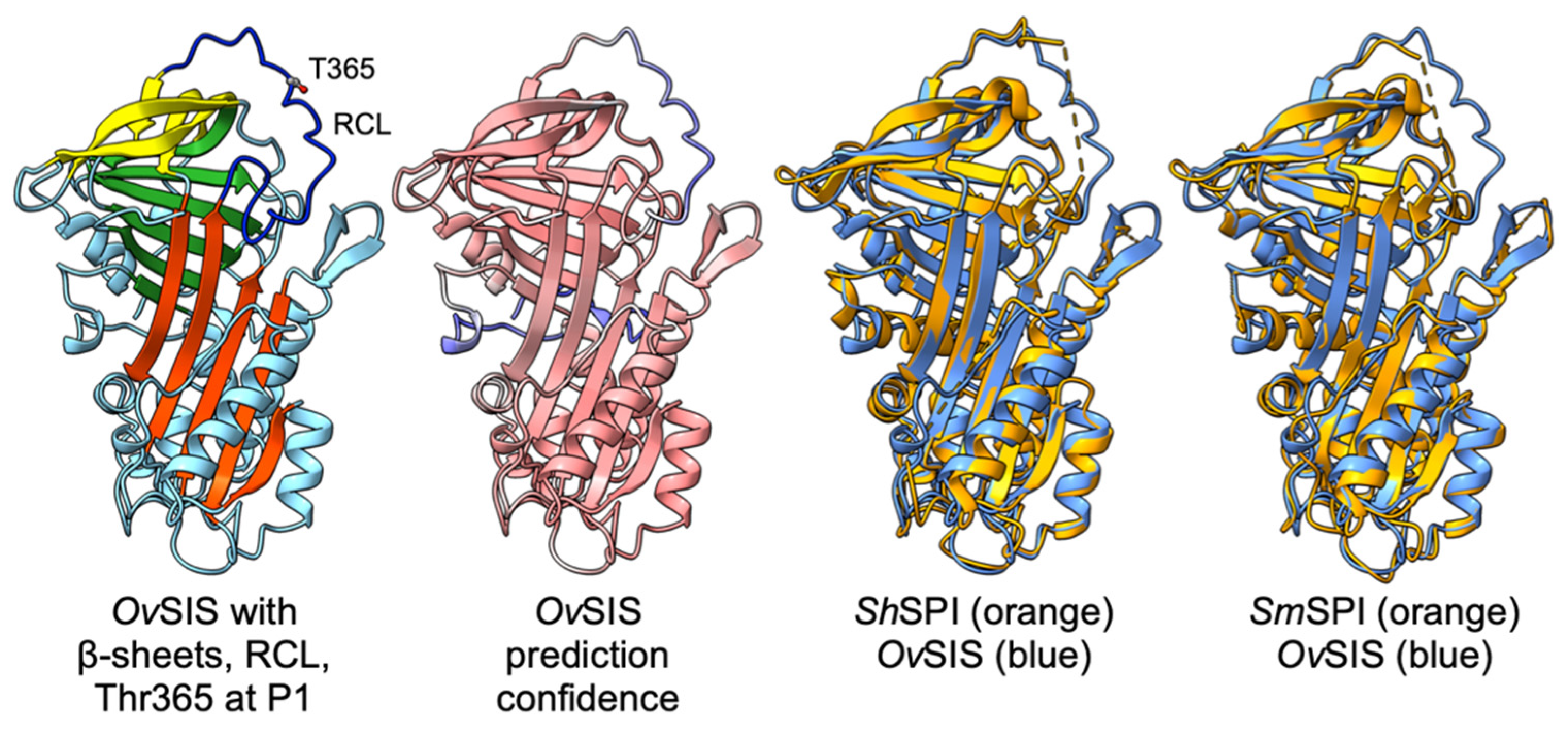
3.1. Molecular Cloning of OvSIS and Analysis of Sequence and Structure
3.2. Phylogenetic Analysis of Serpins in the Genera Opisthorchis/Clonorchis and Schistosoma
3.3. Developmental Expression of OvSIS RNA
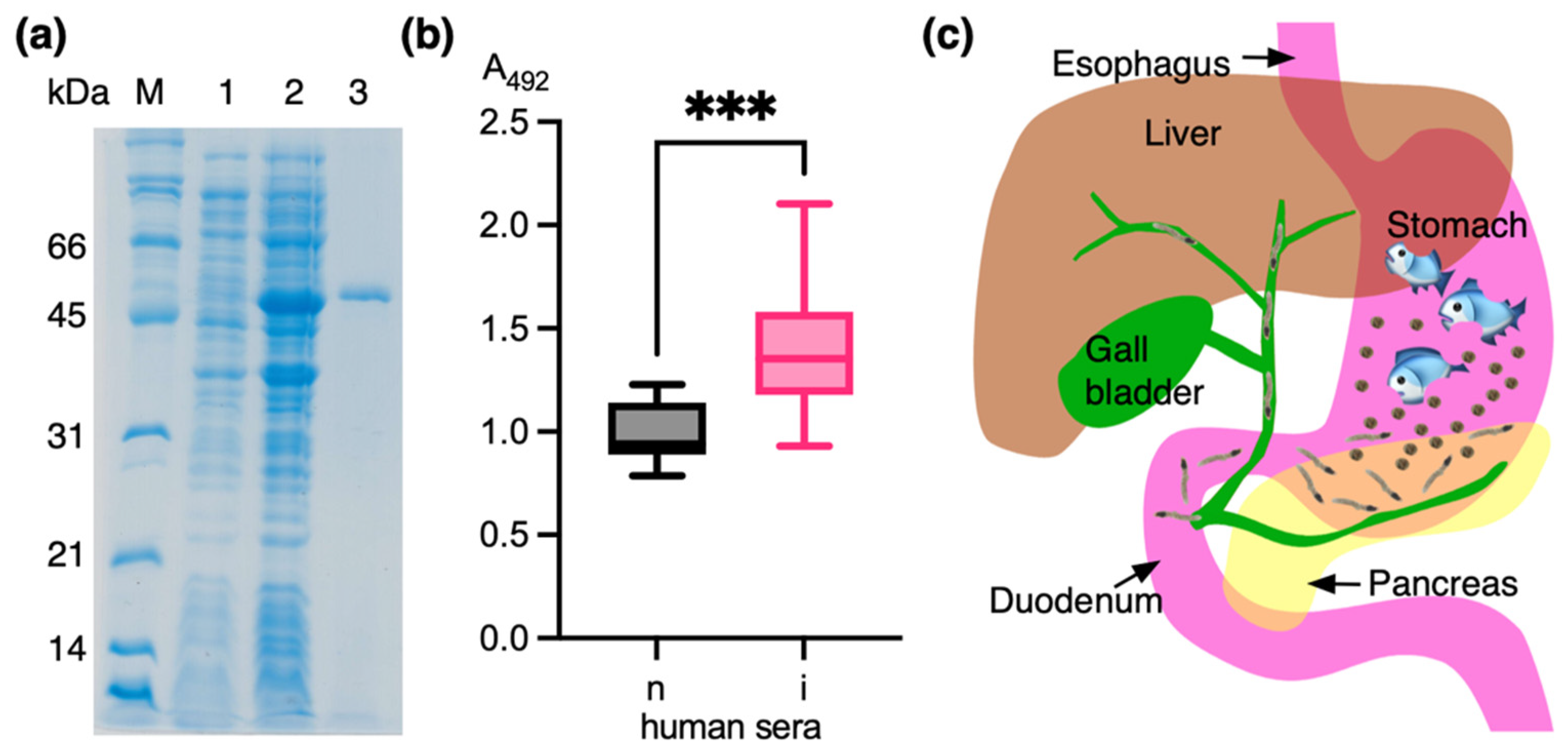
3.4. Analysis of Human Opisthorchiasis Sera Against Recombinant OvSIS
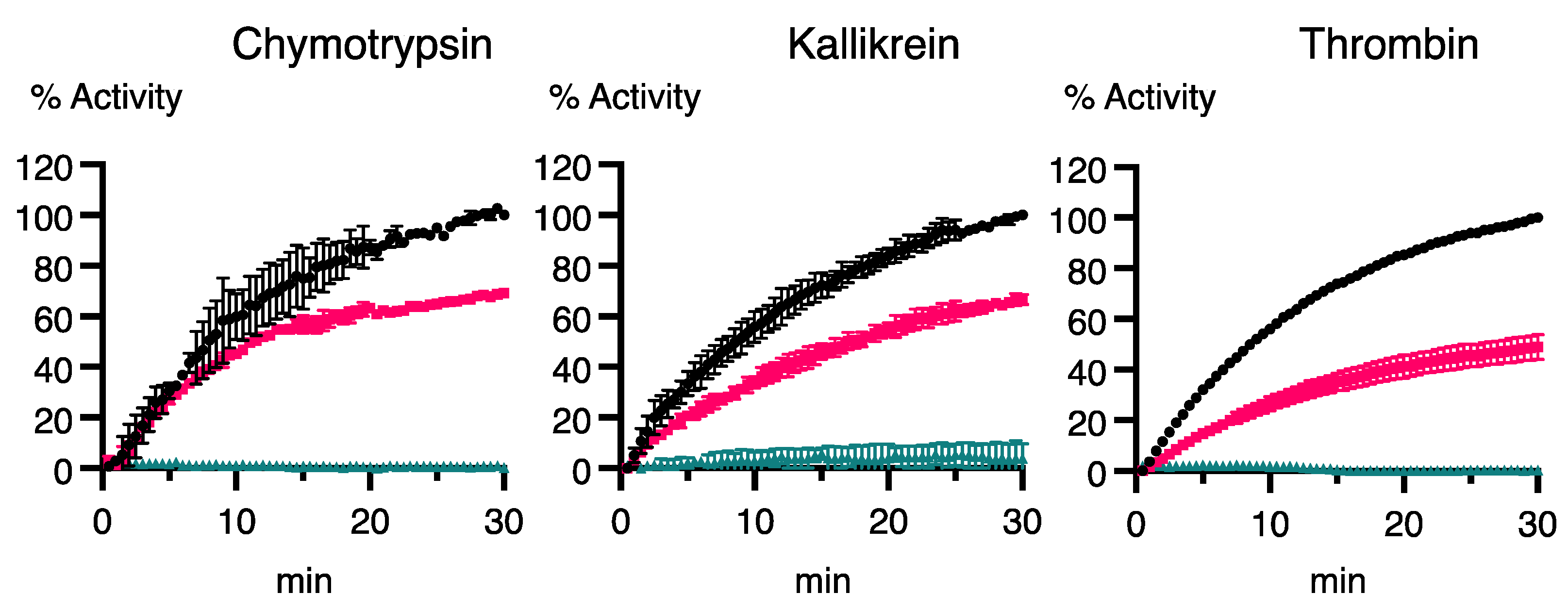
3.5. Inhibition of Human Serine Proteases by Recombinant OvSIS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef]
- Brindley, P.J.; da Costa, J.M.; Sripa, B. Why does infection with some helminths cause cancer. Trends Cancer 2015, 1, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Heit, C.; Jackson, B.C.; McAndrews, M.; Wright, M.W.; Thompson, D.C.; Silverman, G.A.; Nebert, D.W.; Vasiliou, V. Update of the human and mouse SERPIN gene superfamily. Hum. Genom. 2013, 7, 22. [Google Scholar] [CrossRef]
- Molehin, A.J.; Gobert, G.N.; McManus, D.P. Serine protease inhibitors of parasitic helminths. Parasitology 2012, 139, 681–695. [Google Scholar] [CrossRef]
- Molehin, A.J.; Gobert, G.N.; Driguez, P.; McManus, D.P. Functional characterization of SjB10, an intracellular serpin from Schistosoma japonicum. Parasitology 2014, 141, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- De Marco Verissimo, C.; Jewhurst, H.L.; Tikhonova, I.G.; Urbanus, R.T.; Maule, A.G.; Dalton, J.P.; Cwiklinski, K. Fasciola hepatica serine protease inhibitor family (serpins): Purposely crafted for regulating host proteases. PLoS Negl. Trop. Dis. 2020, 14, e0008510. [Google Scholar] [CrossRef]
