Quality Control of Microscopic Diagnosis of Malaria in Healthcare Facilities and Submicroscopic Infections in Mossendjo, the Department of Niari, the Republic of the Congo
Abstract
:1. Introduction
2. Materials and Methods
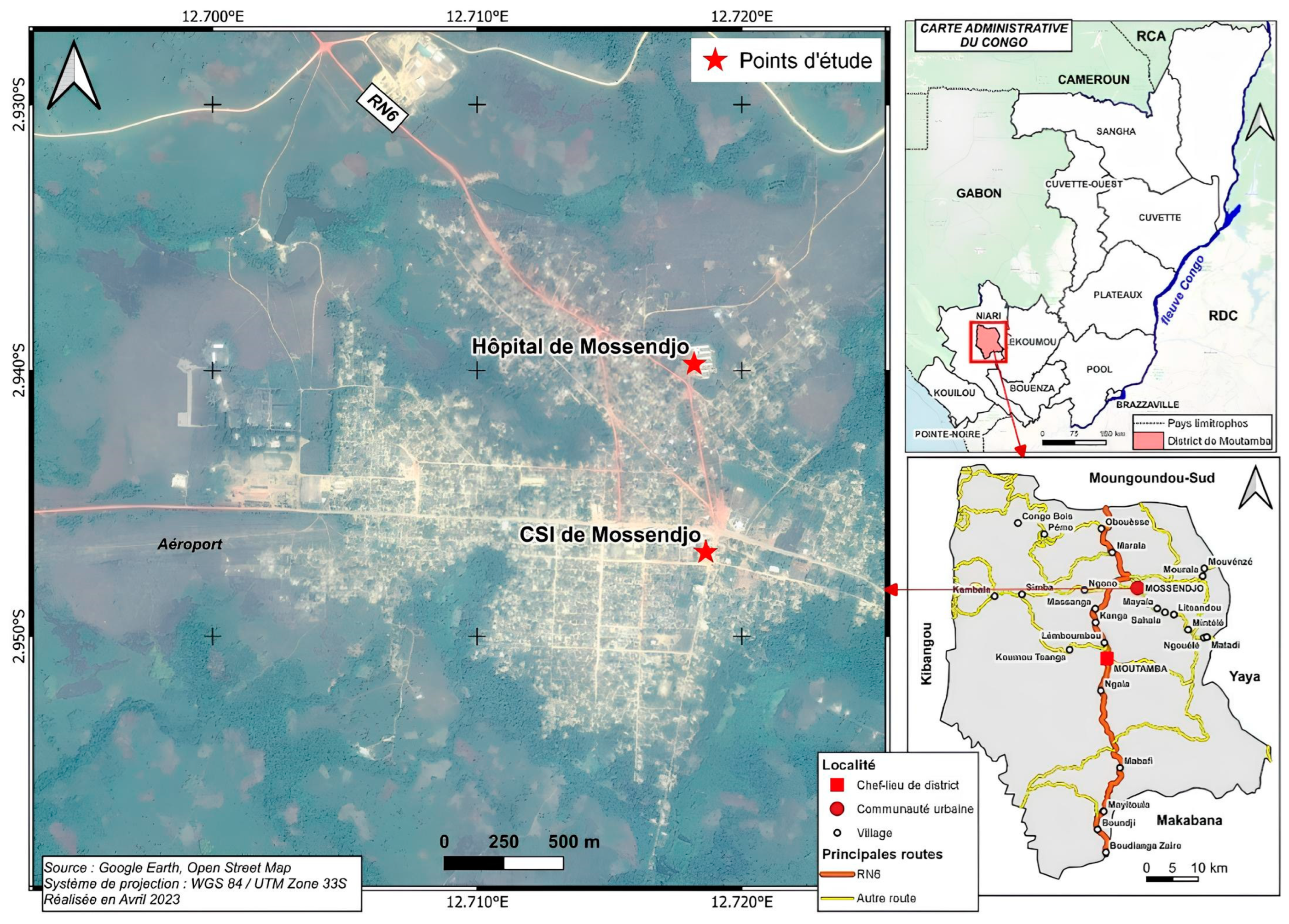
2.1. The Study Areas
2.2. Ethical Clearance and Site Preparation
2.3. Study Population, Blood Samples, and Data Collection
2.4. Detection of Submicroscopic Infection via Parasite Genotyping
2.5. Data Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Quality Control of Malaria Parasite Detection in Health Centers
3.3. Identification of Plasmodial Species
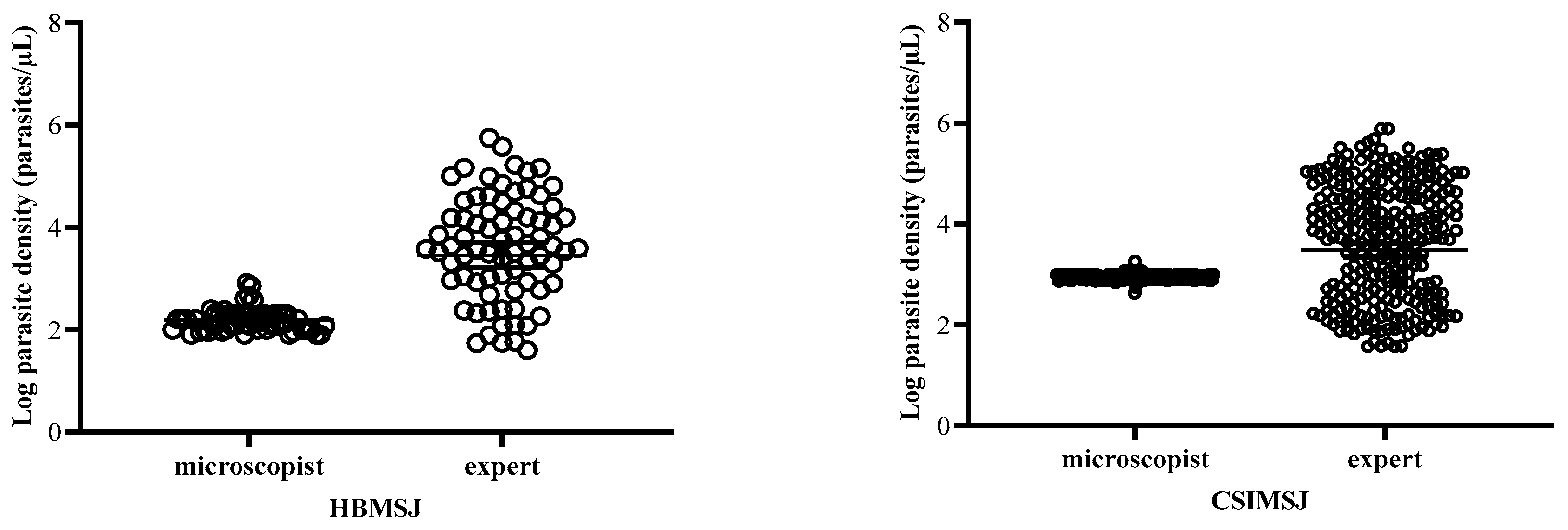
