Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question and Applied Formula
2.2. Eligible Criteria, Article Screening, and Data Extraction
2.3. Data Analysis
3. Results
3.1. Overview on Screened Articles and Relevance of Pathogens in Targeted Populations
3.2. Temporal Evolution of the Selected Papers’ Interests
3.3. Relationship between Screened and Detected Pathogen Species in Target Populations
3.4. Pathogens Detected According to Populations: Highlighting Tick-Borne Zoonotic Pathogens
3.5. Tick Genera and Pathogen Family
3.6. Method Used Most to Detect Bacteria, Parasites, and Viruses in the Target Population
3.7. Tick-Borne Pathogen Distribution: Focus on Viruses and Zoonotic Bacteria and Parasites
4. Discussion
4.1. Tick-Borne Pathogen Research Focus and Implications for Public Health
4.2. Prevalent Pathogens, Vectors and Implications for Further Research and Livestock Farming and Human Health
4.3. Tick-Borne Zoonotic Pathogens in Sub-Saharan Africa and the Need for Integrated One Health Approaches
4.4. Detection of Atypical Pathogens for Sub-Sahara Africa
4.5. Study Limitations Induce Possible Underestimation of the Number of Pathogens Present
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Domain | Family | Genus | Species | Tick | Animal | Human | Countries | Target Loci | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screened | Detected | Screened | Detected | Screened | Detected | |||||||
| Anaplasmatacea | Anaplasma | Ca. Anaplasma ivorensis | 1 | 1 | NA | NA | NA | NA | Ivory_Coast | TtAna | [140] | |
| Ca. Anaplasma africae | NA | NA | 1 | 1 | NA | NA | Senegal | rpoB | [80] | |||
| A. bovis | 1 | 1 | 5 | 5 | NA | NA | Kenya, | 16S, groEL | [141,142] | |||
| Malawi, | [143] | |||||||||||
| South_Africa, | [144] | |||||||||||
| Uganda, | [122] | |||||||||||
| Zambia, | [145] | |||||||||||
| A. capra | 1 | 1 | NA | NA | NA | NA | Ghana | 16S | [146] or [147] | |||
| A. centrale | 3 | 3 | 11 | 11 | NA | NA | Benin, | 16S, groEL, msp2,msp4, rpoB, TtAna | [148] | |||
| Burkina_Faso, | [148] | |||||||||||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [71,148] | |||||||||||
| Ghana, | [148] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [150,151] | |||||||||||
| Nigeria, | [148,152] | |||||||||||
| Senegal, | [80] | |||||||||||
| South_Africa, | [73,153] | |||||||||||
| Sudan, | [154] | |||||||||||
| Tanzania, | [148] | |||||||||||
| Uganda, | [122,148,155,156] | |||||||||||
| Zambia, | [145] | |||||||||||
| A. marginale | 10 | 10 | 32 | 31 | NA | NA | Benin, | 16S, msp1, msp2, msp4, msp5, groEL, rpoB, TtAna, MAR1bB2, pCS20, | [16,24,43,68,148,157] | |||
| Botswana | [75] | |||||||||||
| Burkina_Faso, | [16,24,148] | |||||||||||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [71,72,77,78,148,158] | |||||||||||
| Ghana, | [146,147,148,159] | |||||||||||
| Guinea, | [160] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [141,142,150,151,161,162] | |||||||||||
| Madagascar, | [70] | |||||||||||
| Malawi, | [143] | |||||||||||
| Mozambique, | [163] | |||||||||||
| Nigeria, | [148,152,164,165,166] | |||||||||||
| Senegal, | [80] | |||||||||||
| South_Africa, | [73,144,153,167] | |||||||||||
| Sudan, | [154,168] | |||||||||||
| Tanzania, | [148,169,170] | |||||||||||
| Uganda, | [95,122,148,155,171,172] | |||||||||||
| Zambia, | [145] | |||||||||||
| A. ovis | 3 | 3 | 7 | 7 | NA | NA | Botswana, | 16S, msp4, groEL, rpoB, | [75] | |||
| Ethiopia, | [88] | |||||||||||
| Kenya, | [79] | |||||||||||
| Malawi, | [76] | |||||||||||
| Mozambique, | [84] | |||||||||||
| Senegal, | [80,81] | |||||||||||
| South_Africa, | [144,173] | |||||||||||
| Sudan | [154] | |||||||||||
| A. phagocytophilum | 3 | 3 | 5 | 4 | 1 | 1 | Ethiopia, | 16S, msp2, groEL | [71,72] | |||
| South_Africa, | [34,173,174] | |||||||||||
| Senegal, | [81] | |||||||||||
| Mozambique, | [163] | |||||||||||
| Uganda, | [122] | |||||||||||
| A. platys | 1 | 1 | 1 | 1 | NA | NA | Senegal, | 16S, groEL | [81] | |||
| South_Africa, | [144] | |||||||||||
| A. platys-like | 1 | 1 | 9 | 9 | NA | NA | Cameroon, | 16S, 18S, groEL, msp4, rpoB, | [149] | |||
| Guinea, | [160] | |||||||||||
| Kenya, | [141,142,150,151,162] | |||||||||||
| Malawi, | [143] | |||||||||||
| Nigeria, | [165] | |||||||||||
| Senegal, | [80] | |||||||||||
| Uganda, | [95] | |||||||||||
| Anaplasma sp. Dedessa | NA | NA | 1 | 1 | NA | NA | Ethiopia, | 16S | [158] | |||
| Anaplasma sp. Hadesa | NA | NA | 2 | 2 | NA | NA | Cameroon, | 16S | [149] | |||
| Ethiopia, | [158] | |||||||||||
| Anaplasma sp. Lambwe-1 | NA | NA | 1 | 1 | NA | NA | Kenya, | 16S | [141] | |||
| Anaplasma sp. Omatjenne | 1 | 1 | 3 | 3 | NA | NA | Ethiopia, | 16S | [71,158] | |||
| Uganda, | [122] | |||||||||||
| Zambia, | [145] | |||||||||||
| Anaplasma sp. Saso | NA | NA | 1 | 1 | NA | NA | Ethiopia, | 16S | [158] | |||
| Anaplasma spp. | 4 | 3 | 14 | 14 | 1 | 0 | Angola, | 16S, groEL, msp4, msp5, TtAna | [175] | |||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [88] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [79,141,142,162] | |||||||||||
| Malawi, | [76,143] | |||||||||||
| Mozambique, | [163] | |||||||||||
| Nigeria, | [152,165,176] | |||||||||||
| Senegal, | [35,81] | |||||||||||
| South_Africa, | [177] | |||||||||||
| Sudan, | [154] | |||||||||||
| Tanzania | [178] | |||||||||||
| Anaplasma/Ehrlichia spp. | 2 | 1 | 3 | 3 | NA | NA | Cameroon, | 16S | [149] | |||
| Ethiopia, | [88] | |||||||||||
| Nigeria, | [152,179] | |||||||||||
| South_Africa, | [144] | |||||||||||
| Ehrlichia | Ca. Ehrlichia rustica | 1 | 1 | NA | NA | NA | NA | Ivory_Coast, | TtAna | [140] | ||
| Ca. Ehrlichia urmitei | 1 | 1 | NA | NA | NA | NA | Ivory_Coast, | TtAna | [140] | |||
| E. canis | 4 | 4 | 2 | 2 | NA | NA | Ghana, | 16S, dsbA, groEL | [146,147] | |||
| Malawi, | [76] | |||||||||||
| Senegal, | [80] | |||||||||||
| South_Africa, | [120,180] | |||||||||||
| Zambia, | [145] | |||||||||||
| E. chaffeensis | 1 | 1 | 1 | 1 | NA | NA | South_Africa, | 16S, dsbA | [120] | |||
| Zambia, | [145] | |||||||||||
| E. minasensis | 2 | 2 | 3 | 3 | NA | NA | Ethiopia, | 16S, dsbA | [158] | |||
| Ghana, | [146,147] | |||||||||||
| Kenya, | [142,150,151] | |||||||||||
| South_Africa, | [120] | |||||||||||
| E. muris | 1 | 1 | NA | NA | NA | NA | South_Africa, | dsbA | [120] | |||
| E. ruminantium | 15 | 15 | 20 | 16 | NA | NA | Benin, | 16S, dsbA, groEL, pCS20, TtAna | [16,24,43,68,148,157] | |||
| Burkina_Faso, | [16,24,148] | |||||||||||
| Cameroon, | [149,181,182] | |||||||||||
| Ethiopia, | [71,72,77,78,88,148,158] | |||||||||||
| Ghana, | [148] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [79] | |||||||||||
| Malawi, | [76] | |||||||||||
| Mozambique, | [183] | |||||||||||
| Nigeria, | [148,152,184,185] | |||||||||||
| Senegal, | [81] | |||||||||||
| South_Africa, | [144,167,173,174,180,186,187] | |||||||||||
| Tanzania, | [148,170] | |||||||||||
| Uganda, | [95,122,148,156] | |||||||||||
| Zambia, | [145] | |||||||||||
| Ehrlichia spp. | 8 | 7 | 7 | 4 | NA | NA | Angola, | 16S, dsbA, groEL, gltA, TtAna | [175] | |||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [88] | |||||||||||
| Guinea, | [160] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [142,150,151,162] | |||||||||||
| Malawi, | [143] | |||||||||||
| South_Africa, | [120,144,180] | |||||||||||
| Sudan, | [154] | |||||||||||
| Tanzania, | [178] | |||||||||||
| Coxiellacea | Coxiella | C. burnetii | 11 | 8 | 2 | 1 | 4 | 2 | Angola, | COX, htpB, IS1111 | [175] | |
| Ethiopia, | [188,189] | |||||||||||
| Ghana, | [146,147] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [190] | |||||||||||
| Madagascar, | [191] | |||||||||||
| Sao_Tome | [192] | |||||||||||
| Senegal, | [35,193] | |||||||||||
| South_Africa, | [167,174,194,195] | |||||||||||
| Zanzibar | [196] | |||||||||||
| Coxiella spp. | 4 | 3 | NA | NA | NA | NA | Angola, | 16S, rpoB, groEL | [175] | |||
| Sao_Tome, | [192] | |||||||||||
| South_Africa, | [197] | |||||||||||
| Tanzania | [178] | |||||||||||
| Rickettsiacea | Rickettsia | Ca. Rickettsia barbariae | 1 | 1 | NA | NA | NA | NA | Cameroon | ompB | [121] | |
| R. aeschlimannii | 5 | 5 | NA | NA | NA | NA | Angola, | ompA, ompB, RaescSca1 | [175] | |||
| Cameroon, | [121] | |||||||||||
| Ghana, | [146,147] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Zambia, | [198] | |||||||||||
| R. africae | 21 | 21 | 1 | 1 | 1 | 0 | Angola, | 16S, gltA, ompA, ompB, 17 kDa, poT15-dam2 | [175] | |||
| Burkina_Faso, | [199] | |||||||||||
| Cameroon, | [121,149] | |||||||||||
| Comoros, | [200] | |||||||||||
| Djibouti, | [201] | |||||||||||
| Ethiopia, | [88,199,202] | |||||||||||
| Ghana, | [146,147] | |||||||||||
| Guinea, | [18] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [31,203] | |||||||||||
| Liberia, | [18] | |||||||||||
| Madagascar, | [204] | |||||||||||
| Mozambique, | [84,205] | |||||||||||
| Nigeria, | [206] | |||||||||||
| South_Africa, | [105,186,207,208] | |||||||||||
| Sudan, | [209] | |||||||||||
| Tanzania, | [200] | |||||||||||
| Zambia, | [198] | |||||||||||
| R. massiliae | 4 | 4 | NA | NA | NA | NA | Cameroon, | Hypothetical protein, 23S-5S, ompB | [121] | |||
| Ivory_Coast, | [140] | |||||||||||
| Nigeria, | [206,210] | |||||||||||
| R. rickettsii | 1 | 1 | NA | NA | NA | NA | South_Africa | 16S | [197] | |||
| R. sibirica | 1 | 1 | NA | NA | NA | NA | Cameroon | ompB | [121] | |||
| Rickettsia spp. | 27 | 25 | 6 | 2 | 2 | 1 | Angola, | 16S, gltA, ompA, ompB, 17 kDa, | [175] | |||
| Benin, | [43,68,211] | |||||||||||
| Burkina_Faso, | [199] | |||||||||||
| Cameroon, | [121,149] | |||||||||||
| Djibouti, | [201] | |||||||||||
| Ethiopia, | [88,188,199,212] | |||||||||||
| Ghana, | [146,147] | |||||||||||
| Guinea, | [18] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Kenya, | [141,162,213] | |||||||||||
| Liberia, | [18] | |||||||||||
| Mozambique, | [84,205] | |||||||||||
| Nigeria, | [152,176,206] | |||||||||||
| Sao_Tome, | [192] | |||||||||||
| Senegal, | [35] | |||||||||||
| South_Africa, | [167,173,174,194,208,214,215] | |||||||||||
| Sudan, | [209] | |||||||||||
| Tanzania, | [178] | |||||||||||
| Togo, | [211] | |||||||||||
| Uganda, | [46] | |||||||||||
| Zambia, | [198] | |||||||||||
| Zanzibar | [196] | |||||||||||
| R. felis | NA | NA | 1 | 1 | 1 | 1 | Cameroon, | 16S | [149] | |||
| Ethiopia, | [216] | |||||||||||
| R. bellii | NA | NA | NA | NA | 1 | 1 | Ethiopia, | 16S | [216] | |||
| Wolbachia | Ca. Wolbachia ivorensis | 1 | 1 | NA | NA | NA | Ivory_Coast, | TtAna | [140] | |||
| Spirochaetaceae | Borrelia | Ca. Borrelia africana | 1 | 1 | NA | NA | NA | NA | Ivory_Coast, | Bor ITS4 | [140] | |
| Ca. Borrelia ivorensis | 1 | 1 | NA | NA | NA | NA | Ivory_Coast, | Bor ITS4 | [140] | |||
| B. burgdorferi | 1 | 0 | NA | NA | NA | NA | [174] | |||||
| B. theileri | 1 | 1 | 1 | 1 | NA | NA | Cameroon, | 18S, flaB | [149] | |||
| Mali, | [217] | |||||||||||
| Borrelia spp. | 5 | 3 | 2 | 1 | 2 | 1 | Angola, | 16S, flaB, Bor ITS4 | [175] | |||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [212,216] | |||||||||||
| Ivory_Coast, | [140] | |||||||||||
| Madagascar, | [218] | |||||||||||
| Tanzania, | [178] | |||||||||||
| Uganda | [95] | |||||||||||
| B. crocidurae | NA | NA | NA | NA | 1 | 1 | Senegal, | glpQ | [35] | |||
| Parasite | Babesidae | Babesia | B. bigemina | 8 | 7 | 24 | 23 | NA | NA | Angola, | 16S, 18S, bs1, ITS1, ITS2, ama1, cytb, rap1a, speI_avaI | [175] |
| Benin, | [16,24,43,68,148,157] | |||||||||||
| Burkina_Faso, | [16,24,148,219] | |||||||||||
| Ethiopia, | [77,78,148,158] | |||||||||||
| Ghana, | [148,159] | |||||||||||
| Guinea, | [160] | |||||||||||
| Kenya, | [150,151,161,162,220] | |||||||||||
| Lesitho, | [221] | |||||||||||
| Malawi, | [143] | |||||||||||
| Nigeria, | [148,152,185] | |||||||||||
| South_Africa, | [144,167,222,223,224] | |||||||||||
| Sudan, | [168] | |||||||||||
| Tanzania, | [148,169,170] | |||||||||||
| Uganda, | [95,122,148,156,171,172] | |||||||||||
| Zambia, | [145] | |||||||||||
| B. bovis | 4 | 3 | 21 | 14 | 1 | NA | Benin, | 16S, 18S, BoF2, cytb, rap1, sbp2, sbp4 | [16,24,43,68,148,157] | |||
| Burkina_Faso, | [16,24,148,219] | |||||||||||
| Ethiopia, | [77,78,148] | |||||||||||
| Ghana, | [148,225,226] | |||||||||||
| Kenya, | [161,220] | |||||||||||
| Lesitho, | [221] | |||||||||||
| Mozambique, | [227] | |||||||||||
| Nigeria, | [148,152] | |||||||||||
| South_Africa, | [167,173,222,223,224,227] | |||||||||||
| Sudan, | [168] | |||||||||||
| Tanzania, | [148,169,170] | |||||||||||
| Uganda, | [95,148,171,172] | |||||||||||
| Zambia, | [145] | |||||||||||
| B. caballi | 2 | 2 | 1 | 1 | NA | NA | Ethiopia, | 18S | [188] | |||
| South_Africa, | [144] | |||||||||||
| Zambia, | [145] | |||||||||||
| B. divergens | NA | NA | 1 | 0 | 1 | NA | Ghana | 18S | [225] | |||
| B. microti | 1 | 1 | NA | NA | NA | NA | Uganda, | 18S | [156] | |||
| B. motasi | 1 | 1 | NA | NA | NA | NA | Lesitho, | NA | [221] | |||
| B. occultans | 1 | 1 | 1 | 1 | NA | NA | Burkina_Faso, | 18S | [219] | |||
| South_Africa, | [144] | |||||||||||
| B. ovis | 1 | 1 | 1 | 0 | NA | NA | Kenya, | NA | [79] | |||
| Lesitho, | [221] | |||||||||||
| B. rossi | 1 | 1 | NA | NA | NA | NA | Uganda, | 18S | [156] | |||
| Babesia sp. sable | 1 | 1 | 1 | 1 | NA | NA | South_Africa, | 18S | [144] | |||
| Zambia, | [145] | |||||||||||
| Babesia spp. | 2 | 2 | 2 | 0 | NA | NA | Angola, | 18S, ITS1, ITS2 | [175] | |||
| Kenya, | [141] | |||||||||||
| Sudan, | [228] | |||||||||||
| Tanzania, | [178] | |||||||||||
| B. gibsoni | NA | NA | 2 | 2 | NA | NA | Malawi, | 18S | [76] | |||
| Zambia, | [145] | |||||||||||
| B. canis | NA | NA | NA | NA | 1 | NA | Ghana | 18S | [225] | |||
| Babesia sp. mymensingh | NA | NA | 1 | 1 | NA | NA | Uganda, | ama1 | [171,172] | |||
| Theileridae | Theileria | T. orientalis | 2 | 2 | 7 | 4 | NA | NA | Benin, | 18S, mpsp | [157] | |
| Burkina_Faso, | [219] | |||||||||||
| Ethiopia, | [77,158,188,229,230] | |||||||||||
| Kenya, | [161] | |||||||||||
| South_Africa, | [231] | |||||||||||
| T. velifera | 5 | 5 | 14 | 14 | NA | NA | Benin, | 16S, 18S | [16,24] | |||
| Burkina_Faso, | [16,24,219] | |||||||||||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [77,158,188,229] | |||||||||||
| Kenya, | [141,150,151,161,162] | |||||||||||
| Malawi, | [143] | |||||||||||
| Mozambique, | [84] | |||||||||||
| Nigeria, | [152] | |||||||||||
| South_Africa, | [231] | |||||||||||
| Sudan, | [232] | |||||||||||
| Uganda, | [122,156] | |||||||||||
| Zambia, | [145] | |||||||||||
| T. annulata | 5 | 3 | 7 | 5 | NA | NA | Benin, | 16S, 18S, tams1 | [16,24,43,68,157] | |||
| Burkina_Faso, | [16,24,219] | |||||||||||
| Ethiopia, | [77,229] | |||||||||||