- Sánchez Di Maggio, L.; Tirloni, L.; Uhl, M.; Carmona, C.; Logullo, C.; Mulenga, A.; da Silva Vaz, I.; Berasain, P. Serpins in Fasciola hepatica: Insights into host-parasite interactions. Int. J. Parasitol. 2020, 50, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Becerro-Recio, D.; Serrat, J.; López-García, M.; Molina-Hernández, V.; Pérez-Arévalo, J.; Martínez-Moreno, Á.; Sotillo, J.; Simón, F.; González-Miguel, J.; Siles-Lucas, M. Study of the migration of Fasciola hepatica juveniles across the intestinal barrier of the host by quantitative proteomics in an ex vivo model. PLoS Negl. Trop. Dis. 2022, 16, e0010766. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, D.; Wang, L.; Liang, C.; Hu, X.; Wang, X.; Chen, J.; Xu, J.; Yu, X. Molecular cloning and characterization of a novel serpin gene of Clonorchis sinensis, highly expressed in the stage of metacercaria. Parasitol. Res. 2009, 106, 221–225. [Google Scholar] [CrossRef]
- Kang, J.M.; Sohn, W.M.; Ju, J.W.; Kim, T.S.; Na, B.K. Identification and characterization of a serine protease inhibitor of Clonorchis sinensis. Acta Trop. 2010, 116, 134–140. [Google Scholar] [CrossRef]
- Lei, H.; Tian, Y.; Chen, W.; Wang, X.; Li, X.; Mao, Q.; Sun, J.; Li, R.; Xu, Y.; Liang, C.; et al. The biochemical and immunological characterization of two serpins from Clonorchis sinensis. Mol. Biol. Rep. 2013, 40, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, D.; Wang, L.; Liang, C.; Hu, X.; Xu, J.; Huang, Y.; Yu, X. Comparison of two serpins of Clonorchis sinensis by bioinformatics, expression, and localization in metacercaria. Pathog. Glob. Health 2014, 108, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Geadkaew, A.; von Bülow, J.; Beitz, E.; Tesana, S.; Vichasri Grams, S.; Grams, R. Bi-functionality of Opisthorchis viverrini aquaporins. Biochimie 2015, 108, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Nagarajan, N.; Lin, S.J.; Korhonen, P.K.; Jex, A.R.; Hall, R.S.; Safavi-Hemami, H.; Kaewkong, W.; Bertrand, D.; Gao, S.; et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. 2014, 5, 4378. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Geadkaew-Krenc, A.; Grams, R.; Phadungsil, W.; Chaibangyang, W.; Kosa, N.; Adisakwattana, P.; Dekumyoy, P. Evaluation of Rhophilin Associated Tail Protein (ROPN1L) in the human liver fluke Opisthorchis viverrini for diagnostic approach. Korean J. Parasitol. 2020, 58, 475–479. [Google Scholar] [CrossRef]
- Granzin, J.; Huang, Y.; Topbas, C.; Huang, W.; Wu, Z.; Misra, S.; Hazen, S.L.; Blanton, R.E.; Lee, X.; Weiergraber, O.H. Three-dimensional structure of a schistosome serpin revealing an unusual configuration of the helical subdomain. Acta Crystallogr. D. Biol. Crystallogr. 2012, 68, 686–694. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, S.; Di Pisa, F.; Fassi, E.M.A.; Cretich, M.; Musicò, A.; Frigerio, R.; Mussida, A.; Bombaci, M.; Grifantini, R.; Colombo, G.; et al. Structure, Immunoreactivity, and In Silico Epitope Determination of SmSPI S. mansoni Serpin for Immunodiagnostic Application. Vaccines 2021, 9, 322. [Google Scholar] [CrossRef]
- Wu, R.; Ding, F.; Wang, R.; Shen, R.; Zhang, X.; Luo, S.; Su, C.; Wu, Z.; Xie, Q.; Berger, B.; et al. High-resolution de novo structure prediction from primary sequence. bioRxiv 2022. [Google Scholar] [CrossRef]