3.4. Estimation of Parasitemia at the Health Centers
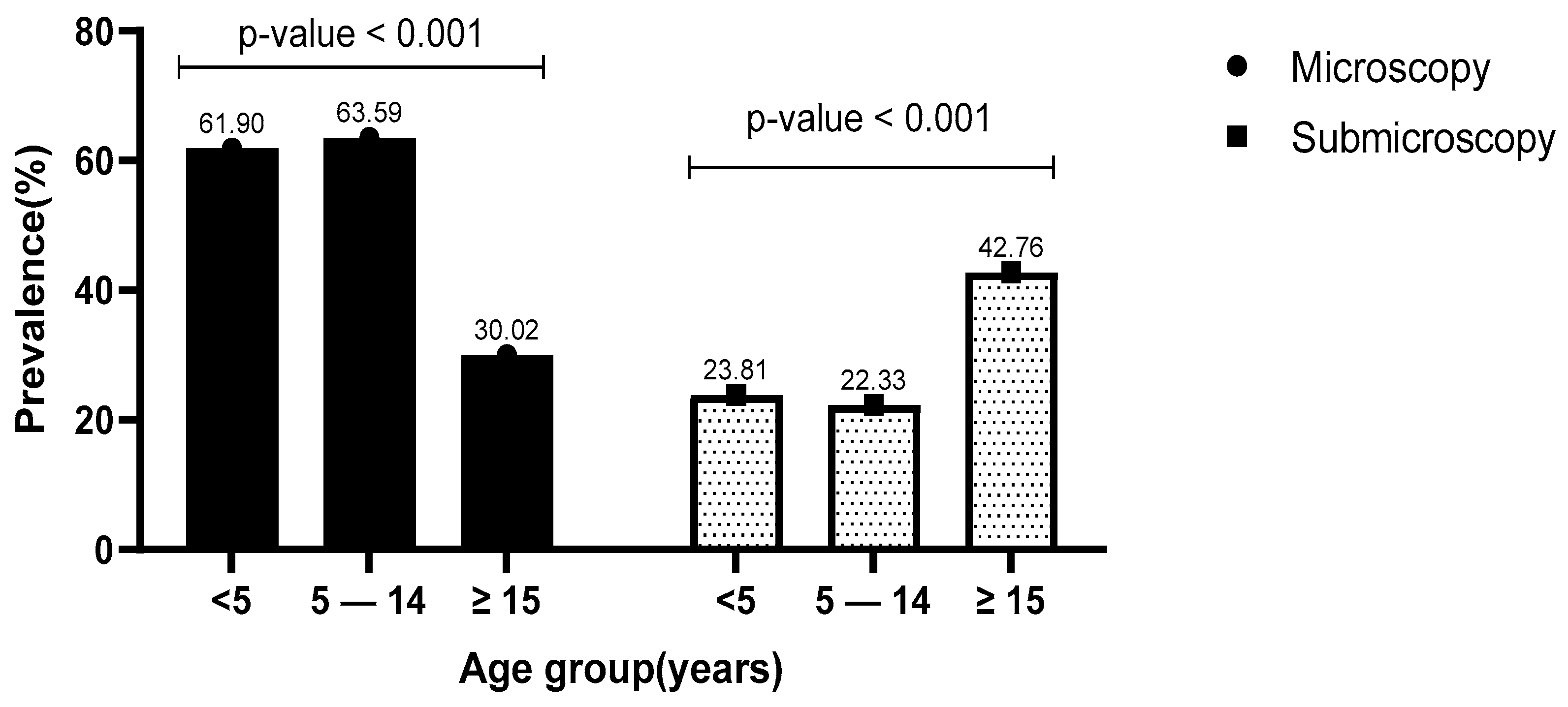
3.5. Prevalence of Microscopic and Submicroscopic Infections in Relation to Age
3.6. Relationship of Microscopic Infection, Parasite Density, and Submicroscopic Infections with Demographic and Clinical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 10 January 2024).
- Musiime, A.K.; Smith, D.L.; Kilama, M.; Rek, J.; Arinaitwe, E.; Nankabirwa, J.I.; Kamya, M.R.; Conrad, M.D.; Dorsey, G.; Akol, A.M.; et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 2019, 18, 445. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 20 February 2022).
- WHO. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://iris.who.int/bitstream/handle/10665/252038/9789241511711-eng.pdf (accessed on 4 April 2024).
- Long, E.G. Requirements for Diagnosis of Malaria at Different Levels of the Laboratory Network in Africa. Am. J. Clin. Pathol. 2009, 131, 858–860. [Google Scholar] [CrossRef] [PubMed]
- WHO. Malaria Microscopy Quality Assurance Manual—Version 2, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; 140p, Available online: https://www.who.int/docs/default-source/documents/publications/gmp/malaria-microscopy-quality-assurance-manual.pdf (accessed on 22 June 2023).