| Guinea, | [160] | |||||||||||
| Nigeria, | [233] | |||||||||||
| South_Africa, | [144] | |||||||||||
| Sudan, | [228,232,234] | |||||||||||
| T. bicornis | 1 | 1 | NA | NA | NA | NA | South_Africa, | 18S | [144] | |||
| T. buffeli | 1 | 1 | 1 | 1 | NA | NA | South_Africa, | 18S | [144] | |||
| Zambia, | [145] | |||||||||||
| T. mutans | 6 | 6 | 19 | 19 | NA | NA | Benin, | 16S, 18S | [16,24,43,68,157] | |||
| Burkina_Faso, | [25,26,107] | |||||||||||
| Cameroon, | [149,235] | |||||||||||
| Ethiopia, | [77,78,158,188,229,236] | |||||||||||
| Kenya, | [141,150,151,161] | |||||||||||
| Malawi, | [76,143] | |||||||||||
| Nigeria, | [152] | |||||||||||
| South_Africa, | [144,167] | |||||||||||
| Sudan, | [232] | |||||||||||
| Tanzania, | [170] | |||||||||||
| Uganda, | [122,156] | |||||||||||
| Zambia, | [145] | |||||||||||
| T. ovis | 2 | 1 | 7 | 7 | NA | NA | Ethiopia, | 18S | [229] | |||
| Kenya, | [161] | |||||||||||
| Lesitho, | [221] | |||||||||||
| Malawi, | [76] | |||||||||||
| South_Africa, | [144,173] | |||||||||||
| Sudan, | [228,237] | |||||||||||
| Tanzania, | [170] | |||||||||||
| T. parva | 2 | 2 | 32 | 30 | NA | NA | Benin, | 18S, p104, COI | [148,157] | |||
| Burkina_Faso, | [148,219] | |||||||||||
| Burundi, | [238,239] | |||||||||||
| Cameroon, | [235] | |||||||||||
| Congo, | [240] | |||||||||||
| Ethiopia, | [77,78,148] | |||||||||||
| Ghana, | [148] | |||||||||||
| Guinea, | [160] | |||||||||||
| Kenya, | [150,151,161,162] | |||||||||||
| Lesitho, | [221] | |||||||||||
| Malawi, | [143] | |||||||||||
| Nigeria, | [148] | |||||||||||
| Rwanda, | [241] | |||||||||||
| South_Africa, | [167,242] | |||||||||||
| Sudan, | [243] | |||||||||||
| Tanzania, | [148,169,170,244,245,246,247] | |||||||||||
| Uganda, | [95,122,148,156,171,172,248,249,250,251,252] | |||||||||||
| Zambia, | [145,253] | |||||||||||
| T. separata | 2 | 2 | 4 | 4 | NA | NA | Ethiopia, | 18S | [229] | |||
| Malawi, | [76] | |||||||||||
| South_Africa, | [144] | |||||||||||
| Sudan, | [228,237] | |||||||||||
| Uganda, | [156] | |||||||||||
| T. taurotragi | 2 | 2 | 12 | 10 | NA | NA | Benin, | 18S | [157] | |||
| Burkina_Faso, | [219] | |||||||||||
| Ethiopia, | [77,78] | |||||||||||
| Kenya, | [150,151,161] | |||||||||||
| Malawi, | [143] | |||||||||||
| Nigeria, | [152] | |||||||||||
| South_Africa, | [144,167] | |||||||||||
| Tanzania, | [170,254] | |||||||||||
| Uganda, | [122] | |||||||||||
| Zambia, | [145] | |||||||||||
| T. equi | NA | NA | 1 | 1 | NA | NA | Zambia, | 18S | [145] | |||
| T. lestoquardi | NA | NA | 4 | 4 | NA | NA | Sudan, | 18S, msp | [228,234,237,255] | |||
| Theileria sp. Buffalo | NA | NA | 3 | 3 | NA | NA | Kenya, | 18S | [161] | |||
| South_Africa, | [242] | |||||||||||
| Zambia, | [145] | |||||||||||
| Theileria sp. Kudu | 1 | 1 | 1 | 1 | NA | NA | South_Africa, | 18S | [144] | |||
| Zambia, | [145] | |||||||||||
| Theileria sp. Sable | 1 | 1 | 1 | 1 | NA | NA | South_Africa, | 18S | [144] | |||
| Zambia, | [145] | |||||||||||
| Theileria spp. | 5 | 4 | 10 | 10 | NA | NA | Agola, | 18S, cytb | [175] | |||
| Cameroon, | [149] | |||||||||||
| Ethiopia, | [229] | |||||||||||
| Ghana, | [159] | |||||||||||
| Kenya, | [79,141,161] | |||||||||||
| Malawi, | [76] | |||||||||||
| Nigeria, | [256] | |||||||||||
| South_Africa, | [144,231] | |||||||||||
| Tanzania, | [170,178] | |||||||||||
| Uganda, | [95,156] | |||||||||||
| Theileridae/ Babesidae | Theileria/ Babesia | Theileria/Babesia spp. | 1 | 1 | 5 | 4 | 1 | Cameroon, | 18S | [149] | ||
| Ghana, | [225] | |||||||||||
| Nigeria, | [176,179] | |||||||||||
| South_Africa, | [144] | |||||||||||
| Sudan, | [232] | |||||||||||
| Virus | Alphaviridae | Alphavirus | Alphavirus | 1 | 0 | 1 | 0 | NA | NA | Guinea, | L_segment | [257] |
| Kenya | [150,151] | |||||||||||
| Bunyaviridae | Phlebovirus | Balambala tick virus | 1 | 1 | NA | NA | NA | NA | Ghana, | L_segment | [258] | |
| BDTPV | 1 | 1 | NA | NA | NA | NA | Kenya, | RdRp | [259] | |||
| BOGV | 1 | 1 | NA | NA | NA | NA | Kenya, | RdRp | [259] | |||
| Bole tick virus | 1 | 1 | NA | NA | NA | NA | Kenya, | RdRp | [259] | |||
| PERV | 1 | 1 | NA | NA | NA | NA | Kenya, | RdRp | [259] | |||
| Phlebovirus | 4 | 1 | 1 | 0 | NA | NA | Burkina_Faso, | RdRp, N_segment, S_segment | [260] | |||
| Ghana, | [261] | |||||||||||
| Guinea, | [257] | |||||||||||
| Kenya, | [150,151,259] | |||||||||||
| Phlebovirus DSP4 | 1 | 1 | NA | NA | NA | NA | Kenya, | RdRp | [259] | |||
| Rift Valley Fever Virus | 1 | 0 | NA | NA | NA | NA | Burkina_Faso | G2 | [260] | |||
| Shibuyunji virus | 1 | 1 | NA | NA | NA | NA | Zambia, | L_segment | [262] | |||
| Flaviridae | Flavivirus | Flavivirus | 2 | 0 | 1 | 0 | NA | NA | Burkina_Faso, | NS5, L_segment | [260] | |
| Guinea, | [257] | |||||||||||
| Kenya | [150,151] | |||||||||||
| JMTV | 1 | 1 | NA | NA | NA | NA | Kenya, | NS5 | [263] | |||
| Nairoviridae | Nairovirus | Nairovirus | 1 | 1 | 1 | 0 | NA | NA | Ghana, | L_segment | [261] | |
| Kenya | [150,151] | |||||||||||
| Orthonairovirus | Orthonairovirus | 1 | 0 | NA | NA | NA | NA | Guinea | NA | [257] | ||
| CCHFV | 7 | 6 | 1 | 0 | 3 | 0 | Burkina_Faso, Cameroon, Kenya, Mauritania, Senegal, South_Africa, Uganda, Zambia, | L_segment, N_segment, S_segment | [260] [264] [31] [265] [32,33] [266] [267] | |||
| DUGV | 4 | 4 | NA | NA | NA | NA | Cameroon, | L_segment, S_segment | [264] | |||
| Ghana, | [258,261] | |||||||||||
| Nigeria, | [268] | |||||||||||
| Peribunyaviridae | Orthobunyavirus | Ngari virus | 1 | 1 | NA | NA | NA | NA | Guinea, | NgvS | [257] | |
| Orthobunyavirus | 1 | 1 | 1 | 0 | NA | NA | Guinea, | S_segment | [257] | |||
| Kenya | [150,151] | |||||||||||
| Poxyviridae | Parapoxvirus | Parapoxvirus | 1 | 1 | NA | NA | NA | NA | Burkina_Faso, | B2L/J6R | [260] | |
| BPSV | 1 | 1 | NA | NA | NA | NA | Burkina_Faso, | BPSV_J6R | [260] | |||
| PCPV | 1 | 1 | NA | NA | NA | NA | Burkina_Faso, | PCPV_J6R | [260] | |||
| Orthopoxvirus | Orthopoxvirus | 1 | 0 | NA | NA | NA | NA | Burkina_Faso, | HA(J7R) | [260] | ||
| Togaviridae | Orbivirus | Orbivirus | 1 | 0 | NA | NA | NA | NA | Guinea | NA | [257] | |
| KPTV | NA | NA | 1 | 1 | NA | NA | Kenya | segment 2 | [269] | |||
| Thogotovirus | Dhori virus | NA | NA | 1 | 0 | NA | NA | Kenya | S_segment | [150,151] | ||
| Thogotovirus | NA | NA | 1 | 0 | NA | NA | Keny | M_segment | [150,151] | |||
References
- Muhammad, A.; Piyumali, K.P.; Abdullah, I.; Shumaila, M. Ticks and Tick-Borne Pathogens. In Ticks and Tick-Borne pathogens; IntechOpen.: London, UK, 2018; Volume 9, pp. 3–9. ISBN 1-78985-765-1. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The Global Importance of Ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.B.; Sarma, K.; Saravanan, M. Upcoming of the Integrated Tick Control Program of Ruminants with Special Emphasis on Livestock Farming System in India. Ticks Tick-Borne Dis. 2013, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Fuente, J.D.L. Overview: Ticks as Vectors of Pathogens That Cause Disease in Humans and Animals. Front. Biosci. 2008, 6938. [Google Scholar] [CrossRef] [PubMed]
- Chitanga, S.; Gaff, H.; Mukaratirwa, S. Tick-Borne Pathogens of Potential Zoonotic Importance in the Southern African Region. J. South Afr. Vet. Assoc. 2014, 85, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; De La Fuente, J. The Ecology of Ticks and Epidemiology of Tick-Borne Viral Diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial Nature of Tick-Borne Diseases. Mbio 2019, 10, e02055-19. [Google Scholar] [CrossRef] [PubMed]
- Burn, L.; Tran, T.M.P.; Pilz, A.; Vyse, A.; Fletcher, M.A.; Angulo, F.J.; Gessner, B.D.; Moïsi, J.C.; Jodar, L.; Stark, J.H. Incidence of Lyme Borreliosis in Europe from National Surveillance Systems (2005-2020). Vector Borne Zoonotic Dis. 2023, 23, 156–171. [Google Scholar] [CrossRef] [PubMed]
- CDC Lyme Disease Surveillance and Data. Available online: https://www.cdc.gov/lyme/data-research/facts-stats/index.html (accessed on 8 August 2024).
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel Thogotovirus Associated with Febrile Illness and Death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Godsey, M.S.; Lambert, A.; Panella, N.A.; Burkhalter, K.L.; Harmon, J.R.; Lash, R.R.; Ashley, D.C.; Nicholson, W.L. First Detection of Heartland Virus (Bunyaviridae: Phlebovirus) from Field Collected Arthropods. Am. J. Trop. Med. Hyg. 2013, 89, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- Al-Abri, S.S.; Abaidani, I.A.; Fazlalipour, M.; Mostafavi, E.; Leblebicioglu, H.; Pshenichnaya, N.; Memish, Z.A.; Hewson, R.; Petersen, E.; Mala, P.; et al. Current Status of Crimean-Congo Haemorrhagic Fever in the World Health Organization Eastern Mediterranean Region: Issues, Challenges, and Future Directions. Int J. Infect. Dis. 2017, 58, 82–89. [Google Scholar] [CrossRef]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A Study of Ticks and Tick-Borne Livestock Pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, e0005681. [Google Scholar] [CrossRef] [PubMed]
- Kivaria, F.M. Climate Change and the Epidemiology of Tick-Borne Diseases of Cattle in Africa. Vet. J. 2010, 184, 7–8. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Zannou, O.M.; Biguezoton, A.S.; Yao, K.P.; Belem, A.M.G.; Farougou, S.; Oosthuizen, M.; Saegerman, C.; Lempereur, L. Cross Border Transhumance Involvement in Ticks and Tick-Borne Pathogens Dissemination and First Evidence of Anaplasma centrale in Burkina Faso. Ticks Tick-Borne Dis. 2021, 12, 101781. [Google Scholar] [CrossRef]
- Zannou, O.M.; Ouedraogo, A.S.; Biguezoton, A.S.; Lempereur, L.; Patrick Yao, K.; Abatih, E.; Zoungrana, S.; Lenaert, M.; Toe, P.; Farougou, S.; et al. First Digital Characterization of the Transhumance Corridors through Benin Used by Cattle Herds from Burkina Faso and Associated Risk Scoring Regarding the Invasion of Rhipicephalus (Boophilus) Microplus. Transbound. Emerg. Dis. 2021, 68, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Diatta, G.; Zolia, Y.; Balde, M.C.; Kohar, H.; Trape, J.-F.; Raoult, D. Tick-Borne Rickettsiae in Guinea and Liberia. Ticks Tick-Borne Dis. 2012, 3, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Biguezoton, A.S. Invasion Biologique & Écologie De La Santé Vétérinaire: Le Cas Des Communautés De Tiques Et Pathogènes Associés Au Bénin Et Au Burkina Faso À L’heure De Leur Invasion Par La Tique Du Bétail Rhipicephalus (Boophilus) Microplus. Ph.D. Thesis, Université Montpellier, Montpellier, France, 2016. [Google Scholar]
- Wanzala, W. Distribution of Ticks and Tick-Borne Pathogens, Hosts, Habitat and Diseases in Kenya and Some Parts of Africa: A Mini Review. J. Anim. Res. Nutr. 2023, 8. [Google Scholar]
- Diarra, A.Z.; Kelly, P.; Davoust, B.; Parola, P. Tick-Borne Diseases of Humans and Animals in West Africa. Pathogens 2023, 12, 1276. [Google Scholar] [CrossRef] [PubMed]
- Farougou, S.; Tassou, A.W.; Tchabode, D.M.; Kpodekon, M.; Boko, C.; Youssao, A.K.I. Tiques et Hémoparasites Du Bétail Dans Le Nord-Bénin. Rev. Méd. Vét. 2007, 158, 463–467. [Google Scholar]
- Ntiamoa-Baidu, Y.; Carr-Saunders, C.; Matthews, B.E.; Preston, P.M.; Walker, A.R. An Updated List of the Ticks of Ghana and an Assessment of the Distribution of the Ticks of Ghanaian Wild Mammals in Different Vegetation Zones. Bull. Entomol. Res. 2004, 94, 245–260. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Zannou, O.M.; Biguezoton, A.S.; Kouassi, P.Y.; Belem, A.; Farougou, S.; Oosthuizen, M.; Saegerman, C.; Lempereur, L. Cattle Ticks and Associated Tick-Borne Pathogens in Burkina Faso and Benin: Apparent Northern Spread of Rhipicephalus microplus in Benin and First Evidence of Theileria velifera and Theileria annulata. Ticks Tick-Borne Dis. 2021, 12, 101733. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, R.; Brandful, J.A.M.; Zayed, A.; Adjei, A.; Watany, N.; Fahmy, N.T.; Hughes, R.; Doman, B.; Voegborlo, S.V.; Aziati, D.; et al. Crimean-Congo Hemorrhagic Fever Virus in Livestock Ticks and Animal Handler Seroprevalence at an Abattoir in Ghana. BMC Infect. Dis. 2016, 16, 324. [Google Scholar] [CrossRef]
- Ogo, N.I.; de Mera, I.G.F.; Galindo, R.C.; Okubanjo, O.O.; Inuwa, H.M.; Agbede, R.I.S.; Torina, A.; Alongi, A.; Vicente, J.; Gortázar, C.; et al. Molecular Identification of Tick-Borne Pathogens in Nigerian Ticks. Vet. Parasitol. 2012, 187, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Vial, H.J.; Gorenflot, A. Chemotherapy against Babesiosis. Vet. Parasitol. 2006, 138, 147–160. [Google Scholar] [CrossRef]
- Burimuah, V.; Sylverken, A.; Owusu, M.; El-Duah, P.; Yeboah, R.; Lamptey, J.; Frimpong, Y.O.; Agbenyega, O.; Folitse, R.; Tasiame, W.; et al. Sero-Prevalence, Cross-Species Infection and Serological Determinants of Prevalence of Bovine Coronavirus in Cattle, Sheep and Goats in Ghana. Vet. Microbiol. 2020, 241, 108544. [Google Scholar] [CrossRef] [PubMed]
- Cossu, C.A.; Collins, N.E.; Oosthuizen, M.C.; Menandro, M.L.; Bhoora, R.V.; Vorster, I.; Cassini, R.; Stoltsz, H.; Quan, M.; van Heerden, H. Distribution and Prevalence of Anaplasmataceae, Rickettsiaceae and Coxiellaceae in African Ticks: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Chiuya, T.; Villinger, J.; Falzon, L.C.; Alumasa, L.; Amanya, F.; Bastos, A.D.S.; Fèvre, E.M.; Masiga, D.K. Molecular Screening Reveals Non-Uniform Malaria Transmission in Western Kenya and Absence of Rickettsia africae and Selected Arboviruses in Hospital Patients. Malar. J. 2022, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Mhamadi, M.; Badji, A.; Dieng, I.; Gaye, A.; Ndiaye, E.H.; Ndiaye, M.; Mhamadi, M.; Touré, C.T.; Mbaye, M.R.; Barry, M.A.; et al. Crimean—Congo Hemorrhagic Fever Virus Survey in Humans, Ticks, and Livestock in Agnam (Northeastern Senegal) from February 2021 to March 2022. Trop. Med. Infect. Dis. 2022, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Diallo, M.; et al. Concurrent Malaria and Arbovirus Infections in Kedougou, Southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef]
- Kolo, A.O.; Collins, N.E.; Brayton, K.A.; Chaisi, M.; Blumberg, L.; Frean, J.; Gall, C.A.; Wentzel, J.M.; Wills-Berriman, S.; De Boni, L.; et al. Anaplasma phagocytophilum and Other Anaplasma spp. In Various Hosts in the Mnisi Community, Mpumalanga Province, South Africa. Microorganisms 2020, 8, 1812. [Google Scholar] [CrossRef] [PubMed]
- El Hadji Ibrahima, N.; Diatta, G.; Adama Zan, D.; Bassene, H.; Sokhna, C.; Parola, P. Quantitative Polymerase Chain Reaction from Malaria Rapid Diagnostic Tests to Detect Borrelia Crocidurae, the Agent of Tick-Borne Relapsing Fever, in Febrile Patients in Senegal. Am. J. Trop. Med. Hyg. 2023, 108, 968–976. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 January 2024).