- Huber, R.; Carrell, R.W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry 1989, 28, 8951–8966. [Google Scholar] [CrossRef]
- Beitz, E. TEXshade: Shading and labeling of multiple sequence alignments using LATEX2 epsilon. Bioinformatics 2000, 16, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.B.; Kim, T.S.; Yang, H.J. Early cysteine protease activity in excretory bladder triggers metacercaria excystment of Paragonimus westermani. J. Parasitol. 2005, 91, 953–954. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Blanton, R.E.; Licate, L.S.; Aman, R.A. Characterization of a native and recombinant Schistosoma haematobium serine protease inhibitor gene product. Mol. Biochem. Parasitol. 1994, 63, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; King, C.L.; Ogundipe, J.O.; Licate, L.S.; Blanton, R.E. Preferential recognition by human IgE and IgG4 of a species-specific Schistosoma haematobium serine protease inhibitor. J. Infect. Dis. 1995, 171, 416–422. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, S.; Song, G.; Xu, Y.; Dissous, C. Characterization of a novel vaccine candidate and serine proteinase inhibitor from Schistosoma japonicum (Sj serpin). Vet. Parasitol. 2005, 131, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pakchotanon, P.; Molee, P.; Nuamtanong, S.; Limpanont, Y.; Chusongsang, P.; Limsomboon, J.; Chusongsang, Y.; Maneewatchararangsri, S.; Chaisri, U.; Adisakwattana, P. Molecular characterization of serine protease inhibitor isoform 3, SmSPI, from Schistosoma mansoni. Parasitol. Res. 2016, 115, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Salter, J.P.; Choe, Y.; Albrecht, H.; Franklin, C.; Lim, K.C.; Craik, C.S.; McKerrow, J.H. Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J. Biol. Chem. 2002, 277, 24618–24624. [Google Scholar] [CrossRef] [PubMed]


| Enzyme | Substrate | Buffer |
|---|---|---|
| 1 nM Chymotrypsin | 15 μM Suc-Ala-Ala-Pro-Phe-MCA | 100 mM Tris-HCl pH 7.4, 10 mM CaCl2 |
| 1 nM Kallikrein | 30 μM Z-Phe-Arg-MCA | 100 mM Tris-HCl pH 7.4, 10 mM CaCl2, 0.3 M NaCl |
| 0.1 U Thrombin | 30 μM Boc-Val-Pro-Arg-MCA | 100 mM Tris-HCl pH 7.4, 10 mM CaCl2, 0.3 M NaCl |
| Species | O. viverrini | C. sinensis | O. felineus |
|---|---|---|---|
| O. viverrini | ----- | 83.9 | 82.1 |
| C. sinensis | 90.1 | ----- | 87.1 |
| O. felineus | 88.8 | 92.1 | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salang, R.; Phadungsil, W.; Geadkaew-Krenc, A.; Grams, R. Investigation of a Serine Protease Inhibitor Active in the Infectious Stage of the Human Liver Fluke Opisthorchis viverrini. Pathogens 2024, 13, 678. https://doi.org/10.3390/pathogens13080678
Salang R, Phadungsil W, Geadkaew-Krenc A, Grams R. Investigation of a Serine Protease Inhibitor Active in the Infectious Stage of the Human Liver Fluke Opisthorchis viverrini. Pathogens. 2024; 13(8):678. https://doi.org/10.3390/pathogens13080678
Chicago/Turabian StyleSalang, Rosnanee, Wansika Phadungsil, Amornrat Geadkaew-Krenc, and Rudi Grams. 2024. "Investigation of a Serine Protease Inhibitor Active in the Infectious Stage of the Human Liver Fluke Opisthorchis viverrini" Pathogens 13, no. 8: 678. https://doi.org/10.3390/pathogens13080678