- Varo, R.; Balanza, N.; Mayor, A.; Bassat, Q. Diagnosis of clinical malaria in endemic settings. Expert Rev. Anti. Infect. Ther. 2021, 19, 79–92. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Update on Malaria Diagnostics and Test Utilization. J. Clin. Microbiol. 2017, 55, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Msellem, M.I.; Mårtensson, A.; Rotllant, G.; Bhattarai, A.; Strömberg, J.; Kahigwa, E.; Garcia, M.; Petzold, M.; Olumese, P.; Ali, A.; et al. Influence of Rapid Malaria Diagnostic Tests on Treatment and Health Outcome in Fever Patients, Zanzibar—A Crossover Validation Study. PLoS Med. 2009, 6, e1000070. [Google Scholar] [CrossRef] [PubMed]
- Gerstl, S.; Dunkley, S.; Mukhtar, A.; De Smet, M.; Baker, S.; Maikere, J. Assessment of two malaria rapid diagnostic tests in children under five years of age, with follow-up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone. Malar. J. 2010, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Kalinga, A.K.; Mwanziva, C.; Chiduo, S.; Mswanya, C.; Ishengoma, D.I.; Francis, F.; Temu, L.; Mahikwano, L.; Mgata, S.; Amoo, G.; et al. Comparison of visual and automated Deki Reader interpretation of malaria rapid diagnostic tests in rural Tanzanian military health facilities. Malar. J. 2018, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.M.; Korevaar, D.A.; Leeflang, M.M.G.; Mens, P.F. Molecular malaria diagnostics: A systematic review and meta-analysis. Crit. Rev. Clin. Lab. Sci. 2016, 53, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, H.; Ofori, M.F.; Kusi, K.A.; Adu, B.; Owusu-Yeboa, E.; Kyei-Baafour, E.; Arku, A.T.; Bosomprah, S.; Alifrangis, M.; Quakyi, I.A. The prevalence of submicroscopic Plasmodium falciparum gametocyte carriage and multiplicity of infection in children, pregnant women and adults in a low malaria transmission area in Southern Ghana. Malar. J. 2018, 17, 331. [Google Scholar] [CrossRef] [PubMed]
- PNLP. Programme Nationale de Lutte Contre le Paludisme. In National Report of Malaria in Republic of Congo; Ministry of Public Health Brazzaville: Brazzaville, Congo, 2021; Available online: https://files.aho.afro.who.int/afahobckpcontainer/production/files/Rapport_Annuel_dActivites_du_PNLP_2021.pdf (accessed on 15 December 2023).
- Mayengue, P.I.; Kouhounina Batsimba, D.; Dossou-Yovo, L.R.; Niama, R.F.; Macosso, L.; Pembet Singana, B.; Louzolo, I.; Bongolo Loukabou, N.C.; Sekangue Obili, G.; Kobawila, S.C. Evaluation of routine microscopy performance for malaria diagnosis at three different health centers in Brazzaville, Republic of Congo. Malar. Res. Treat. 2018, 2018, 4914358. [Google Scholar] [CrossRef]
- Ntoumi, F.; Vouvoungui, J.; Ibara, R.; Landry, M.; Sidibé, A. Malaria burden and case management in the Republic of Congo: Limited use and application of rapid diagnostic tests results. BMC Public Health 2013, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Baina, M.T.; Lissom, A.; Assioro Doulamo, N.V.; Djontu, J.C.; Umuhoza, D.M.; Mbama-Ntabi, J.D.; Diafouka-Kietela, S.; Mayela, J.; Missontsa, G.; Wondji, C.; et al. Comparative study of Plasmodium falciparum msp-1 and msp-2 Genetic Diversity in Isolates from Rural and Urban Areas in the South of Brazzaville, Republic of Congo. Pathogens 2023, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Massamba, J.E.; Djontu, J.C.; Vouvoungui, C.J.; Kobawila, C.; Ntoumi, F. Plasmodium falciparum multiplicity of infection and pregnancy outcomes in Congolese women from southern Brazzaville, Republic of Congo. Malar. J. 