- Gouda, H.N.; Charlson, F.; Sorsdahl, K.; Ahmadzada, S.; Ferrari, A.J.; Erskine, H.; Leung, J.; Santamauro, D.; Lund, C.; Aminde, L.N.; et al. Burden of Non-Communicable Diseases in Sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Glob. Health 2019, 7, e1375–e1387. [Google Scholar] [CrossRef] [PubMed]
- Happold, D.C.D. The Interactions between Humans and Mammals in Africa in Relation to Conservation: A Review. Biodivers. Conserv. 1995, 4, 395–414. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Kwak, M.L.; Nonaka, N.; Nakao, R. Human-Biting Ticks and Zoonotic Tick-Borne Pathogens in North Africa: Diversity, Distribution, and Trans-Mediterranean Public Health Challenges. One Health 2023, 16, 100547. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Lotric-Furlan, S.; Korva, M.; Avsic-Zupanc, T. African Tick-Bite Fever in Traveler Returning to Slovenia from Uganda. Emerg. Infect. Dis. 2016, 22, 1848–1849. [Google Scholar] [CrossRef] [PubMed]
- Ledwaba, M.B.; Nozipho, K.; Tembe, D.; Onyiche, T.E.; Chaisi, M.E. Distribution and Prevalence of Ticks and Tick-Borne Pathogens of Wild Animals in South Africa: A Systematic Review. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100088. [Google Scholar] [CrossRef]
- Onyiche, T.E.; MacLeod, E.T. Hard Ticks (Acari: Ixodidae) and Tick-Borne Diseases of Sheep and Goats in Africa: A Review. Ticks Tick-Borne Dis. 2023, 14, 102232. [Google Scholar] [CrossRef] [PubMed]
- Adjou Moumouni, P.F.; Terkawi, M.A.; Jirapattharasate, C.; Cao, S.; Liu, M.; Nakao, R.; Umemiya-Shirafuji, R.; Yokoyama, N.; Sugimoto, C.; Fujisaki, K.; et al. Molecular Detection of Spotted Fever Group Rickettsiae in Amblyomma variegatum Ticks from Benin. Ticks Tick-Borne Dis. 2016, 7, 828–833. [Google Scholar] [CrossRef]
- Diseko, L.J.; Tsotetsi-Khambule, A.M.; Onyiche, T.E.; Ramatla, T.; Thekisoe, O.; Gcebe, N. Coxiella burnetii Infections from Animals and Ticks in South Africa: A Systematic Review. Vet. Res. Commun. 2024, 48, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mangena, M.; Gcebe, N.; Pierneef, R.; Thompson, P.N.; Adesiyun, A.A. Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa. Pathogens 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Qiu, Y.; Igarashi, M.; Magona, J.W.; Zhou, L.; Ito, K.; Sugimoto, C. High Prevalence of Spotted Fever Group Rickettsiae in Amblyomma variegatum from Uganda and Their Identification Using Sizes of Intergenic Spacers. Ticks Tick-Borne Dis. 2013, 4, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Sears, K.; Dinkel, K.D.; Knowles, D.P.; Fry, L.M. Changes in the Molecular and Functional Phenotype of Bovine Monocytes during Theileria parva Infection. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Mutai, B.; Njaanake, K.; Gathii, K.; Estambale, B.B.; Waitumbi, J.N. Bacteriome in Ticks Collected from Domestic Livestock in Kenya. AiM 2022, 12, 67–82. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Tick-Borne Infections of Animals and Humans: A Common Ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kasi, K.K.; Arnim, F.; Schulz, A.; Rehman, A.; Chudhary, A.; Oneeb, M.; Sas, M.A.; Jamil, T.; Maksimov, P.; Sauter-Louis, C.; et al. Crimean-Congo Haemorrhagic Fever Virus in Ticks Collected from Livestock in Balochistan, Pakistan. Transbound. Emerg. Dis. 2020, 67, 1543–1552. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Ragiadakou, D.; Kouris, G.; Papadopoulos, B.; Chaniotis, B.; Tselentis, Y. Ticks, Tick-Borne Rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann. New York Acad. Sci. 2006, 1078, 389–399. [Google Scholar] [CrossRef]
- Cowdry, E.V. Studies on the Etiology of Heartwater. J. Exp. Med. 1925, 42, 253–274. [Google Scholar] [CrossRef]
- Hurtado, O.J.B.; Giraldo-Ríos, C. Economic and Health Impact of the Ticks in Production Animals. In Ticks and Tick-Borne pathogens; Abubakar, M., Perera, P.K., Eds.; IntechOpen: London, UK, 2018; Volume 9, pp. 133–151. ISBN 1-78985-765-1. [Google Scholar]
- Allsopp, M.T.E.P.; Van Strijp, M.F.; Faber, E.; Josemans, A.I.; Allsopp, B.A. Ehrlichia ruminantium Variants Which Do Not Cause Heartwater Found in South Africa. Vet. Microbiol. 2007, 120, 158–166. [Google Scholar] [CrossRef]
- Some, M.V.; Biguezoton, A.S.; Githaka, N.; Adakal, H.; Dayo, G.-K.; Belem, A.; Zoungrana, S.; Stachurski, F.; Chevillon, C. The Potential of Rhipicephalus micropluss as a Vector of Ehrlichia ruminantium in West Africa. Ticks Tick-Borne Dis. 2023, 14, 102117. [Google Scholar] [CrossRef] [PubMed]
- Vilela, V.L.R.; Feitosa, T.F.; Bezerra, R.A.; Klafke, G.M.; Riet-Correa, F. Multiple Acaricide-Resistant Rhipicephalus microplus in the Semi-Arid Region of Paraíba State, Brazil. Ticks Tick-Borne Dis. 2020, 11, 101413. [Google Scholar] [CrossRef]
- Yessinou, R.E.; Akpo, Y.; Ossè, R.; Adoligbe, C.; Cassini, R.; Akogbeto, M.; Farougou, S. Molecular Characterization of Pyrethroids Resistance Mechanisms in Field Populations of Rhipicephalus microplus (Acari: Ixodidae) in District of Kpinnou and Opkara, Benin. Int. J. Acarol. 2018, 44, 198–203. [Google Scholar] [CrossRef]
- Louw, M.; Allsopp, M.; Meyer, E.C.; Wasserman, E. Ehrlichia ruminantium, an Emerging Human Pathogen-a Further Report. South Afr. Med. J. 2005, 95, 948–950. [Google Scholar] [CrossRef]
- Allsopp, B.A. Natural History of Ehrlichia ruminantium. Vet. Parasitol. 2010, 167, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gajadhar, A.A.; Lobanov, V.; Scandrett, W.B.; Campbell, J.; Al-Adhami, B. A Novel Ehrlichia Genotype Detected in Naturally Infected Cattle in North America. Vet. Parasitol. 2010, 173, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, H.; Collins, N.E.; Brayton, K.A.; Rademeyer, C.; Allsopp, B.A. Characterization of a Major Outer Membrane Protein Multigene Family in Ehrlichia ruminantium. Gene 2004, 330, 159–168. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The Natural History of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef]
- Spare, M.R.; Hanzlicek, G.A.; Wootten, K.L.; Anderson, G.A.; Thomson, D.U.; Sanderson, M.W.; Ganta, R.R.; Reif, K.E.; Raghavan, R.K. Bovine Anaplasmosis Herd Prevalence and Management Practices as Risk-Factors Associated with Herd Disease Status. Vet. Parasitol. 2020, 277, 100021. [Google Scholar] [CrossRef] [PubMed]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic Diversity and Molecular Epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- De Waal, D.T. Anaplasmosis Control and Diagnosis in South Africa. Ann. New York Acad. Sci. 2000, 916, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Adjou Moumouni, P.F.; Guo, H.; Gao, Y.; Liu, M.; Ringo, A.E.; Galon, E.M.; Vudriko, P.; Umemiya-Shirafuji, R.; Inoue, N.; Suzuki, H.; et al. Identification and Genetic Characterization of Piroplasmida and Anaplasmataceae Agents in Feeding Amblyomma variegatum Ticks from Benin. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Teshale, S.; Geysen, D.; Ameni, G.; Dorny, P.; Berkvens, D. Survey of Anaplasma phagocytophilum and Anaplasma spp. “Omatjenne” Infection in Cattle in Africa with Special Reference to Ethiopia. Parasit Vectors 2018, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Pothmann, D.; Poppert, S.; Rakotozandrindrainy, R.; Hogan, B.; Mastropaolo, M.; Thiel, C.; Silaghi, C. Prevalence and Genetic Characterization of Anaplasma marginale in Zebu Cattle (Bos indicus) and Their Ticks (Amblyomma variegatum, Rhipicephalus microplus) from Madagascar. Ticks Tick-Borne Dis. 2016, 7, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Teshale, S.; Geysen, D.; Ameni, G.; Asfaw, Y.; Berkvens, D. Improved Molecular Detection of Ehrlichia and Anaplasma Species Applied to Amblyomma Ticks Collected from Cattle and Sheep in Ethiopia. Ticks Tick-Borne Dis. 2015, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Abichu, G.; Takács, N.; Gyuranecz, M.; Farkas, R.; Fernández De Mera, I.G.; De La Fuente, J. Molecular Screening for Anaplasmataceae in Ticks and Tsetse Flies from Ethiopia. Acta Vet. Hung. 2016, 64, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hove, P.; Chaisi, M.E.; Brayton, K.A.; Ganesan, H.; Catanese, H.N.; Mtshali, M.S.; Mutshembele, A.M.; Oosthuizen, M.C.; Collins, N.E. Co-Infections with Multiple Genotypes of Anaplasma marginale in Cattle Indicate Pathogen Diversity. Parasites Vectors 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Amaro-Estrada, I.; Taqadus, A.; Swelum, A.A.; Alqhtani, A.H.; Asif, M.; Sajid, M.; Khan, A.U.; Tariq, A.; Anjum, S.; et al. Molecular Prevalence and Associated Risk Factors of Anaplasma Ovis in Pakistani Sheep. Front. Vet. Sci. 2023, 10, 1096418. [Google Scholar] [CrossRef]
- Berthelsson, J.; Ramabu, S.S.; Lysholm, S.; Aspán, A.; Wensman, J.J. Anaplasma Ovis Infection in Goat Flocks around Gaborone, Botswana. Comp. Clin. Pathol. 2020, 29, 167–172. [Google Scholar] [CrossRef]
- Chatanga, E.; Kainga, H.; Razemba, T.; Ssuna, R.; Swennen, L.; Hayashida, K.; Sugimoto, C.; Katakura, K.; Nonaka, N.; Nakao, R. Molecular Detection and Characterization of Tick-Borne Hemoparasites and Anaplasmataceae in Dogs in Major Cities of Malawi. Parasitol. Res. 2021, 120, 267–276. [Google Scholar] [CrossRef]
- Ringo, A.E.; Rizk, M.A.; Adjou Moumouni, P.F.; Liu, M.; Galon, E.M.; Li, Y.; Ji, S.; Tumwebaze, M.; Byamukama, B.; Thekisoe, O.; et al. Molecular Detection and Characterization of Tick-Borne Haemoparasites among Cattle on Zanzibar Island, Tanzania. Acta Trop. 2020, 211, 105598. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Nonga, H.E.; Galon, E.M.; Ji, S.; Rizk, M.A.; El-Sayed, S.A.E.-S.; Mohanta, U.K.; Ma, Z.; Chikufenji, B.; Do, T.T.; et al. Molecular Investigation of Tick-Borne Haemoparasites Isolated from Indigenous Zebu Cattle in the Tanga Region, Tanzania. Animals 2022, 12, 3171. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Aboge, G.O.; Adjou Moumouni, P.F.; Hun Lee, S.; Jirapattharasate, C.; Liu, M.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; et al. Molecular Detection and Genetic Characterisation of Pathogenic Theileria, Anaplasma and Ehrlichia Species among Apparently Healthy Sheep in Central and Western Kenya. Onderstepoort J. Vet. Res. 2019, 86, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, M.; Davoust, B.; Sambou, M.; Bassene, H.; Scandola, P.; Ameur, T.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular Investigation and Phylogeny of Species of the Anaplasmataceae Infecting Animals and Ticks in Senegal. Parasit Vectors 2019, 12, 495. [Google Scholar] [CrossRef]
- Djiba, M.L.; Mediannikov, O.; Mbengue, M.; Thiongane, Y.; Molez, J.-F.; Seck, M.T.; Fenollar, F.; Raoult, D.; Ndiaye, M. Survey of Anaplasmataceae Bacteria in Sheep from Senegal. Trop. Anim. Health Prod. 2013, 45, 1557–1561. [Google Scholar] [CrossRef]
- Aouadi, A.; Leulmi, H.; Boucheikhchoukh, M.; Benakhla, A.; Raoult, D.; Parola, P. Molecular Evidence of Tick-Borne Hemoprotozoan-Parasites (Theileria ovis and Babesia ovis) and Bacteria in Ticks and Blood from Small Ruminants in Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Belkahia, H.; Ben Said, M.; Ghribi, R.; Selmi, R.; Ben Asker, A.; Yahiaoui, M.; Bousrih, M.; Daaloul-Jedidi, M.; Messadi, L. Molecular Detection, Genotyping and Phylogeny of Anaplasma spp. in Rhipicephalus Ticks from Tunisia. Acta Trop. 2019, 191, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Matsimbe, A.M.; Magaia, V.; Sanches, G.S.; Neves, L.; Noormahomed, E.; Antunes, S.; Domingos, A. Molecular Detection of Pathogens in Ticks Infesting Cattle in Nampula Province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91–102. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Vasić, A.; Schäfer, M.; Biu, A.A.; Ogo, N.I.; Thekisoe, O.; Silaghi, C. Prevalence and Molecular Characterization of Ticks and Tick-Borne Pathogens of One-Humped Camels (Camelus dromedarius) in Nigeria. Parasit Vectors 2020, 13, 428. [Google Scholar] [CrossRef]
- Oundo, J.W.; Villinger, J.; Jeneby, M.; Ong’amo, G.; Otiende, M.Y.; Makhulu, E.E.; Musa, A.A.; Ouso, D.O.; Wambua, L. Pathogens, Endosymbionts, and Blood-Meal Sources of Host-Seeking Ticks in the Fast-Changing Maasai Mara Wildlife Ecosystem. PLoS ONE 2020, 15, e0228366. [Google Scholar] [CrossRef] [PubMed]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular Identification of Protozoal and Bacterial Organisms in Domestic Animals and Their Infesting Ticks from North-Eastern Algeria. Ticks Tick-Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef] [PubMed]
- Teshale, S.; Kumsa, B.; Menandro, M.L.; Cassini, R.; Martini, M. Anaplasma, Ehrlichia and Rickettsial Pathogens in Ixodid Ticks Infesting Cattle and Sheep in Western Oromia, Ethiopia. Exp. Appl. Acarol. 2016, 70, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Omondi, D.; Masiga, D.K.; Fielding, B.C.; Kariuki, E.; Ajamma, Y.U.; Mwamuye, M.M.; Ouso, D.O.; Villinger, J. Molecular Detection of Tick-Borne Pathogen Diversities in Ticks from Livestock and Reptiles along the Shores and Adjacent Islands of Lake Victoria and Lake Baringo, Kenya. Front. Vet. Sci. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine Babesiosis: An Insight into the Global Perspective on the Disease Distribution by Systematic Review and Meta-Analysis. Veterinary Parasitology 2020, 283, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Jackson, L.; Vos, A.D.; Jorgensen, W. Babesiosis of Cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; George, J.C.; Hansmann, Y.; Degeilh, B.; Joncour, G.; Jourdain, E.; Malandrin, L.; Umhang, G.; Vayssier-Taussat, M.; Vial, L.; et al. Principales Maladies Transmises Par Les Tiques: Épidémiologie, Clinique et Diagnostic. In Tiques Et Maladies à Tiques: Biologie, Écologie Évolutive, Épidémiologie; Actiques: Marseille, France, 2015; pp. 193–237. ISBN 978-2-7099-2100-8. [Google Scholar]
- Gharbi, M.; Darghouth, M.A.; Elati, K.; AL-Hosary, A.A.T.; Ayadi, O.; Salih, D.A.; El Hussein, A.M.; Mhadhbi, M.; Khamassi Khbou, M.; Hassan, S.M.; et al. Current Status of Tropical Theileriosis in Northern Africa: A Review of Recent Epidemiological Investigations and Implications for Control. Transbound. Emerg. Dis. 2020, 67, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Nene, V.; Kiara, H.; Lacasta, A.; Pelle, R.; Svitek, N.; Steinaa, L. The Biology of Theileria parva and Control of East Coast Fever: Current Status and Future Trends. Ticks Tick-Borne Dis. 2016, 7, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Byamukama, B.; Vudriko, P.; Tumwebaze, M.A.; Tayebwa, D.S.; Byaruhanga, J.; Angwe, M.K.; Li, J.; Galon, E.M.; Ringo, A.; Liu, M.; et al. Molecular Detection of Selected Tick-Borne Pathogens Infecting Cattle at the Wildlife–Livestock Interface of Queen Elizabeth National Park in Kasese District, Uganda. Ticks Tick-Borne Dis. 2021, 12, 101772. [Google Scholar] [CrossRef] [PubMed]
- Chaisi, M.E.; Janssens, M.E.; Vermeiren, L.; Oosthuizen, M.C.; Collins, N.E.; Geysen, D. Evaluation of a Real-Time PCR Test for the Detection and Discrimination of Theileria Species in the African Buffalo (Syncerus caffer). PLoS ONE 2013, 8, e75827. [Google Scholar] [CrossRef] [PubMed]
- Theiler, A. Piroplasma mutans (N. Spec.) of South African Cattle. J. Comp. Pathol. Ther. 1906, 19, 292–300. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Thumbi, S.M.; Jennings, A.; Chase-Topping, M.; Callaby, R.; Kiara, H.; Oosthuizen, M.C.; Mbole-Kariuki, M.N.; Conradie, I.; Handel, I.G.; et al. Co-Infections Determine Patterns of Mortality in a Population Exposed to Parasite Infection. Sci. Adv. 2015, 1, e1400026. [Google Scholar] [CrossRef] [PubMed]
- Irvin, A.D.; Brown, C.G.D.; Burridge, M.J.; Cunningham, M.P.; Musoke, A.J.; Pierce, M.A.; Purnell, R.E.; Radley, D.E. A Pathogenic Theilerial Syndrome of Cattle in the Narok District of Kenya. Trop. Anim. Health Prod. 1972, 4, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.; Pedersen, V.; Odeke, G.M.; Kamya, E.P.; Brown, C.G.D. East Coast Fever Immunisation Trials in Uganda: Field Exposure of Zebu Cattle Immunized with Three Isolates of Theileria parva. Trop. Anim. Health Prod. 1977, 9, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, D.R.; Trees, A.J.; Bowyer, W.A.; Bergman, J.R.; Daft, J.; Wall, A.E. East Coast Fever: Field Challenge of Cattle Immunised against Theileria parva (Muguga). Trop. Anim. Health Prod. 1972, 4, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The Role of Ticks in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus: A Review of Published Field and Laboratory Studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Beati, L.; Mason, P.R.; Matthewman, L.A.; Roux, V.; Raoult, D. Rickettsia africae spp. Nov., the Etiological Agent of African Tick Bite Fever. Int. J. Syst. Bacteriol. 1996, 46, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Jensenius, M.; Fournier, P.-E.; Vene, S.; Hoel, T.; Hasle, G.; Henriksen, A.Z.; Hellum, K.B.; Raoult, D.; Myrvang, B. Norwegian African Tick Bite Fever Study Group African Tick Bite Fever in Travelers to Rural Sub-Equatorial Africa. Clin. Infect. Dis. 2003, 36, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Mazhetese, E.; Lukanji, Z.; Byaruhanga, C.; Neves, L.; Morar-Leather, D. Rickettsia africae Infection Rates and Transovarial Transmission in Amblyomma hebraeum Ticks in Mnisi, Bushbuckridge, South Africa. Exp. Appl. Acarol. 2022, 86, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Mason, P.R.; Manning, T.; Slater, S. Role of Cattle in the Epidemiology of Tick-Bite Fever in Zimbabwe. J. Clin. Microbiol. 1991, 29, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum: A Widespread Multi-Host Pathogen with Highly Adaptive Strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Franzo, G.; Martini, M.; Mondin, A.; Cassini, R.; Drigo, M.; Pasotto, D.; Vidorin, E.; Menandro, M.L. Ecotyping of Anaplasma phagocytophilum from Wild Ungulates and Ticks Shows Circulation of Zoonotic Strains in Northeastern Italy. Animals 2021, 11, 310. [Google Scholar] [CrossRef]
- Hamidinejat, H.; Jallali, M.; Bahrami, S.; Bagheri, M. First Molecular Survey of Anaplasma phagocytophilum in Hard Ticks (Ixodidae) from Southwestern Iran. J. Vector. Borne Dis. 2021, 58, 115. [Google Scholar] [CrossRef]
- Severo, M.S.; Stephens, K.D.; Kotsyfakis, M.; Pedra, J.H. Anaplasma phagocytophilum: Deceptively Simple or Simply Deceptive? Future Microbiol. 2012, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Altay, K.; Erol, U.; Sahin, O.F. Anaplasma capra: A New Emerging Tick-Borne Zoonotic Pathogen. Vet. Res. Commun. 2024, 48, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Y.-C.; Ma, L.; Jia, N.; Jiang, B.-G.; Jiang, R.-R.; Huo, Q.-B.; Wang, Y.-W.; Liu, H.-B.; Chu, Y.-L. Human Infection with a Novel Tick-Borne Anaplasma Species in China: A Surveillance Study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Pfäffle, M.P.; Petney, T.N. Genus Dermacentor Koch, 1844. In Ticks of Europe and North Africa: A Guide to Species Identification; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 279–280. ISBN 978-3-319-63760-0. [Google Scholar]
- Walker, A.; Bouattour, A.; Camicas, J.L.; Estrada-Peña, A.; Horak, I.; Latif, A.; Pegram, R.G.; Preston, P.M. Species of Ticks. In Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, Scotland, 2014; pp. 46–221. ISBN 0-9545173-0-X. [Google Scholar]
- Estrada-Peña, A.; Pfäffle, M.P.; Petney, T.N. Genus Ixodes Latreille, 1795. In Ticks of Europe and North Africa: A Guide to Species Identification; Estrada-Peña, A., Mihalca, A.D., Petney, T.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 79–90. ISBN 978-3-319-63760-0. [Google Scholar]
- Lilak, A.A.; Pecor, D.B.; Matulis, G.; Potter, A.M.; Wofford, R.N.; Kearney, M.F.; Mitchell, S.; Jaradat, F.; Kano, A.; Zimmerman, D.M.; et al. Data Release: Targeted Systematic Literature Search for Tick and Tick-Borne Pathogen Distributions in Six Countries in Sub-Saharan Africa from 1901 to 2020. Parasites Vectors 2024, 17, 84. [Google Scholar] [CrossRef]
- Woldehiwet, Z. The Natural History of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Hojgaard, A.; Foster, E.; Maes, S.E.; Osikowicz, L.M.; Parise, C.M.; Villalpando, J.; Eisen, R.J. Geographic Variation in the Distribution of Anaplasma phagocytophilum Variants in Host-Seeking Ixodes Scapularis Nymphs and Adults in the Eastern United States Elucidated Using next Generation Sequencing. Ticks Tick-Borne Dis. 2024, 15, 102360. [Google Scholar] [CrossRef] [PubMed]
- Nováková, M.; Šmajs, D.; Nováková, M.; Šmajs, D. Rickettsial Endosymbionts of Ticks. In Ticks and Tick-Borne Pathogens; IntechOpen: London, UK, 2018; pp. 81–94. ISBN 978-1-78985-766-5. [Google Scholar]
- Iweriebor, B.C.; Mmbaga, E.J.; Adegborioye, A.; Igwaran, A.; Obi, L.C.; Okoh, A.I. Genetic Profiling for Anaplasma and Ehrlichia Species in Ticks Collected in the Eastern Cape Province of South Africa. BMC Microbiol. 2017, 17, 45. [Google Scholar] [CrossRef]
- Vanegas, A.; Keller, C.; Krüger, A.; Manchang, T.K.; Hagen, R.M.; Frickmann, H.; Veit, A.; Achukwi, M.D.; Krücken, J.; Poppert, S. Molecular Detection of Spotted Fever Group Rickettsiae in Ticks from Cameroon. Ticks Tick-Borne Dis. 2018, 9, 1049–1056. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.E.; Knobel, D.; Chaisi, M.E.; Vorster, I.; Steyn, H.C.; Oosthuizen, M.C. Molecular Investigation of Tick-Borne Haemoparasite Infections among Transhumant Zebu Cattle in Karamoja Region, Uganda. Vet. Parasitol. Reg. Stud. Rep. 2016, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rollins, R.E.; Schaper, S.; Kahlhofer, C.; Frangoulidis, D.; Strauß, A.F.T.; Cardinale, M.; Springer, A.; Strube, C.; Bakkes, D.K.; Becker, N.S.; et al. Ticks (Acari: Ixodidae) on Birds Migrating to the Island of Ponza, Italy, and the Tick-Borne Pathogens They Carry. Ticks Tick-Borne Dis. 2021, 12, 101590. [Google Scholar] [CrossRef] [PubMed]