2022, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Koukouikila-Koussounda, F.; Ntoumi, F. Malaria epidemiological research in the Republic of Congo. Malar. J. 2016, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Mayengue, P.I.; Niama, R.F.; Kouhounina Batsimba, D.; Malonga-Massanga, A.; Louzolo, I.; Loukabou Bongolo, N.C.; Macosso, L.; Ibara Ottia, R.; Kimbassa Ngoma, G.; Dossou-Yovo, L.R.; et al. No polymorphisms in K13-propeller gene associated with artemisinin resistance in Plasmodium falciparum isolated from Brazzaville, Republic of Congo. BMC Infect. Dis. 2018, 18, 538. [Google Scholar] [CrossRef]
- Chipwaza, B.; Sumaye, R.D. High malaria parasitemia among outpatient febrile children in low endemic area, East-Central Tanzania in 2013. BMC Res Notes 2020, 13, 251. [Google Scholar] [CrossRef]
- Mayengue, P.I.; Kouhounina Batsimba, D.; Niama, R.F.; Ibara Ottia, R.; Malonga-Massanga, A.; Fila-Fila, G.P.U.; Ahombo, G.; Kobawila, S.C.; Parra, H.J. Variation of prevalence of malaria, parasite density and the multiplicity of Plasmodium falciparum infection throughout the year at three different health centers in Brazzaville, Republic of Congo. BMC Infect. Dis. 2020, 20, 190. [Google Scholar] [CrossRef]
- Ntoumi, F.; Ngoundou-Landji, J.; Lekoulou, F.; Luty, A.; Deloron, P.; Ringwald, P. Site-based study on polymorphism of Plasmodium falciparum MSP-1 and MSP-2 genes in isolates from two villages in Central Africa. Parassitologia 2000, 42, 197–203. [Google Scholar]
- McHugh, M. Interrater reliability: The kappa statistic. Biochem. Medica: Časopis Hrvat. Društva Med. Biokem. HDMB 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Opoku Afriyie, S.; Addison, T.K.; Gebre, Y.; Mutala, A.-H.; Antwi, K.B.; Abbas, D.A.; Addo, K.A.; Tweneboah, A.; Ayisi-Boateng, N.K.; Koepfli, C.; et al. Accuracy of diagnosis among clinical malaria patients: Comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar. J. 2023, 22, 76. [Google Scholar] [CrossRef]
- Mukadi, P.; Gillet, P.; Lukuka, A.; Atua, B.; Kahodi, S.; Lokombe, J.; Muyembe, J.-J.; Jacobs, J. External quality assessment of malaria microscopy in the Democratic Republic of the Congo. Malar. J. 2011, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Nega, D.; Abebe, A.; Abera, A.; Gidey, B.; G/Tsadik, A.; Tasew, G. Comprehensive competency assessment of malaria microscopists and laboratory diagnostic service capacity in districts stratified for malaria elimination in Ethiopia. PLoS ONE 2020, 15, e0235151. [Google Scholar] [CrossRef] [PubMed]
- Ochwedo, K.O.; Omondi, C.J.; Magomere, E.O.; Olumeh, J.O.; Debrah, I.; Onyango, S.A.; Orondo, P.W.; Ondeto, B.M.; Atieli, H.E.; Ogolla, S.O.; et al. Hyper-prevalence of submicroscopic Plasmodium falciparum infections in a rural area of western Kenya with declining malaria cases. Malar. J. 2021, 20, 472. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, P.; de Lucio, A.; Romay-Barja, M.; Herrador, Z.; González, V.; García, L.; Fernández-Martínez, A.; Santana-Morales, M.; Ncogo, P.; Valladares, B.; et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar. J. 2018, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Mbama Ntabi, J.D.; Lissom, A.; Djontu, J.C.; Diafouka-Kietela, S.; Vouvoungui, C.; Boumpoutou, R.K.; Mayela, J.; Nguiffo-Nguete, D.; Nkemngo, F.N.; Ndo, C.; et al. Prevalence of non-Plasmodium falciparum species in southern districts of Brazzaville in The Republic of the Congo. Parasit. Vectors 2022, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Bousema, T.; Okell, L.; Felger, I.; Drakeley, C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat. Rev. Microbiol. 2014, 12, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Bousema, J.T.; Gouagna, L.C.; Otieno, S.; van de Vegte-Bolmer, M.; Omar, S.A.; Sauerwein, R.W. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop. Med. Hyg. 2007, 76, 470–474. [Google Scholar] [CrossRef]