- Tsapko, N.V. Importation of Hyalomma rufipes Koch, 1844, Vectors of Crimean-Congo Haemorrhagic Fever Virus to the South Russia by Migratory Birds: Epidemiological Aspect. Russ. J. Biol. Invasions 2022, 13, 264–269. [Google Scholar] [CrossRef]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.-E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted Fever Rickettsia Species in Hyalomma and Ixodes Ticks Infesting Migratory Birds in the European Mediterranean Area. Parasites Vectors 2014, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, I.; Di Domenico, M.; Capobianco Dondona, G.; Di Gennaro, A.; Polci, A.; Capobianco Dondona, A.; Mancuso, E.; Cammà, C.; Savini, G.; Cecere, J.G.; et al. Assessing the Role of Migratory Birds in the Introduction of Ticks and Tick-Borne Pathogens from African Countries: An Italian Experience. Ticks Tick-Borne Dis. 2019, 10, 101272. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G.C. Importation of Hyalomma Marginatum, Vector of Crimean-Congo Haemorrhagic Fever Virus, into the United Kingdom by Migratory Birds. Ticks Tick-Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, E.; Toma, L.; Pascucci, I.; d’Alessio, S.G.; Marini, V.; Quaglia, M.; Riello, S.; Ferri, A.; Spina, F.; Serra, L.; et al. Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy. Pathogens 2022, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Yessinou, R.E.; Adehan, S.; Hedegbetan, G.C.; Cassini, R.; Mantip, S.E.; Farougou, S. Molecular Characterization of Rickettsia spp., Bartonella spp., and Anaplasma phagocytophilum in Hard Ticks Collected from Wild Animals in Benin, West Africa. Trop. Anim. Health Prod. 2022, 54, 306. [Google Scholar] [CrossRef] [PubMed]
- Madder, M.; Adehan, S.; De Deken, R.; Adehan, R.; Lokossou, R. New Foci of Rhipicephalus microplus in West Africa. Exp. Appl. Acarol. 2012, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Biguezoton, A.; Adehan, S.; Adakal, H.; Zoungrana, S.; Farougou, S.; Chevillon, C. Community Structure, Seasonal Variations and Interactions between Native and Invasive Cattle Tick Species in Benin and Burkina Faso. Parasites Vectors 2016, 9, 43. [Google Scholar] [CrossRef]
- Biguezoton, A.; Noel, V.; Adehan, S.; Adakal, H.; Dayo, G.-K.; Zoungrana, S.; Farougou, S.; Chevillon, C. Ehrlichia ruminantium Infects Rhipicephalus microplus in West Africa. Parasites Vectors 2016, 9, 354. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Emerging Technologies for the Clinical Microbiology Laboratory. Clin. Microbiol. Rev. 2014, 27, 783–822. [Google Scholar] [CrossRef]
- Goldberg, B.; Sichtig, H.; Geyer, C.; Ledeboer, N.; Weinstock, G.M. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. Mbio 2015, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, L.; Chai, Y.; Xu, J. Advantages and Challenges of Metagenomic Sequencing for the Diagnosis of Pulmonary Infectious Diseases. Clin. Respir. J. 2022, 16, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vicente, S.; Jain, K.; Tagliafierro, T.; Gokden, A.; Kapoor, V.; Guo, C.; Horn, E.J.; Lipkin, W.I.; Tokarz, R. Capture Sequencing Enables Sensitive Detection of Tick-Borne Agents in Human Blood. Front. Microbiol. 2022, 13, 837621. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.-L.; Alexander, D.; Chen, J.C.-Y.; Adam, H.; Walker, M.; Ali, J.; Forbes, J.; Taboada, E.; Barker, D.O.R.; Graham, M.; et al. Clinical Metagenomics Is Increasingly Accurate and Affordable to Detect Enteric Bacterial Pathogens in Stool. Microorganisms 2022, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Cangi, N.; Pinarello, V.; Bournez, L.; Lefrançois, T.; Albina, E.; Neves, L.; Vachiéry, N. Efficient High-Throughput Molecular Method to Detect Ehrlichia ruminantium in Ticks. Parasites Vectors 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Vayssier-Taussat, M.; Greub, G. Tick-Borne Pathogen Detection: What’s New? Microbes Infect. 2018, 20, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; Kacou N’Douba, A.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef] [PubMed]
- Okal, M.N.; Odhiambo, B.K.; Otieno, P.; Bargul, J.L.; Masiga, D.; Villinger, J.; Kalayou, S. Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for pro-Active Surveillance in the Wildlife–Livestock Interface. Microorganisms 2020, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.G.; Aboge, G.O.; Kariuki, H.W.; Kanduma, E.G.; Gakuya, D.W.; Maingi, N.; Mulei, C.M.; Mainga, A.O. Molecular Prevalence of Emerging Anaplasma and Ehrlichia Pathogens in Apparently Healthy Dairy Cattle in Peri-Urban Nairobi, Kenya. BMC Vet. Res. 2020, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Chatanga, E.; Maganga, E.; Mohamed, W.M.A.; Ogata, S.; Pandey, G.S.; Abdelbaset, A.E.; Hayashida, K.; Sugimoto, C.; Katakura, K.; Nonaka, N.; et al. High Infection Rate of Tick-Borne Protozoan and Rickettsial Pathogens of Cattle in Malawi and the Development of a Multiplex PCR for Babesia and Theileria Species Identification. Acta Trop. 2022, 231, 106413. [Google Scholar] [CrossRef] [PubMed]
- Berggoetz, M.; Schmid, M.; Ston, D.; Wyss, V.; Chevillon, C.; Pretorius, A.-M.; Gern, L. Protozoan and Bacterial Pathogens in Tick Salivary Glands in Wild and Domestic Animal Environments in South Africa. Ticks Tick-Borne Dis. 2014, 5, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Tembo, S.; Collins, N.E.; Sibeko-Matjila, K.P.; Troskie, M.; Vorster, I.; Byaruhanga, C.; Oosthuizen, M.C. Occurrence of Tick-Borne Haemoparasites in Cattle in the Mungwi District, Northern Province, Zambia. Ticks Tick-Borne Dis. 2018, 9, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Addo, S.O.; Baako, B.O.A.; Bentil, R.E.; Addae, C.A.; Behene, E.; Asoala, V.; Sallam, M.; Mate, S.; Dunford, J.C.; Larbi, J.A.; et al. Molecular Survey of Anaplasma and Ehrlichia Species in Livestock Ticks from Kassena-Nankana, Ghana; with a First Report of Anaplasma capra and Ehrlichia minasensis. Arch Microbiol. 2023, 205, 92. [Google Scholar] [CrossRef] [PubMed]
- Addo, S.O.; Bentil, R.E.; Baako, B.O.A.; Yartey, K.N.; Behene, E.; Asiamah, B.; Nyarko, A.A.; Asoala, V.; Sallam, M.; Mate, S.; et al. Occurrence of Rickettsia spp. and Coxiella burnetii in Ixodid Ticks in Kassena-Nankana, Ghana. Exp. Appl. Acarol. 2023, 90, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.J.A.; Kumsa, B.; Kimbita, E.; Frank, M.N.; Muhanguzi, D.; Jongejan, F.; Adehan, S.B.; Toure, A.; Aboagye-Antwi, F.; Ogo, N.I.; et al. Tick-Borne Pathogens and Body Condition of Cattle in Smallholder Rural Livestock Production Systems in East and West Africa. Parasites Vectors 2023, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Abanda, B.; Paguem, A.; Abdoulmoumini, M.; Kingsley, M.T.; Renz, A.; Eisenbarth, A. Molecular Identification and Prevalence of Tick-Borne Pathogens in Zebu and Taurine Cattle in North Cameroon. Parasit Vectors 2019, 12, 448. [Google Scholar] [CrossRef]
- Chiuya, T.; Villinger, J.; Masiga, D.K.; Ondifu, D.O.; Murungi, M.K.; Wambua, L.; Bastos, A.D.S.; Fèvre, E.M.; Falzon, L.C. Molecular Prevalence and Risk Factors Associated with Tick-Borne Pathogens in Cattle in Western Kenya. BMC Vet. Res. 2021, 17, 363. [Google Scholar] [CrossRef] [PubMed]
- Chiuya, T.; Masiga, D.K.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-Borne Pathogens, Including Crimean-Congo Haemorrhagic Fever Virus, at Livestock Markets and Slaughterhouses in Western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-Borne Pathogens of Zoonotic and Veterinary Importance in Nigerian Cattle. Parasites Vectors 2016, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Chaisi, M.E.; Baxter, J.R.; Hove, P.; Choopa, C.N.; Oosthuizen, M.C.; Brayton, K.A.; Khumalo, Z.T.H.; Mutshembele, A.M.; Mtshali, M.S.; Collins, N.E. Comparison of Three Nucleic Acid-Based Tests for Detecting Anaplasma marginale and Anaplasma centrale in Cattle. Onderstepoort J. Vet. Res. 2017, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eisawi, N.M.; El Hussein, A.R.M.; Hassan, D.A.; Musa, A.B.; Hussien, M.O.; Enan, K.A.; Bakheit, M.A. A Molecular Prevalence Survey on Anaplasma Infection among Domestic Ruminants in Khartoum State, Sudan. Trop. Anim. Health Prod. 2020, 52, 1845–1852. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.E.; Knobel, D.L.; Khumalo, Z.T.H.; Chaisi, M.E.; Oosthuizen, M.C. Molecular Detection and Phylogenetic Analysis of Anaplasma marginale and Anaplasma centrale amongst Transhumant Cattle in North-Eastern Uganda. Ticks Tick-Borne Dis. 2018, 9, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Byaruhanga, C.; Akure, P.C.; Lubembe, D.M.; Sibeko-Matjila, K.; Troskie, M.; Oosthuizen, M.C.; Stoltsz, H. Molecular Detection and Characterisation of Protozoan and Rickettsial Pathogens in Ticks from Cattle in the Pastoral Area of Karamoja, Uganda. Ticks Tick-Borne Dis. 2021, 12, 101709. [Google Scholar] [CrossRef] [PubMed]
- Adjou Moumouni, P.F.; Aplogan, G.L.; Katahira, H.; Gao, Y.; Guo, H.; Efstratiou, A.; Jirapattharasate, C.; Wang, G.; Liu, M.; Ringo, A.E.; et al. Prevalence, Risk Factors, and Genetic Diversity of Veterinary Important Tick-Borne Pathogens in Cattle from Rhipicephalus microplus-Invaded and Non-Invaded Areas of Benin. Ticks Tick-borne Dis. 2018, 9, 450–464. [Google Scholar] [CrossRef]
- Hailemariam, Z.; Krücken, J.; Baumann, M.; Ahmed, J.S.; Clausen, P.-H.; Nijhof, A.M. Molecular Detection of Tick-Borne Pathogens in Cattle from Southwestern Ethiopia. PLoS ONE 2017, 12, e0188248. [Google Scholar] [CrossRef]
- Beckley, C.S.; Shaban, S.; Palmer, G.H.; Hudak, A.T.; Noh, S.M.; Futse, J.E. Disaggregating Tropical Disease Prevalence by Climatic and Vegetative Zones within Tropical West Africa. PLoS ONE 2016, 11, e0152560. [Google Scholar] [CrossRef] [PubMed]
- Makenov, M.T.; Toure, A.H.; Korneev, M.G.; Sacko, N.; Porshakov, A.M.; Yakovlev, S.A.; Radyuk, E.V.; Zakharov, K.S.; Shipovalov, A.V.; Boumbaly, S.; et al. Rhipicephalus microplus and Its Vector-Borne Haemoparasites in Guinea: Further Species Expansion in West Africa. Parasitol. Res. 2021, 120, 1563–1570. [Google Scholar] [CrossRef]
- Adjou Moumouni, P.F.; Aboge, G.O.; Terkawi, M.A.; Masatani, T.; Cao, S.; Kamyingkird, K.; Jirapattharasate, C.; Zhou, M.; Wang, G.; Liu, M.; et al. Molecular Detection and Characterization of Babesia bovis, Babesia bigemina, Theileria Species and Anaplasma marginale Isolated from Cattle in Kenya. Parasit Vectors 2015, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Oundo, J.W.; Masiga, D.; ten Bosch, Q.; Villinger, J.; Koenraadt, C.J.M.; Kalayou, S. Epidemiology of Tick-Borne Pathogens of Cattle and Tick Control Practices in Coastal Kenya. Prev. Vet. Med. 2022, 209, 105777. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.D.J.; Matos, C.A.; Freschi, C.R.; de Souza Ramos, I.A.; Machado, R.Z.; André, M.R. Diversity of Anaplasma Species in Cattle in Mozambique. Ticks Tick-Borne Dis. 2019, 10, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Elelu, N.; Ferrolho, J.; Couto, J.; Domingos, A.; Eisler, M.C. Molecular Diagnosis of the Tick-Borne Pathogen Anaplasma marginale in Cattle Blood Samples from Nigeria Using qPCR. Exp. Appl. Acarol. 2016, 70, 501–510. [Google Scholar] [CrossRef]
- Kamani, J.; Schaer, J.; Umar, A.G.; Pilarshimwi, J.Y.; Bukar, L.; González-Miguel, J.; Harrus, S. Molecular Detection and Genetic Characterization of Anaplasma marginale and Anaplasma Platys in Cattle in Nigeria. Ticks Tick-Borne Dis. 2022, 13, 101955. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Irene, S.; Qasim, A.M.M.M.; Olubade, T.A.; Abasiama, M.S.; Gajibo, A.; Balami, P.U.; Shands, M.; Harrus, S. Nucleotide Sequence Types (ntSTs) of Anaplasma marginale in Cattle in Nigeria Based on the Major Surface Protein 5 (Msp5) Gene. Acta Trop. 2022, 233, 106544. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Adjou Moumouni, P.F.; Thekisoe, O.; Gao, Y.; Liu, M.; Li, J.; Galon, E.M.; Efstratiou, A.; Wang, G.; Jirapattharasate, C.; et al. Genetic Characterization of Tick-Borne Pathogens in Ticks Infesting Cattle and Sheep from Three South African Provinces. Ticks Tick-Borne Dis. 2019, 10, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Satti, R.A.; Awadelkareem, E.A.; Suganuma, K.; Salim, B.; Inoue, N.; Xuan, X.; Rehan, S.; Mossaad, E. Cattle Anaplasmosis and Babesiosis: Major Tick-Borne Diseases Affecting the Cattle Industry in Khartoum State, Sudan. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100632. [Google Scholar] [CrossRef]
- Haji, I.; Simuunza, M.; Kerario, I.I.; Jiang, N.; Chen, Q. Epidemiology of Tick-Borne Pathogens of Cattle and Tick Control Practices among Mixed Farming and Pastoral Communities in Gairo and Monduli Districts, Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2022, 32, 100738. [Google Scholar] [CrossRef]
- Ringo, A.E.; Adjou Moumouni, P.F.; Lee, S.-H.; Liu, M.; Khamis, Y.H.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; Galon, E.M.; et al. Molecular Detection and Characterization of Tick-Borne Protozoan and Rickettsial Pathogens Isolated from Cattle on Pemba Island, Tanzania. Ticks Tick-Borne Dis. 2018, 9, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Tayebwa, D.S.; Vudriko, P.; Tuvshintulga, B.; Guswanto, A.; Nugraha, A.B.; Gantuya, S.; Batiha, G.E.-S.; Musinguzi, S.P.; Komugisha, M.; Bbira, J.S.; et al. Molecular Epidemiology of Babesia Species, Theileria parva, and Anaplasma marginale Infecting Cattle and the Tick Control Malpractices in Central and Eastern Uganda. Ticks Tick-Borne Dis. 2018, 9, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Tuvshintulga, B.; Kothalawala, H.; Silva, S.S.P.; Lan, D.T.B.; Long, P.T.; Ybañez, A.P.; Ybañez, R.H.D.; Benitez, D.F.; Tayebwa, D.S.; et al. Host Range and Geographical Distribution of Babesia Sp. Mymensingh. Transbound. Emerg. Dis. 2020, 67, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Adjou Moumouni, P.F.; Taioe, M.; Jirapattharasate, C.; Liu, M.; Wang, G.; Gao, Y.; Guo, H.; Lee, S.-H.; Zheng, W.; et al. Molecular Analysis of Tick-Borne Protozoan and Rickettsial Pathogens in Small Ruminants from Two South African Provinces. Parasitol. Int. 2018, 67, 144–149. [Google Scholar] [CrossRef]
- Mtshali, K.; Khumalo, Z.T.H.; Nakao, R.; Grab, D.J.; Sugimoto, C.; Thekisoe, O.M.M. Molecular Detection of Zoonotic Tick-Borne Pathogens from Ticks Collected from Ruminants in Four South African Provinces. J. Vet. Med. Sci. 2016, 77, 1573–1579. [Google Scholar] [CrossRef]
- Palomar, A.M.; Molina, I.; Bocanegra, C.; Portillo, A.; Salvador, F.; Moreno, M.; Oteo, J.A. Old Zoonotic Agents and Novel Variants of Tick-Borne Microorganisms from Benguela (Angola), July 2017. Parasit Vectors 2022, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Mofokeng, L.S.; Thekisoe, O.; MacLeod, E.T. Molecular Survey for Tick-Borne Pathogens and Associated Risk Factors in Sheep and Goats in Kano Metropolis, Nigeria. Vet. Parasitol. Reg. Stud. Rep. 2022, 33, 100753. [Google Scholar] [CrossRef] [PubMed]
- Ramabu, S.S.; Kgwatalala, P.M.; Nsoso, S.J.; Gasebonwe, S.; Kgosiesele, E. Anaplasma Infection Prevalence in Beef and Dairy Cattle in the South East Region of Botswana. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 4–8. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kwak, Y.S.; Kim, J.Y.; Nam, S.-H.; Lee, I.-Y.; Mduma, S.; Keyyu, J.; Fyumagwa, R.; Yong, T.-S. Prevalence of Tick-Borne Pathogens from Ticks Collected from Cattle and Wild Animals in Tanzania in 2012. Korean J. Parasitol. 2018, 56, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Happi, A.N.; Osifade, O.; Oluniyi, P.E.; Ogunro, B.N. Comparison of Light Microscopy and Polymerase Chain Reaction for the Detection of Haemoparasites in Cattle in Nigeria. Acta Parasitol. 2020, 65, 44–56. [Google Scholar] [CrossRef]
- Adelabu, O.A.; Iweriebor, B.C.; Okoh, A.I.; Obi, L.C. Phylogenetic Profiling for Zoonotic Ehrlichia spp. from Ixodid Ticks in the Eastern Cape, South Africa. Transbound. Emerg. Dis. 2020, 67, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Esemu, S.N.; Besong, W.O.; Ndip, R.N.; Ndip, L.M. Prevalence of Ehrlichia ruminantium in Adult Amblyomma variegatum Collected from Cattle in Cameroon. Exp. Appl. Acarol. 2013, 59, 377–387. [Google Scholar] [CrossRef]
- Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Detection of Ehrlichia ruminantium Infection in Cattle in Cameroon. BMC Res. Notes 2018, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.A.; Gonçalves, L.R.; de Souza Ramos, I.A.; Mendes, N.S.; Zanatto, D.C.S.; André, M.R.; Machado, R.Z. Molecular Detection and Characterization of Ehrlichia ruminantium from Cattle in Mozambique. Acta Trop. 2019, 191, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Anifowose, O.I.; Takeet, M.I.; Talabi, A.O.; Otesile, E.B. Molecular Detection of Ehrlichia ruminantium in Engorged Amblyomma variegatum and Cattle in Ogun State, Nigeria. J. Parasit. Dis. 2020, 44, 403–410. [Google Scholar] [CrossRef]
- Hector, E.; Elelu, N.; Ferrolho, J.; Couto, J.; Sanches, G.; Antunes, S.; Domingos, A.; Eisler, M. PCR Detection of Ehrlichia ruminantium and Babesia bigemina in Cattle from Kwara State, Nigeria: Unexpected Absence of Infection. Parasitol. Res. 2019, 118, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Berger, L.; Busser, S.; Deetman, I.; Jochems, M.; Leenders, T.; De Sitter, B.; Van Der Steen, F.; Wentzel, J.; Stoltsz, H. Amblyomma hebraeum Is the Predominant Tick Species on Goats in the Mnisi Community Area of Mpumalanga Province South Africa and Is Co-Infected with Ehrlichia ruminantium and Rickettsia africae. Parasites Vectors 2020, 13, 172. [Google Scholar] [CrossRef]
- Steyn, H.C.; Pretorius, A. Genetic Diversity of Ehrlichia ruminantium Field Strain from Selected Farms in South Africa. Onderstepoort J. Vet. Res. 2020, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Abichu, G.; Meli, M.L.; Tánczos, B.; Sulyok, K.M.; Gyuranecz, M.; Gönczi, E.; Farkas, R.; Hofmann-Lehmann, R. Influence of the Biotope on the Tick Infestation of Cattle and on the Tick-Borne Pathogen Repertoire of Cattle Ticks in Ethiopia. PLoS ONE 2014, 9, e106452. [Google Scholar] [CrossRef] [PubMed]
- Kumsa, B.; Socolovschi, C.; Almeras, L.; Raoult, D.; Parola, P. Occurrence and Genotyping of Coxiella burnetii in Ixodid Ticks in Oromia, Ethiopia. Am. J. Trop. Med. Hyg. 2015, 93, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Koka, H.; Sang, R.; Kutima, H.L.; Musila, L. Coxiella burnetii Detected in Tick Samples from Pastoral Communities in Kenya. Biomed Res. Int. 2018, 2018, 8158102. [Google Scholar] [CrossRef] [PubMed]
- Boone, I.; Henning, K.; Hilbert, A.; Neubauer, H.; von Kalckreuth, V.; Dekker, D.M.; Schwarz, N.G.; Pak, G.D.; Krüger, A.; Hagen, R.M.; et al. Are Brucellosis, Q Fever and Melioidosis Potential Causes of Febrile Illness in Madagascar? Acta Trop. 2017, 172, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hsi, T.-E.; Hsiao, S.-W.; Minahan, N.T.; Yen, T.-Y.; de Assunção Carvalho, A.V.; Raoult, D.; Fournier, P.-E.; Tsai, K.-H. Seroepidemiological and Molecular Investigation of Spotted Fever Group Rickettsiae and Coxiella burnetii in Sao Tome Island: A One Health Approach. Transbound. Emerg. Dis. 2020, 67 (Suppl. S2), 36–43. [Google Scholar] [CrossRef]
- Ratmanov, P.; Bassene, H.; Fenollar, F.; Tall, A.; Sokhna, C.; Raoult, D.; Mediannikov, O. The Correlation of Q Fever and Coxiella burnetii DNA in Household Environments in Rural Senegal. Vector Borne Zoonotic Dis. 2013, 13, 70–72. [Google Scholar] [CrossRef]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Luus-Powell, W.J.; Oteo, J.A. Investigation of Rickettsia, Coxiella burnetii and Bartonella in Ticks from Animals in South Africa. Ticks Tick-Borne Dis. 2016, 7, 361–366. [Google Scholar] [CrossRef]
- Sadiki, V.; Gcebe, N.; Mangena, M.L.; Ngoshe, Y.B.; Adesiyun, A.A. Prevalence and Risk Factors of Q Fever (Coxiella burnetii) in Cattle on Farms of Limpopo Province, South Africa. Front. Vet. Sci. 2023, 10, 1101988. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; James, O.C.; Mohamed, A.A.; Joachim, A.; Mubi, M.; Omodior, O. Etiologic Agents of Fever of Unknown Origin Among Patients Attending Mnazi Mmoja Hospital, Zanzibar. J. Commun. Health 2020, 45, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Chigwada, A.D.; Mapholi, N.O.; Ogola, H.J.O.; Mbizeni, S.; Masebe, T.M. Pathogenic and Endosymbiotic Bacteria and Their Associated Antibiotic Resistance Biomarkers in Amblyomma and Hyalomma Ticks Infesting Nguni Cattle (Bos spp.). Pathogens 2022, 11, 432. [Google Scholar] [CrossRef]
- Chitanga, S.; Chambaro, H.M.; Moonga, L.C.; Hayashida, K.; Yamagishi, J.; Muleya, W.; Changula, K.; Mubemba, B.; Simbotwe, M.; Squarre, D.; et al. Rickettsia Lusitaniae in Ornithodoros Porcinus Ticks, Zambia. Pathogens 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; De Meneghi, D.; Adakal, H.; Rodighiero, P.; Pressi, G.; Grego, E. Detection of Rickettsia Aeschlimannii and Rickettsia africae in Ixodid Ticks from Burkina Faso and Somali Region of Ethiopia by New Real-Time PCR Assays. Ticks Tick-Borne Dis. 2016, 7, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Socolovschi, C.; Kernif, T.; Temmam, S.; Lagadec, E.; Tortosa, P.; Parola, P. First Molecular Detection of Rickettsia africae in Ticks from the Union of the Comoros. Parasites Vectors 2014, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Jiang, J.; Maina, A.; Dueger, E.; Zayed, A.; Ahmed, A.A.; Pimentel, G.; Richards, A.L. Evidence of Rickettsia and Orientia Infections among Abattoir Workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Tufa, T.B.; Wölfel, S.; Zubriková, D.; Víchová, B.; Andersson, M.; Rieß, R.; Rutaihwa, L.; Fuchs, A.; Orth, H.M.; Häussinger, D.; et al. Tick Species from Cattle in the Adama Region of Ethiopia and Pathogens Detected. Exp. Appl. Acarol. 2021, 84, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Koka, H.; Sang, R.; Kutima, H.L.; Musila, L. The Detection of Spotted Fever Group Rickettsia DNA in Tick Samples From Pastoral Communities in Kenya. J. Med. Entomol. 2017, 54, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Krüger, A.; Schwarz, N.G.; Rakotozandrindrainy, R.; Rakotondrainiarivelo, J.P.; Razafindrabe, T.; Derschum, H.; Silaghi, C.; Pothmann, D.; Veit, A.; et al. High Detection Rate of Rickettsia africae in Amblyomma variegatum but Low Prevalence of Anti-Rickettsial Antibodies in Healthy Pregnant Women in Madagascar. Ticks Tick-Borne Dis. 2016, 7, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Magaia, V.; Taviani, E.; Cangi, N.; Neves, L. Molecular Detection of Rickettsia africae in Amblyomma Ticks Collected in Cattle from Southern and Central Mozambique. J. Infect. Dev. Ctries. 2020, 14, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Nnabuife, H.E.; Matur, B.; Ogo, N.I.; Goselle, O.; Shittu, I.; Mkpuma, N.; Obishakin, E.; Chima, N.; Kamani, J. Rickettsia africae and Rickettsia Massiliae in Ixodid Ticks Infesting Small Ruminants in Agro-Pastoral Settlements in Plateau State, Nigeria. Exp. Appl. Acarol. 2023, 89, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Kisten, D.; Brinkerhoff, J.; Tshilwane, S.I.; Mukaratirwa, S. A Pilot Study on the Microbiome of Amblyomma hebraeum Tick Stages Infected and Non-Infected with Rickettsia africae. Pathogens 2021, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Pillay, A.; Nyangiwe, N.; Mukaratirwa, S. Low Genetic Diversity and Population Structuring of Amblyomma hebraeum and Rickettsia africae from Coastal and Inland Regions in the Eastern Cape Province of South Africa. Med. Vet. Entomol. 2023, 37, 275–285. [Google Scholar] [CrossRef]