- Shekalaghe, S.A.; Bousema, J.T.; Kunei, K.K.; Lushino, P.; Masokoto, A.; Wolters, L.R.; Mwakalinga, S.; Mosha, F.W.; Sauerwein, R.W.; Drakeley, C.J. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop. Med. Int. Health 2007, 12, 547–553. [Google Scholar] [CrossRef]
- White, N.J. How antimalarial drug resistance affects post-treatment prophylaxis. Malar. J. 2008, 7, 9. [Google Scholar] [CrossRef]
- Nadine, C.P.; Mayengue, P.I.; Brunelle, M.D.; Chyvanelle, A.N.; Grace, N.B.; Etienne, N. Analyse de l’Infection Palustre en Zone Rurale des Environs de Brazzaville: Données d’une Enquête Parasitologique dans des Ménages.: L’infection palustre autour de Brazzaville. Health Sci. Dis. 2022, 23. [Google Scholar]
- van Eijk, A.M.; Sutton, P.L.; Ramanathapuram, L.; Sullivan, S.A.; Kanagaraj, D.; Priya, G.S.L.; Ravishankaran, S.; Asokan, A.; Sangeetha, V.; Rao, P.N.; et al. The burden of submicroscopic and asymptomatic malaria in India revealed from epidemiology studies at three varied transmission sites in India. Sci. Rep. 2019, 9, 17095. [Google Scholar] [CrossRef] [PubMed]
- Walldorf, J.A.; Cohee, L.M.; Coalson, J.E.; Bauleni, A.; Nkanaunena, K.; Kapito-Tembo, A.; Seydel, K.B.; Ali, D.; Mathanga, D.; Taylor, T.E.; et al. School-Age Children Are a Reservoir of Malaria Infection in Malawi. PLoS ONE 2015, 10, e0134061. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Omondi, C.J.; Otambo, W.O.; Odongo, D.; Ochwedo, K.O.; Otieno, A.; Onyango, S.A.; Orondo, P.; Ondeto, B.M.; Lee, M.-C.; Zhong, D.; et al. Asymptomatic and submicroscopic Plasmodium infections in an area before and during integrated vector control in Homa Bay, western Kenya. Malar. J. 2022, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Okell, L.C.; Bousema, T.; Griffin, J.T.; Ouédraogo, A.L.; Ghani, A.C.; Drakeley, C.J. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat. Commun. 2012, 3, 1237. [Google Scholar] [CrossRef]
- Otambo, W.O.; Omondi, C.J.; Ochwedo, K.O.; Onyango, P.O.; Atieli, H.; Lee, M.-C.; Wang, C.; Zhou, G.; Githeko, A.K.; Githure, J.; et al. Risk associations of submicroscopic malaria infection in lakeshore, plateau and highland areas of Kisumu County in western Kenya. PLoS ONE 2022, 17, e0268463. [Google Scholar] [CrossRef]
| Characteristics | CSIMSJ | HBMSJ | Total n (%) |
|---|---|---|---|
| Total number of patients | 650 | 234 | 884 |
| Age groups n (%) | |||
| <5 years | 89 (13.69) | 16 (6.84) | 105 (10.63) |
| 5–14 years | 181 (27.85) | 25 (10.68) | 206 (19.63) |
| ≥15 years | 380 (58.46) | 193 (82.48) | 573 (70.47) |
| Mean age ± SD | 25.96 ± 0.84 | 32.01 ± 1.26 | 28.97 ± 2.11 |
| Gender n (%) | |||
| Male | 282 (43.38) | 96 (41.03) | 378 (42.21) |
| Female | 368 (56.62) | 138 (58.97) | 506 (57.80) |
| Use of mosquito nets n (%) | |||
| Yes | 604 (92.92) | 201 (85.90) | 805 (89.41) |
| No | 44 (6.77) | 31 (13.28) | 75 (10.03) |
| Unspecified | 2 (0.31) | 2 (0.85) | 4 (0.58) |
| Treatment 15 days before consultation n (%) | |||
| Yes | 45 (6.92) | 65 (27.78) | 110 (17.35) |
| No | 602 (92.62) | 168 (71.79) | 770 (82.21) |
| Unspecified | 3 (0.46) | 1 (0.43) | 4 (0.45) |