- Nakao, R.; Qiu, Y.; Salim, B.; Hassan, S.M.; Sugimoto, C. Molecular Detection of Rickettsia africae in Amblyomma variegatum Collected from Sudan. Vector Borne Zoonotic Dis. 2015, 15, 323–325. [Google Scholar] [CrossRef]
- Elelu, N.; Ola-Fadunsin, S.D.; Bankole, A.A.; Raji, M.A.; Ogo, N.I.; Cutler, S.J. Prevalence of Tick Infestation and Molecular Characterization of Spotted Fever Rickettsia Massiliae in Rhipicephalus Species Parasitizing Domestic Small Ruminants in North-Central Nigeria. PLoS ONE 2022, 17, e0263843. [Google Scholar] [CrossRef]
- Yessinou, R.E.; Cazan, C.D.; Panait, L.C.; Mollong, E.; Biguezoton, A.S.; Bonnet, S.I.; Farougou, S.; Groschup, M.H.; Mihalca, A.D. New Geographical Records for Tick-Borne Pathogens in Ticks Collected from Cattle in Benin and Togo. Vet. Med. Sci. 2023, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Abdissa, A.; Socolovschi, C.; Diatta, G.; Trape, J.-F.; Raoult, D. Detection of a New Borrelia Species in Ticks Taken from Cattle in Southwest Ethiopia. Vector-Borne Zoonotic Dis. 2013, 13, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Jiang, J.; Omulo, S.A.; Cutler, S.J.; Ade, F.; Ogola, E.; Feikin, D.R.; Njenga, M.K.; Cleaveland, S.; Mpoke, S.; et al. High Prevalence of Rickettsia africae Variants in Amblyomma variegatum Ticks from Domestic Mammals in Rural Western Kenya: Implications for Human Health. Vector-Borne Zoonotic Dis. 2014, 14, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Iweriebor, B.C.; Igwaran, A.; Adegborioye, A.A.; Mmbaga, E.J.; Okoh, A.I.; Obi, L.C. Molecular Screening of Ticks for the Presence of Rickettsia Species: A Public Health Concern. Asian Pac. J. Trop. Dis. 2017, 7, 199–204. [Google Scholar] [CrossRef]
- Iweriebor, B.C.; Nqoro, A.; Obi, C.L. Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa. Pathogens 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Pérez-Tanoira, R.; Martín-Martín, I.; Prieto-Pérez, L.; Tefasmariam, A.; Tiziano, G.; Escudero, R.; Gil-Zamorano, J.; Gil-Gil, H.; Górgolas, M.; et al. Arthropod-Borne Bacteria Cause Nonmalarial Fever in Rural Ethiopia: A Cross-Sectional Study in 394 Patients. Vector-Borne Zoonotic Dis. 2019, 19, 815–820. [Google Scholar] [CrossRef] [PubMed]
- McCoy, B.N.; Maïga, O.; Schwan, T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014, 5, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Hagen, R.M.; Frickmann, H.; Ehlers, J.; Krüger, A.; Margos, G.; Hizo-Teufel, C.; Fingerle, V.; Rakotozandrindrainy, R.; Kalckreuth, V.V.; Im, J.; et al. Presence of Borrelia spp. DNA in Ticks, but Absence of Borrelia spp. and of Leptospira spp. DNA in Blood of Fever Patients in Madagascar. Acta Trop. 2018, 177, 127–134. [Google Scholar] [CrossRef]
- Moumouni, P.F.A.; Minoungou, G.L.-B.; Dovonou, C.E.; Galon, E.M.; Efstratiou, A.; Tumwebaze, M.A.; Byamukama, B.; Vudriko, P.; Umemiya-Shirafuji, R.; Suzuki, H.; et al. A Survey of Tick Infestation and Tick-Borne Piroplasm Infection of Cattle in Oudalan and Séno Provinces, Northern Burkina Faso. Pathogens 2022, 11, 31. [Google Scholar] [CrossRef]
- Githaka, N.W.; Bishop, R.P.; Šlapeta, J.; Emery, D.; Nguu, E.K.; Kanduma, E.G. Molecular Survey of Babesia Parasites in Kenya: First Detailed Report on Occurrence of Babesia bovis in Cattle. Parasit Vectors 2022, 15, 161. [Google Scholar] [CrossRef]
- Mahlobo, S.I.; Zishiri, O.T. A Descriptive Study of Parasites Detected in Ticks of Domestic Animals in Lesotho. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100611. [Google Scholar] [CrossRef] [PubMed]
- Ganzinelli, S.; Byaruhanga, C.; Primo, M.E.; Lukanji, Z.; Sibeko, K.; Matjila, T.; Neves, L.; Benitez, D.; Enkhbaatar, B.; Nugraha, A.B.; et al. International Interlaboratory Validation of a Nested PCR for Molecular Detection of Babesia bovis and Babesia bigemina, Causative Agents of Bovine Babesiosis. Vet. Parasitol. 2022, 304, 109686. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, P.S.; Tsotetsi, A.M.; Thekisoe, M.M.O.; Mtshali, M.S. Nested PCR Detection and Phylogenetic Analysis of Babesia bovis and Babesia bigemina in Cattle from Peri-Urban Localities in Gauteng Province, South Africa. J. Vet. Med. Sci. 2014, 76, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, M.S.; Mtshali, P.S. Molecular Diagnosis and Phylogenetic Analysis of Babesia bigemina and Babesia bovis Hemoparasites from Cattle in South Africa. BMC Vet. Res. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Ayeh-Kumi, P.F.; Owusu, I.A.; Tetteh-Quarcoo, P.B.; Dayie, N.T.K.D.; Adutwum-Ofosu, K.K.; Amponsah, S.K.; Udofia, E.A.; Afutu, E.; Attah, S.K.; Armah, R.; et al. Preliminary Investigation into Plasmodium-like Piroplasms (Babesia/Theileria) among Cattle, Dogs and Humans in A Malaria-Endemic, Resource-Limited Sub-Saharan African City. Med. Sci. 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Nagano, D.; Sivakumar, T.; De De Macedo, A.C.C.; Inpankaew, T.; Alhassan, A.; Igarashi, I.; Yokoyama, N. The Genetic Diversity of Merozoite Surface Antigen 1 (MSA-1) among Babesia bovis Detected from Cattle Populations in Thailand, Brazil and Ghana. J. Vet. Med. Sci. 2013, 75, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Byaruhanga, C.; Makgabo, S.; Choopa, C.N.; Mulandane, F.C.; Vorster, I.; Troskie, M.; Chaisi, M.E.; Collins, N.E. Genetic Diversity in Babesia bovis from Southern Africa and Estimation of B. Bovis Infection Levels in Cattle Using an Optimised Quantitative PCR Assay. Ticks Tick-Borne Dis. 2023, 14, 102084. [Google Scholar] [CrossRef] [PubMed]
- El Imam, A.H.; Hassan, S.M.; Gameel, A.A.; El Hussein, A.M.; Taha, K.M.; Oosthuizen, M.C. Molecular Identification of Different Theileria and Babesia Species Infecting Sheep in Sudan. Ann. Parasitol. 2016, 62, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gebrekidan, H.; Hailu, A.; Kassahun, A.; Rohoušová, I.; Maia, C.; Talmi-Frank, D.; Warburg, A.; Baneth, G. Theileria Infection in Domestic Ruminants in Northern Ethiopia. Vet. Parasitol. 2014, 200, 31–38. [Google Scholar] [CrossRef]
- Gebrekidan, H.; Gasser, R.B.; Baneth, G.; Yasur-Landau, D.; Nachum-Biala, Y.; Hailu, A.; Jabbar, A. Molecular Characterization of Theileria Orientalis from Cattle in Ethiopia. Ticks Tick-Borne Dis. 2016, 7, 742–747. [Google Scholar] [CrossRef]
- Iweriebor, B.C.; Afolabi, K.O.; Nqoro, A.; Obi, L.C. Emergence of Theileria Species in Ticks from Free-Ranging Domestic Animals in Raymond Mhlaba Local Municipality, South Africa. Heliyon 2022, 8, e09085. [Google Scholar] [CrossRef]
- Mohamed, S.B.; Alagib, A.; AbdElkareim, T.B.; Hassan, M.M.; Johnson, W.C.; Hussein, H.E.; Taus, N.S.; Ueti, M.W. Molecular Detection and Characterization of Theileria spp. Infecting Cattle in Sennar State, Sudan. Parasitol. Res. 2018, 117, 1271–1276. [Google Scholar] [CrossRef]
- Mamman, A.H.; Lorusso, V.; Adam, B.M.; Dogo, G.A.; Bown, K.J.; Birtles, R.J. First Report of Theileria annulata in Nigeria: Findings from Cattle Ticks in Zamfara and Sokoto States. Parasites Vectors 2021, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Taha, K.M.; Salih, D.A.; Ali, A.M.; Omer, R.A.; El Hussein, A.M. Naturally Occurring Infections of Cattle with Theileria Lestoquardi and Sheep with Theileria annulata in the Sudan. Vet. Parasitol. 2013, 191, 143–145. [Google Scholar] [CrossRef]
- Silatsa, B.A.; Simo, G.; Githaka, N.; Kamga, R.; Oumarou, F.; Keambou Tiambo, C.; Machuka, E.; Domelevo, J.-B.; Odongo, D.; Bishop, R.; et al. First Detection of Theileria parva in Cattle from Cameroon in the Absence of the Main Tick Vector Rhipicephalus appendiculatus. Transbound. Emerg. Dis. 2020, 67, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Grego, E.; Callà, G.; Rodighiero, P.; Pressi, G.; Gebre, S.; Zeleke, B.; De Meneghi, D. Ticks and Tick-Borne Pathogens in Livestock from Nomadic Herds in the Somali Region, Ethiopia. Exp. Appl. Acarol. 2012, 56, 391–401. [Google Scholar] [CrossRef]
- Hassan, S.; Skilton, R.A.; Pelle, R.; Odongo, D.; Bishop, R.P.; Ahmed, J.; Seitzer, U.; Bakheit, M.; Hassan, S.M.; El Hussein, A.M. Assessment of the Prevalence of Theileria Lestoquardi in Sheep from the Sudan Using Serological and Molecular Methods. Prev. Vet. Med. 2019, 169, 104697. [Google Scholar] [CrossRef] [PubMed]
- Atuhaire, D.K.; Muleya, W.; Mbao, V.; Niyongabo, J.; Nyabongo, L.; Nsanganiyumwami, D.; Salt, J.; Namangala, B.; Musoke, A.J. Molecular Characterization and Population Genetics of Theileria parva in Burundi’s Unvaccinated Cattle: Towards the Introduction of East Coast Fever Vaccine. PLoS ONE 2021, 16, e0251500. [Google Scholar] [CrossRef]
- Nyabongo, L.; Kanduma, E.G.; Bishop, R.P.; Machuka, E.; Njeri, A.; Bimenyimana, A.V.; Nkundwanayo, C.; Odongo, D.O.; Pelle, R. Prevalence of Tick-Transmitted Pathogens in Cattle Reveals That Theileria parva, Babesia bigemina and Anaplasma marginale Are Endemic in Burundi. Parasites Vectors 2021, 14, 6. [Google Scholar] [CrossRef]
- Amzati, G.S.; Djikeng, A.; Odongo, D.O.; Nimpaye, H.; Sibeko, K.P.; Muhigwa, J.-B.B.; Madder, M.; Kirschvink, N.; Marcotty, T. Genetic and Antigenic Variation of the Bovine Tick-Borne Pathogen Theileria parva in the Great Lakes Region of Central Africa. Parasites Vectors 2019, 12, 588. [Google Scholar] [CrossRef]
- Atuhaire, D.K.; Muleya, W.; Mbao, V.; Bazarusanga, T.; Gafarasi, I.; Salt, J.; Namangala, B.; Musoke, A.J. Sequence Diversity of Cytotoxic T Cell Antigens and Satellite Marker Analysis of Theileria parva Informs the Immunization against East Coast Fever in Rwanda. Parasit Vectors 2020, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, R.; Troskie, P.C.; Josemans, A.I.; Potgieter, F.T.; Maboko, B.B.; Latif, A.A.; Mans, B.J. Investigations into the Carrier-State of Theileria Sp. (Buffalo) in Cattle. Int. J. Parasitol. Parasites Wildl. 2020, 11, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Mwacharo, J.M.; Pelle, R.; Njahira, M.N.; Odongo, D.O.; Mbole-Kariuki, M.N.; Marcellino, W.L.; Malak, A.K.; Kiara, H.; El Hussein, A.R.M.; et al. Genetic Diversity and Population Structure of Theileria parva in South Sudan. Ticks Tick-Borne Dis. 2018, 9, 806–813. [Google Scholar] [CrossRef]
- Allan, F.K.; Sindoya, E.; Adam, K.E.; Byamungu, M.; Lea, R.S.; Lord, J.S.; Mbata, G.; Paxton, E.; Mramba, F.; Torr, S.J.; et al. A Cross-Sectional Survey to Establish Theileria parva Prevalence and Vector Control at the Wildlife-Livestock Interface, Northern Tanzania. Prev. Vet. Med. 2021, 196, 105491. [Google Scholar] [CrossRef] [PubMed]
- Kerario, I.I.; Simuunza, M.C.; Chenyambuga, S.W.; Koski, M.; Hwang, S.-G.; Muleya, W. Prevalence and Risk Factors Associated with Theileria parva Infection in Cattle in Three Regions of Tanzania. Trop. Anim. Health Prod. 2017, 49, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Kimaro, E.G.; Mor, S.M.; Gwakisa, P.; Toribio, J.-A. Seasonal Occurrence of Theileria parva Infection and Management Practices amongst Maasai Pastoralist Communities in Monduli District, Northern Tanzania. Vet. Parasitol. 2017, 246, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Laisser, E.L.K.; Kipanyula, M.J.; Msalya, G.; Mdegela, R.H.; Karimuribo, E.D.; Mwilawa, A.J.; Mwega, E.D.; Kusiluka, L.; Chenyambuga, S.W. Tick Burden and Prevalence of Theileria parva Infection in Tarime Zebu Cattle in the Lake Zone of Tanzania. Trop. Anim. Health Prod. 2014, 46, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Kabi, F.; Masembe, C.; Muwanika, V.; Kirunda, H.; Negrini, R. Geographic Distribution of Non-Clinical Theileria parva Infection among Indigenous Cattle Populations in Contrasting Agro-Ecological Zones of Uganda: Implications for Control Strategies. Parasit Vectors 2014, 7, 414. [Google Scholar] [CrossRef]
- Miyama, T.; Byaruhanga, J.; Okamura, I.; Uchida, L.; Muramatsu, Y.; Mwebembezi, W.; Vudriko, P.; Makita, K. Effect of Chemical Tick Control Practices on Tick Infestation and Theileria parva Infection in an Intensive Dairy Production Region of Uganda. Ticks Tick-Borne Dis. 2020, 11, 101438. [Google Scholar] [CrossRef] [PubMed]
- Muhanguzi, D.; Picozzi, K.; Hatendorf, J.; Thrusfield, M.; Welburn, S.C.; Kabasa, J.D.; Waiswa, C. Prevalence and Spatial Distribution of Theileria parva in Cattle under Crop-Livestock Farming Systems in Tororo District, Eastern Uganda. Parasites Vectors 2014, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Nanteza, A.; Obara, I.; Kasaija, P.; Mwega, E.; Kabi, F.; Salih, D.A.; Njahira, M.; Njuguna, J.; Odongo, D.; Bishop, R.P.; et al. Antigen Gene and Variable Number Tandem Repeat (VNTR) Diversity in Theileria parva Parasites from Ankole Cattle in South-Western Uganda: Evidence for Conservation in Antigen Gene Sequences Combined with Extensive Polymorphism at VNTR Loci. Transbound. Emerg. Dis. 2020, 67, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Oligo, S.; Nanteza, A.; Nsubuga, J.; Musoba, A.; Kazibwe, A.; Lubega, G.W. East Coast Fever Carrier Status and Theileria parva Breakthrough Strains in Recently ITM Vaccinated and Non-Vaccinated Cattle in Iganga District, Eastern Uganda. Pathogens 2023, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Muleya, W.; Namangala, B.; Simuunza, M.; Nakao, R.; Inoue, N.; Kimura, T.; Ito, K.; Sugimoto, C.; Sawa, H. Population Genetic Analysis and Sub-Structuring of Theileria parva in the Northern and Eastern Parts of Zambia. Parasites Vectors 2012, 5, 255. [Google Scholar] [CrossRef] [PubMed]
- Catalano, D.; Biasibetti, E.; Lynen, G.; Di Giulio, G.; De Meneghi, D.; Tomassone, L.; Valenza, F.; Capucchio, M.T. “Ormilo Disease” a Disorder of Zebu Cattle in Tanzania: Bovine Cerebral Theileriosis or New Protozoan Disease? Trop. Anim. Health Prod. 2015, 47, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Salih, D.A.; Njahira, M.N.; Hassan, S.K.; El Hussein, A.M.; Liu, Z.; Yin, H.; Pelle, R.; Skilton, R.A. Genotyping of Theileria Lestoquardi from Sheep and Goats in Sudan to Support Control of Malignant Ovine Theileriosis. Vet. Parasitol. 2017, 239, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Vieira, T.S.W.J.; da Costa Vieira, R.F.; Shekaro, A.; Nahum-Biala, Y.; Olubade, T.; Abasiama, M.S.; Gajibo, U.A.; Bukar, L.; Shand, M.; et al. Molecular Detection of Theileria annulata, Theileria mutans and Theileria velifera but No Evidence of Theileria parva Infected or Vaccinated Cattle in Nigeria despite Extensive Transboundary Migrations. Vet. Parasitol. Reg. Stud. Rep. 2023, 41, 100887. [Google Scholar] [CrossRef] [PubMed]
- Makenov, M.T.; Toure, A.H.; Bayandin, R.B.; Gladysheva, A.V.; Shipovalov, A.V.; Boumbaly, S.; Sacko, N.; Korneev, M.G.; Yakovlev, S.A.; Zhurenkova, O.B.; et al. Ngari Virus (Orthobunyavirus, Peribunyaviridae) in Ixodid Ticks Collected from Cattle in Guinea. Acta Trop. 2021, 214, 105790. [Google Scholar] [CrossRef] [PubMed]
- Amoa-Bosompem, M.; Kobayashi, D.; Faizah, A.N.; Kimura, S.; Antwi, A.; Agbosu, E.; Pratt, D.; Ohashi, M.; Bonney, J.H.K.; Dadzie, S.; et al. Screening for Tick-Borne and Tick-Associated Viruses in Ticks Collected in Ghana. Arch. Virol. 2022, 167, 123–130. [Google Scholar] [CrossRef]
- Ogola, E.O.; Kopp, A.; Bastos, A.D.S.; Slothouwer, I.; Omoga, D.C.A.; Osalla, J.; Sang, R.; Torto, B.; Junglen, S.; Tchouassi, D.P. Phlebovirus Diversity in Ticks from Livestock in Arid Ecologies in Kenya. Ticks Tick-Borne Dis. 2023, 14, 102087. [Google Scholar] [CrossRef]
- Ouedraogo, A.; Luciani, L.; Zannou, O.; Biguezoton, A.; Pezzi, L.; Thirion, L.; Belem, A.; Saegerman, C.; Charrel, R.; Lempereur, L. Detection of Two Species of the Genus Parapoxvirus (Bovine Papular Stomatitis Virus and Pseudocowpox Virus) in Ticks Infesting Cattle in Burkina Faso. Microorganisms 2020, 8, 644. [Google Scholar] [CrossRef]
- Kobayashi, D.; Ohashi, M.; Osei, J.H.N.; Agbosu, E.; Opoku, M.; Agbekudzi, A.; Joannides, J.; Fujita, R.; Sasaki, T.; Bonney, J.H.K.; et al. Detection of a Novel Putative Phlebovirus and First Isolation of Dugbe Virus from Ticks in Accra, Ghana. Ticks Tick-Borne Dis. 2017, 8, 640–645. [Google Scholar] [CrossRef]
- Simulundu, E.; Mbambara, S.; Chambaro, H.M.; Sichibalo, K.; Kajihara, M.; Nalubamba, K.S.; Sawa, H.; Takada, A.; Changula, K.; Chitanga, S. Prevalence and Genetic Diversity of Shibuyunji Virus, a Novel Tick-Borne Phlebovirus Identified in Zambia. Arch. Virol. 2021, 166, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Ogola, E.O.; Kopp, A.; Bastos, A.D.S.; Slothouwer, I.; Marklewitz, M.; Omoga, D.; Rotich, G.; Getugi, C.; Sang, R.; Torto, B.; et al. Jingmen Tick Virus in Ticks from Kenya. Viruses 2022, 14, 1041. [Google Scholar] [CrossRef]
- Simo Tchetgna, H.; Yousseu, F.S.; Cosset, F.-L.; de Freitas, N.B.; Kamgang, B.; McCall, P.J.; Ndip, R.N.; Legros, V.; Wondji, C.S. Molecular and Serological Evidence of Crimean-Congo Hemorrhagic Fever Orthonairovirus Prevalence in Livestock and Ticks in Cameroon. Front. Cell. Infect. Microbiol. 2023, 13, 1132495. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Barry, Y.; Stoek, F.; Pickin, M.J.; Ba, A.; Chitimia-Dobler, L.; Haki, M.L.; Doumbia, B.A.; Eisenbarth, A.; Diambar, A.; et al. Detection of Crimean-Congo Hemorrhagic Fever Virus in Blood-Fed Hyalomma Ticks Collected from Mauritanian Livestock. Parasites Vectors 2021, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Wampande, E.M.; Waiswa, P.; Allen, D.J.; Hewson, R.; Frost, S.D.W.; Stubbs, S.C.B. Phylogenetic Characterization of Crimean-Congo Hemorrhagic Fever Virus Detected in African Blue Ticks Feeding on Cattle in a Ugandan Abattoir. Microorganisms 2021, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, M.; Simuunza, M.; Saasa, N.; Dautu, G.; Mori-Kajihara, A.; Qiu, Y.; Nakao, R.; Eto, Y.; Furumoto, H.; Hang’ombe, B.M.; et al. Serologic and Molecular Evidence for Circulation of Crimean-Congo Hemorrhagic Fever Virus in Ticks and Cattle in Zambia. PLoS Neglected Trop. Dis. 2021, 15, e0009452. [Google Scholar] [CrossRef] [PubMed]
- Daodu, O.B.; Eisenbarth, A.; Schulz, A.; Hartlaub, J.; Olopade, J.O.; Oluwayelu, D.O.; Groschup, M.H. Molecular Detection of Dugbe Orthonairovirus in Cattle and Their Infesting Ticks (Amblyomma and Rhipicephalus (Boophilus)) in Nigeria. PLoS Negl. Trop. Dis. 2021, 15, e0009905. [Google Scholar] [CrossRef]
- Omoga, D.C.A.; Tchouassi, D.P.; Venter, M.; Ogola, E.O.; Langat, S.; Getugi, C.; Eibner, G.; Kopp, A.; Slothouwer, I.; Torto, B.; et al. Characterization of a Novel Orbivirus from Cattle Reveals Active Circulation of a Previously Unknown and Pathogenic Orbivirus in Ruminants in Kenya. Msphere 2023, 8, e0048822. [Google Scholar] [CrossRef] [PubMed]


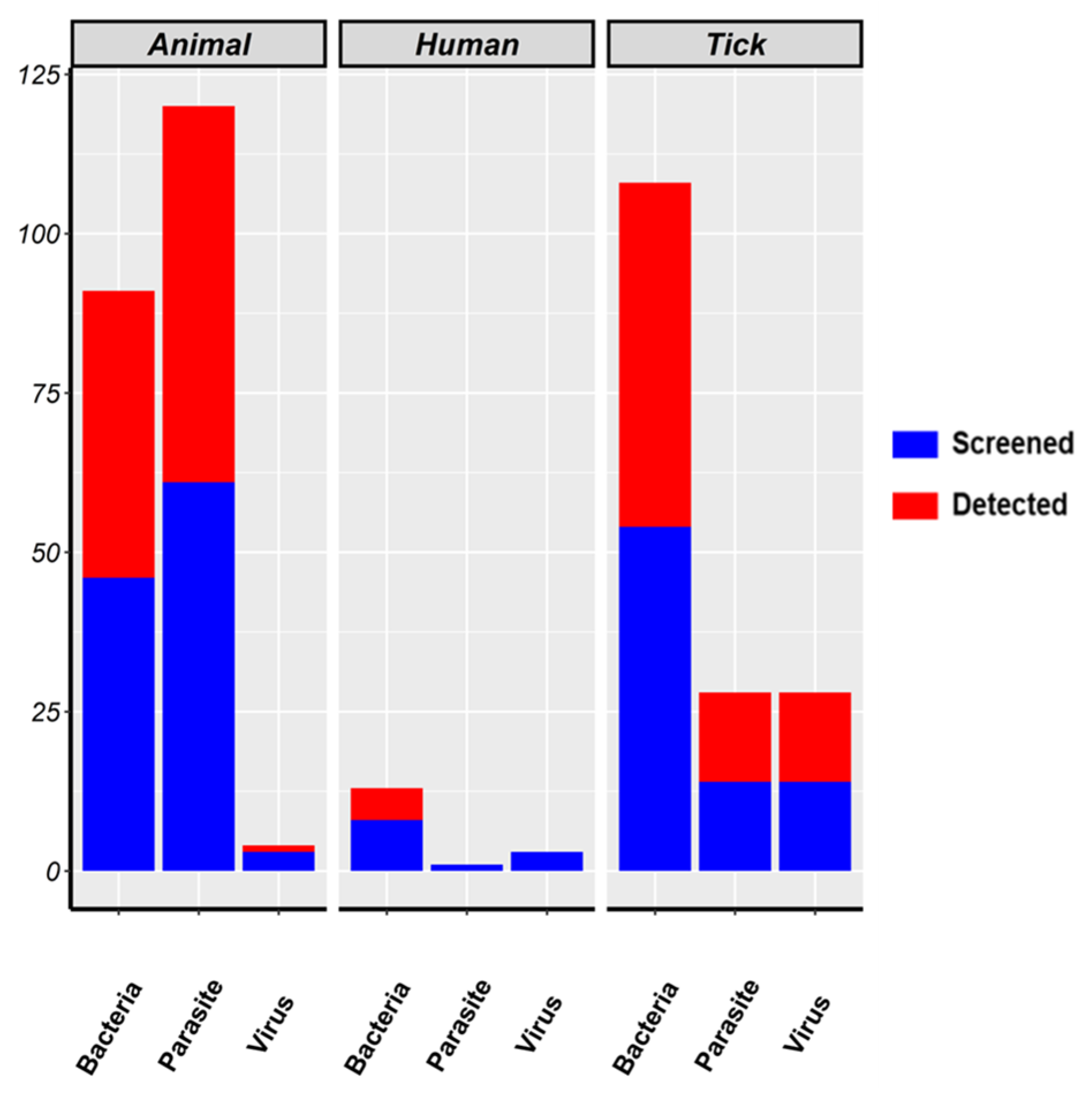

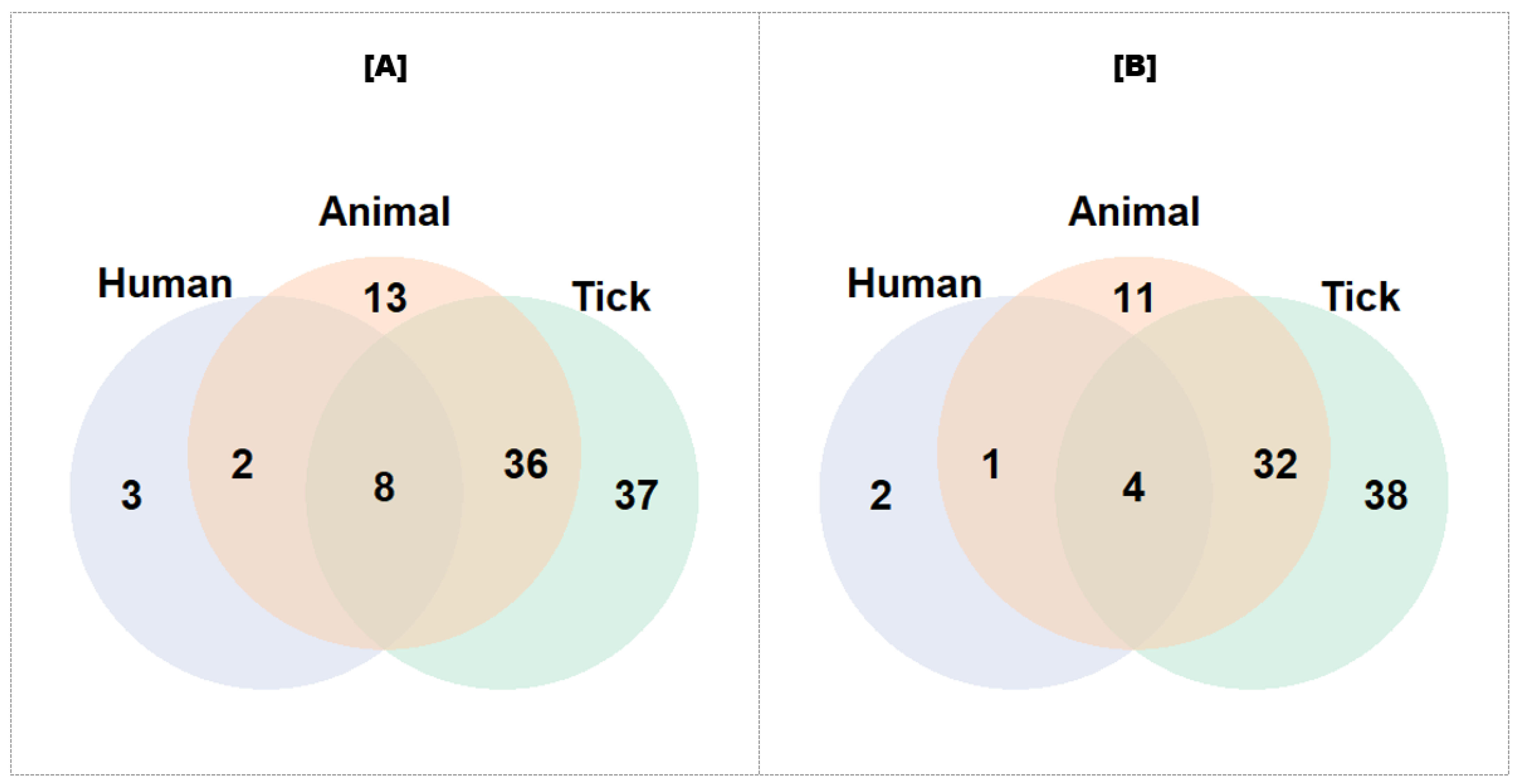

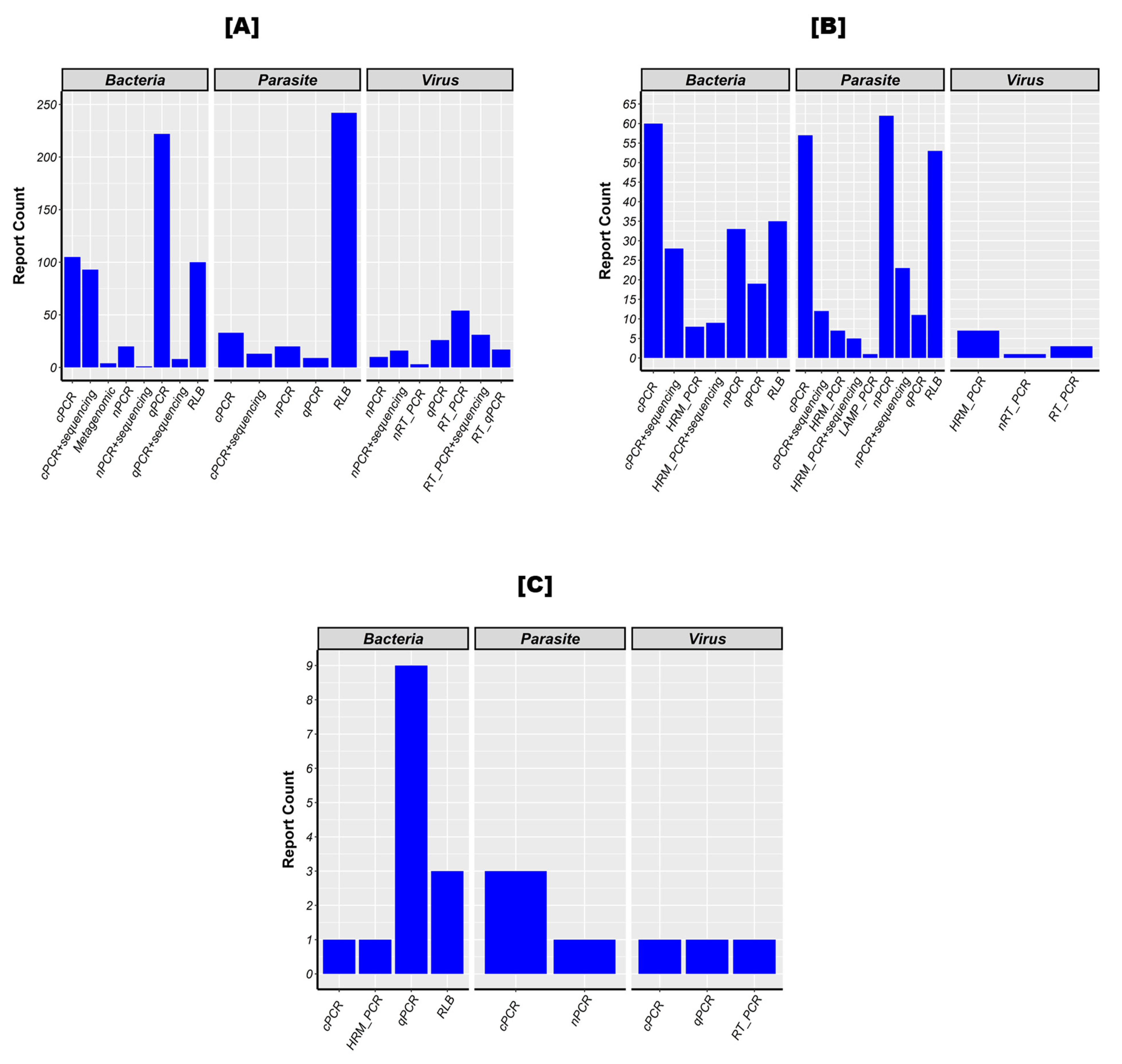
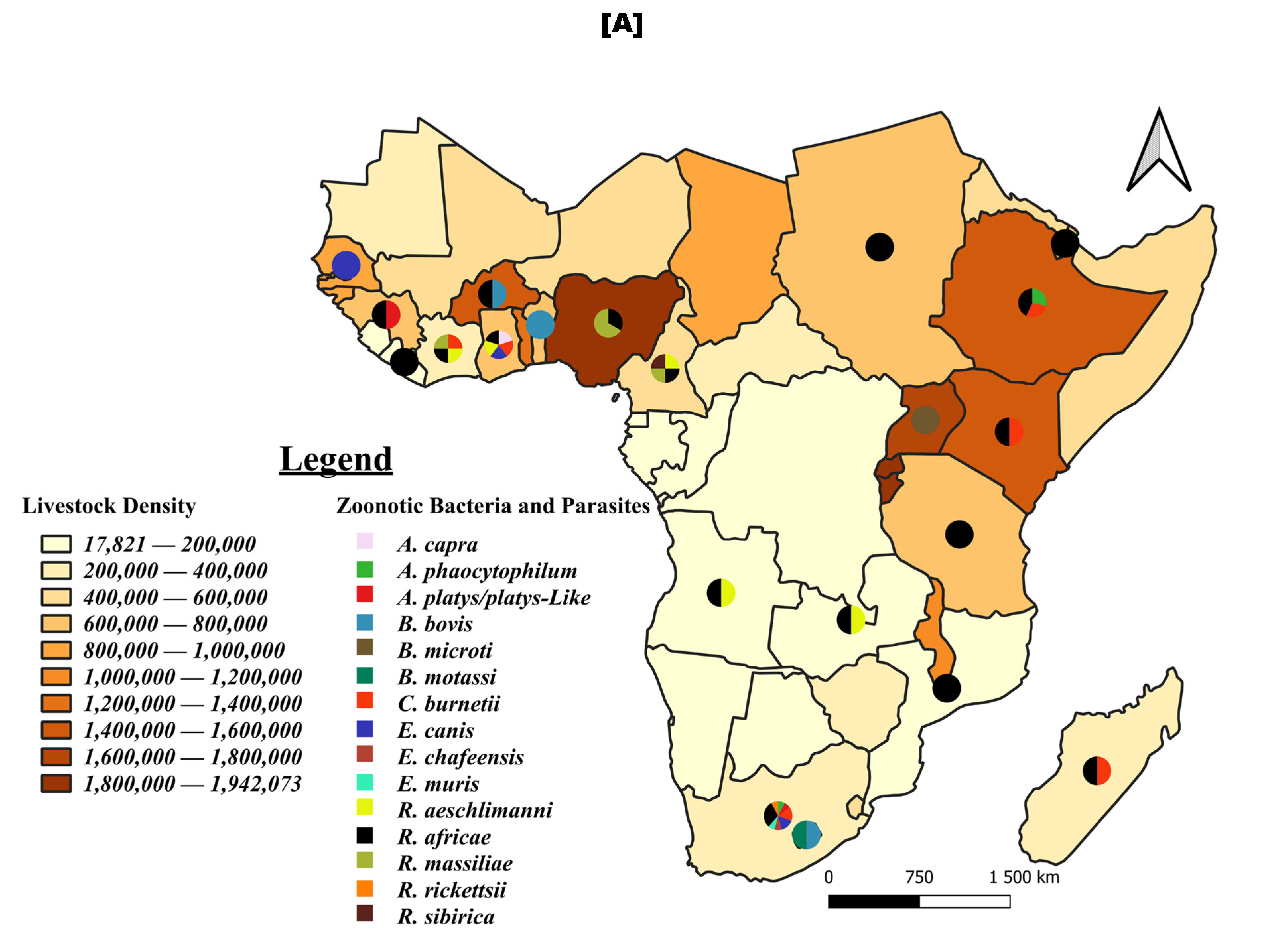
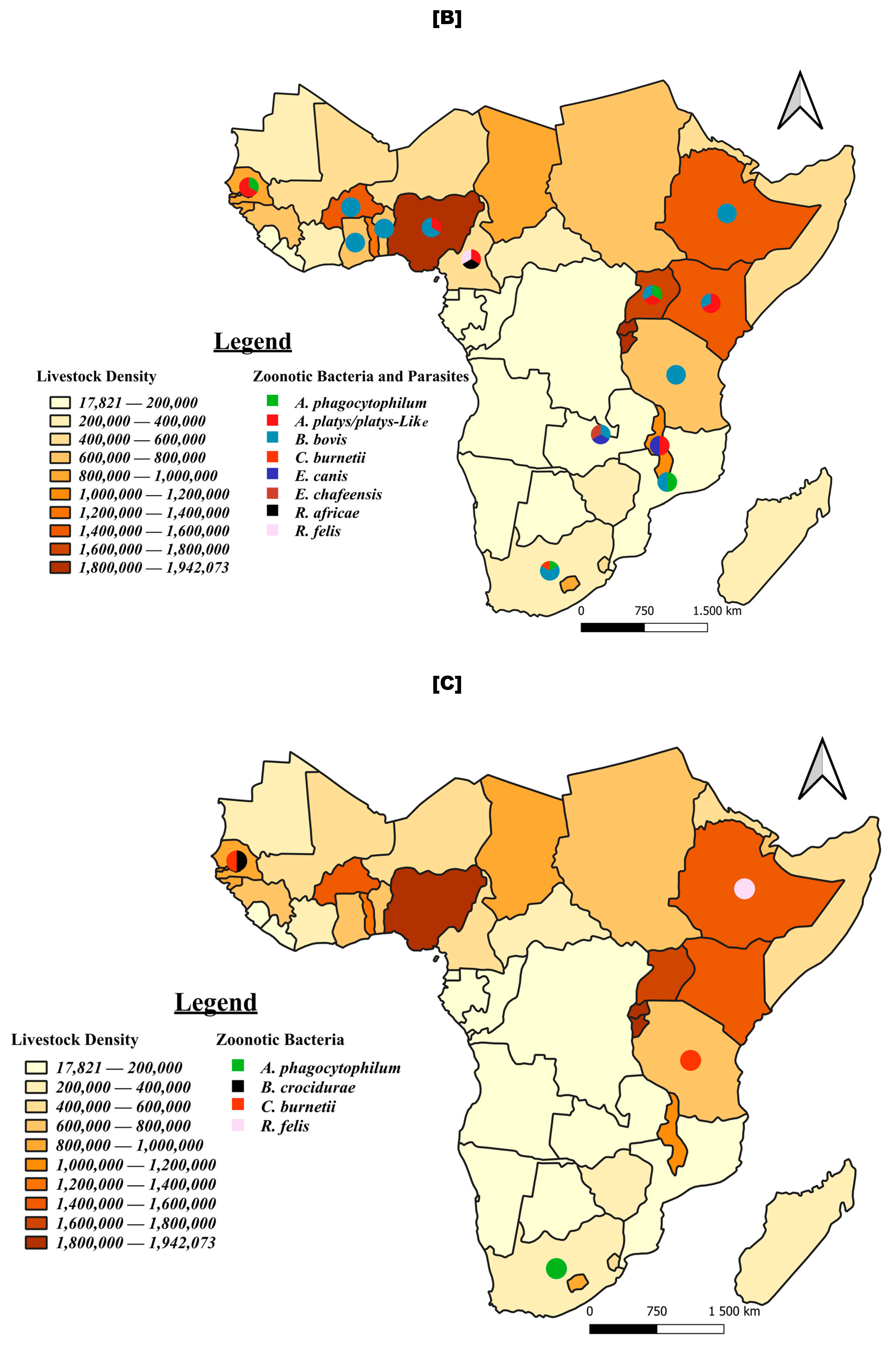
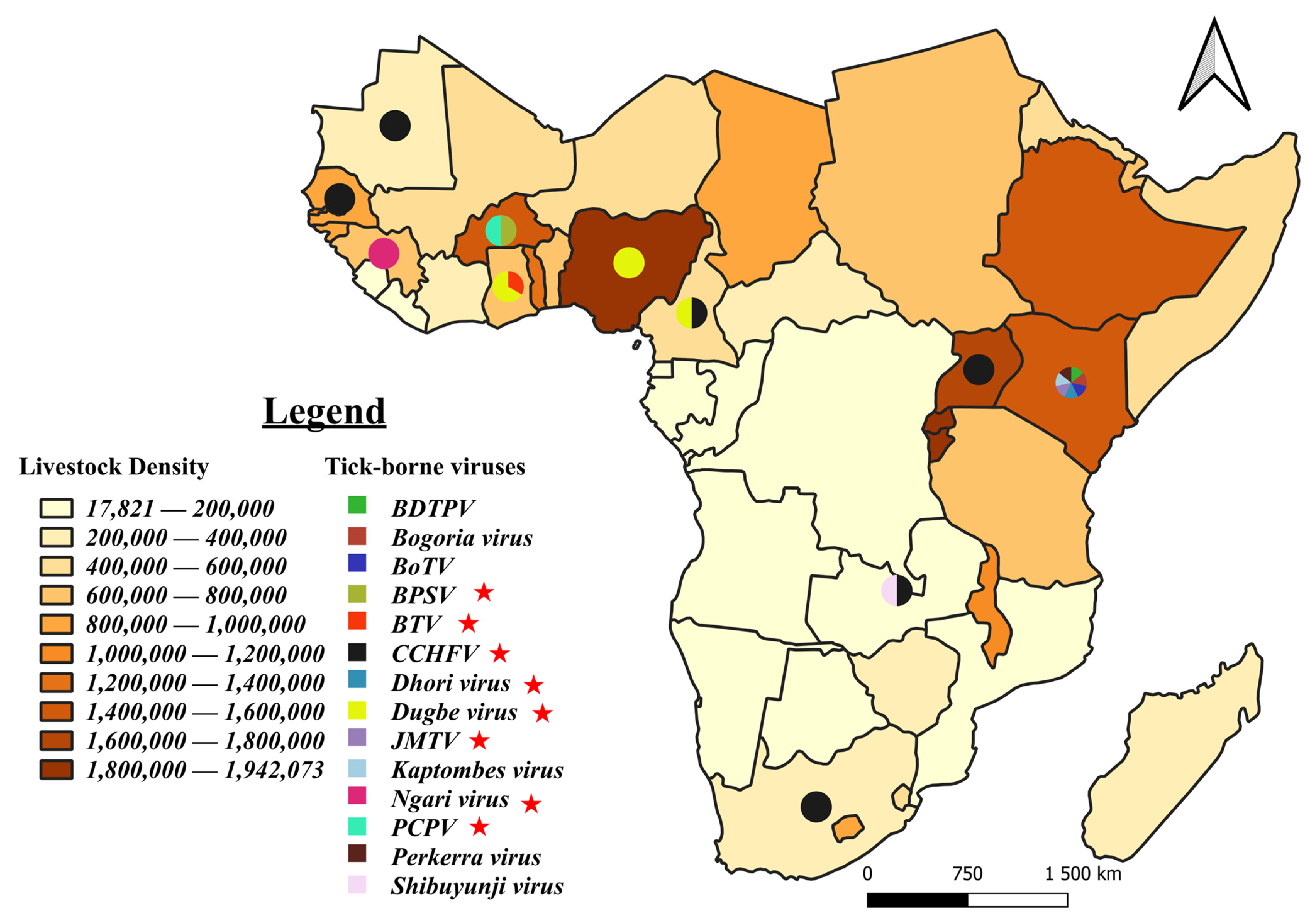
| Data Base | Search String |
|---|---|
| PubMed | “africa south of the sahara”[MeSH Terms] AND (“Tick”[Title/Abstract] OR “Cattle”[Title/Abstract] OR “Goat”[Title/Abstract] OR “Sheep”[Title/Abstract] OR “Human”[Title/Abstract]) AND (“anaplasma”[Title/Abstract] OR “rickettsia”[Title/Abstract] OR “ehrlichia”[Title/Abstract] OR “coxiella”[Title/Abstract] OR “wolbachia”[Title/Abstract] OR “babesia”[Title/Abstract] OR “borrelia”[Title/Abstract] OR “theileria”[Title/Abstract] OR “arboviruses”[MeSH Terms]) AND (2012:2023[pdat]) |
| Scopus | TITLE-ABS-KEY (africa AND NOT (algeria OR egypt OR libya OR morocco OR tunisia)) AND (tick OR cattle OR sheep OR goat) AND (anaplasma OR ehrlichia OR rickettsia OR coxiella OR wolbachia OR babesia OR borrelia OR theileria OR virus OR arbovirus OR “tick-borne virus”)) AND PUBYEAR > 2011 AND PUBYEAR < 2024 AND (LIMIT-TO (DOCTYPE, “ar”)) |
| TITLE-ABS-KEY (africa AND NOT (algeria OR egypt OR libya OR morocco OR tunisia)) AND human AND (anaplasma OR ehrlichia OR rickettsia OR coxiella OR wolbachia OR babesia OR borrelia OR theileria OR arbovirus OR “tick-borne virus”)) AND PUBYEAR > 2011 AND PUBYEAR < 2024 AND (LIMIT-TO (DOCTYPE, “ar”)) | |
| Science Direct |
|
| |
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djiman, T.A.; Biguezoton, A.S.; Saegerman, C. Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health. Pathogens 2024, 13, 697. https://doi.org/10.3390/pathogens13080697
Djiman TA, Biguezoton AS, Saegerman C. Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health. Pathogens. 2024; 13(8):697. https://doi.org/10.3390/pathogens13080697
Chicago/Turabian StyleDjiman, Tidjani A., Abel S. Biguezoton, and Claude Saegerman. 2024. "Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health" Pathogens 13, no. 8: 697. https://doi.org/10.3390/pathogens13080697
APA StyleDjiman, T. A., Biguezoton, A. S., & Saegerman, C. (2024). Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health. Pathogens, 13(8), 697. https://doi.org/10.3390/pathogens13080697