| Fever n (%) | |||
| Yes | 524 (80.62) | 123 (52.56) | 647 (66.59) |
| No | 123 (18.92) | 99 (42.31) | 222 (42.31) |
| Unspecified | 3 (0.46) | 12 (5.13) | 15 (2.80) |
| Microscopic infections n (%) | 289 (44.46) | 79 (33.76) | 368 (39.11) |
| Mean geometric of parasite density | 4845.50 ± 5582.91 | 4019.79 ± 4251.38 | 4432.65 ± 4917.45 |
| Site | Quality Control | Sensitivity % (CI: 95%) | Specificity % (CI: 95%) | PPV % (CI: 95%) | NPV % (CI: 95%) | ||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| CSIMSJ | Positive | 143 (22%) | 41 (6.31%) | 49.5 (43.7–55.2) a | 88.6 (85.4–91.9) | 77.7 (71.9–82.6) | 68.7 (66.0–71.2) |
| Negative | 146 (22.46%) | 320 (49.23%) | |||||
| HBMSJ | Positive | 26 (11.11%) | 32 (13.67%) | 32.9 (22.5–43.3) b | 79.4 (73.2–85.7) | 44.8 (34.3–55.8) | 69.9 (66.1–73.4) |
| Negative | 53 (22.65%) | 123 (52.56%) | |||||
| Species | CSIMSJ Microscopists n (%) | Experts n (%) | HBMSJ Microscopists n (%) | Experts n (%) | All Sites n (%) | Experts n (%) |
|---|---|---|---|---|---|---|
| Pf | 184 (100%) | 261 (90.31%) | 58 (100%) | 70 (88.61%) | 242 (100%) | 331 (89.94%) |
| Pm | 0 | 15 (5.19%) | 0 | 1 (1.27%) | 0 | 16 (4.35%) |
| Po | 0 | 8 (2.77%) | 0 | 2 (2.53%) | 0 | 10 (2.72%) |
| Pf + Pm | 0 | 4 (1.38%) | 0 | 6 (7.59%) | 0 | 10 (2.72%) |
| Pf + Po | 0 | 1 (0.35%) | 0 | 0 | 0 | 1 (0.27%) |
| Characteristics | Prevalence of Microscopic Infection % (n/N) | p-Value | Mean of Log 10 Parasite Density + SD | p-Value | Prevalence of Submicroscopic Infections % (n/N) | p-Value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 42.27 (156/378) | 0.851 | 8.65 ± 2.73 | 0.128 | 33.33 (126/378) | 0.196 |
| Female | 41.90 (212/506) | 8.23 ± 2.41 | 37.55 (190/506) | |||
| Use of mosquito nets * | ||||||
| Yes | 39.63 (319/805) | <0.001 | 8.65 ± 2.32 | 0.898 | 37.38 (299/805) | 0.005 |
| No | 60 (45/75) | 8.66 ± 2.33 | 21.25 (17/75) | |||
| Treatment 15 days before consultation * | ||||||
| Yes | 40.91 (45/110) | 0.917 | 8.88 ± 2.31 | 0.308 | 30.91 (34/110) | 0.165 |
| No | 41.43 (319/770) | 8.34 ± 2.59 | 36.62 (282/770) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fila-Fila, G.P.U.; Koukouikila-Koussounda, F.; Niama, F.R.; Bissombolo Madingou, L.P.; Demboux, J.E.; Mandiangou, A.F.; Vembe Mahounga, S.; Doniama, A.J.; Dossou-Yovo, L.R.; Casimiro, P.N.; et al. Quality Control of Microscopic Diagnosis of Malaria in Healthcare Facilities and Submicroscopic Infections in Mossendjo, the Department of Niari, the Republic of the Congo. Pathogens 2024, 13, 709. https://doi.org/10.3390/pathogens13080709
Fila-Fila GPU, Koukouikila-Koussounda F, Niama FR, Bissombolo Madingou LP, Demboux JE, Mandiangou AF, Vembe Mahounga S, Doniama AJ, Dossou-Yovo LR, Casimiro PN, et al. Quality Control of Microscopic Diagnosis of Malaria in Healthcare Facilities and Submicroscopic Infections in Mossendjo, the Department of Niari, the Republic of the Congo. Pathogens. 2024; 13(8):709. https://doi.org/10.3390/pathogens13080709
Chicago/Turabian StyleFila-Fila, Grâce Petula Urielle, Felix Koukouikila-Koussounda, Fabien Roch Niama, Lauriate Prudencie Bissombolo Madingou, Jordy Exaucé Demboux, Aldi Fred Mandiangou, Stéphane Vembe Mahounga, Ahmed Jordy Doniama, Louis Régis Dossou-Yovo, Prisca Nadine Casimiro, and et al. 2024. "Quality Control of Microscopic Diagnosis of Malaria in Healthcare Facilities and Submicroscopic Infections in Mossendjo, the Department of Niari, the Republic of the Congo" Pathogens 13, no. 8: 709. https://doi.org/10.3390/pathogens13080709







