Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Serological Testing
2.3. Epidemiological Data Analysis
2.4. Molecular Analysis
2.5. Genotyping of Brucella Samples
3. Results
3.1. Animal Brucellosis Epidemic Profile during 2015–2022
3.2. Real-Time PCR Analysis
3.3. MLVA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gwida, M.; Al Dahouk, S.; Melzer, F.; Rösler, U.; Neubauer, H.; Tomaso, H. Brucellosis—Regionally Emerging Zoonotic Disease? Croat. Med. J. 2010, 51, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; El-Diasty, M.; Melzer, F.; Schmoock, G.; Moustafa, S.A.; El-Beskawy, M.; Khater, D.F.; Hamdy, M.E.R.; Zaki, H.M.; Ferreira, A.C.; et al. MLVA-16 Genotyping of Brucella abortus and Brucella melitensis isolates from Different Animal Species in Egypt: Geographical Relatedness and the Mediterranean Lineage. Pathogens 2020, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.L.; Goenka, R. Host Immune Responses to the Intracellular Bacteria Brucella: Does the Bacteria Instruct the Host to Facilitate Chronic Infection? Crit. Rev. Immunol. 2006, 26, 407–442. [Google Scholar] [CrossRef]
- Jamil, T.; Akar, K.; Erdenlig, S.; Murugaiyan, J.; Sandalakis, V.; Boukouvala, E.; Psaroulaki, A.; Melzer, F.; Neubauer, H.; Wareth, G. Spatio-Temporal Distribution of Brucellosis in European Terrestrial and Marine Wildlife Species and Its Regional Implications. Microorganisms 2022, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Garofolo, G.; Di Giannatale, E.; De Massis, F.; Zilli, K.; Ancora, M.; Cammà, C.; Calistri, P.; Foster, J.T. Investigating genetic diversity of Brucella abortus and Brucella melitensis in Italy with MLVA-16. Infect. Genet. Evol. 2013, 19, 59–70. [Google Scholar] [CrossRef]
- Lytras, T.; Danis, K.; Dounias, G. Incidence Patterns and Occupational Risk Factors of Human Brucellosis in Greece, 2004–2015. Int. J. Occup. Environ. Med. 2016, 7, 221–226. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Brucellosis. In ECDC. Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2023. [Google Scholar]
- Katsiolis, A.; Papanikolaou, E.; Stournara, A.; Giakkoupi, P.; Papadogiannakis, E.; Zdragas, A.; Giadinis, N.D.; Petridou, E. Molecular detection of Brucella spp. in ruminant herds in Greece. Trop. Anim. Health Prod. 2022, 54, 173. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zahoor, M. An Overview of Brucellosis in Cattle and Humans, and its Serological and Molecular Diagnosis in Control Strategies. Trop. Med. Infect. Dis. 2018, 3, 65. [Google Scholar] [CrossRef]
- Kumar, V.; Bansal, N.; Nanda, T.; Kumar, A.; Kumari, R.; Maan, S. PCR Based Molecular Diagnostic Assays for Brucellosis: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2666–2681. [Google Scholar] [CrossRef]
- Kurmanov, B.; Zincke, D.; Su, W.; Hadfield, T.L.; Aikimbayev, A.; Karibayev, T.; Berdikulov, M.; Orynbayev, M.; Nikolich, M.P.; Blackburn, J.K. Assays for Identification and Differentiation of Brucella Species: A Review. Microorganisms 2022, 10, 1584. [Google Scholar] [CrossRef]
- Hinić, V.; Brodard, I.; Thomann, A.; Cvetnić, Ž.; Makaya, P.V.; Frey, J.; Abril, C. Novel identification and differentiation of Brucella melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae suitable for both conventional and real-time PCR systems. J. Microbiol. Methods 2008, 75, 375–378. [Google Scholar]
- Hinić, V.; Brodard, I.; Thomann, A.; Holub, M.; Miserez, R.; Abril, C. IS711-based real-time PCR assay as a tool for detection of Brucella spp. in wild boars and comparison with bacterial isolation and serology. BMC Vet. Res. 2009, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Le Flèche, P.; Jacques, I.; Grayon, M.; Al Dahouk, S.; Bouchon, P.; Denoeud, F.; Nöckler, K.; Neubauer, H.; Guilloteau, L.A.; Vergnaud, G. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006, 6, 9. [Google Scholar] [CrossRef]
- Al Dahouk, S.; Le Flèche, P.; Nöckler, K.; Jacques, I.; Grayon, M.; Scholz, H.C.; Tomaso, H.; Vergnaud, G.; Neubauer, H. Evaluation of Brucella MLVA typing for human brucellosis. J. Microbiol. Methods 2007, 69, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Fouskis, I.; Sandalakis, V.; Christidou, A.; Tsatsaris, A.; Tzanakis, N.; Tselentis, Y.; Psaroulaki, A. The epidemiology of Brucellosis in Greece, 2007–2012: A ‘One Health’ approach. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 124–135. [Google Scholar] [CrossRef] [PubMed]
- World Organization of Animal Health. Terrestrial Manual 2018, Chapter 3.1.4.—Brucellosis (Infection with Brucella abortus, B. melitensis and B. suis); World Organization of Animal Health: Paris, France, 2022; pp. 1–47. [Google Scholar]
- Probert, W.S.; Schrader, K.N.; Khuong, N.Y.; Bystrom, S.L.; Graves, M.H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbiol. 2004, 42, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Ogunkolade, W.; Bustin, S.A. SPUD: A quantitative PCR assay for the detection of inhibitors in nucleic acid preparations. Anal. Biochem. 2006, 351, 308–310. [Google Scholar] [CrossRef]
- Brangsch, H.; Sandalakis, V.; Babetsa, M.; Boukouvala, E.; Ntoula, A.; Makridaki, E.; Christidou, A.; Psaroulaki, A.; Akar, K.; Gürbilek, S.E.; et al. Genotype diversity of brucellosis agents isolated from humans and animals in Greece based on whole-genome sequencing. BMC Infect. Dis. 2023, 23, 529. [Google Scholar] [CrossRef]
- Pal, M.; Gizaw, F.; Fekadu, G.; Alemayehu, G.; Kandi, V. Public Health and Economic Importance of Bovine Brucellosis: An Overview. Am. J. Epidemiol. Infect. Dis. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Katsiolis, A.; Papadopoulos, D.K.; Giantsis, I.A.; Papageorgiou, K.; Zdragas, A.; Giadinis, N.D.; Petridou, E. Brucella spp. distribution, hosting ruminants from Greece, applying various molecular identification techniques. BMC Vet. Res. 2022, 18, 202. [Google Scholar] [CrossRef]
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Godfroid, J. Brucellosis in livestock and wildlife: Zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health 2017, 75, 34. [Google Scholar] [CrossRef] [PubMed]
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Vourvidis, D.; Kyrma, A.; Linou, M.; Edouard, S.; Angelakis, E. Sero-epidemiology investigation of Coxiella burnetii in domestic ruminants throughout most Greek regions. Vet. Med. Sci. 2021, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
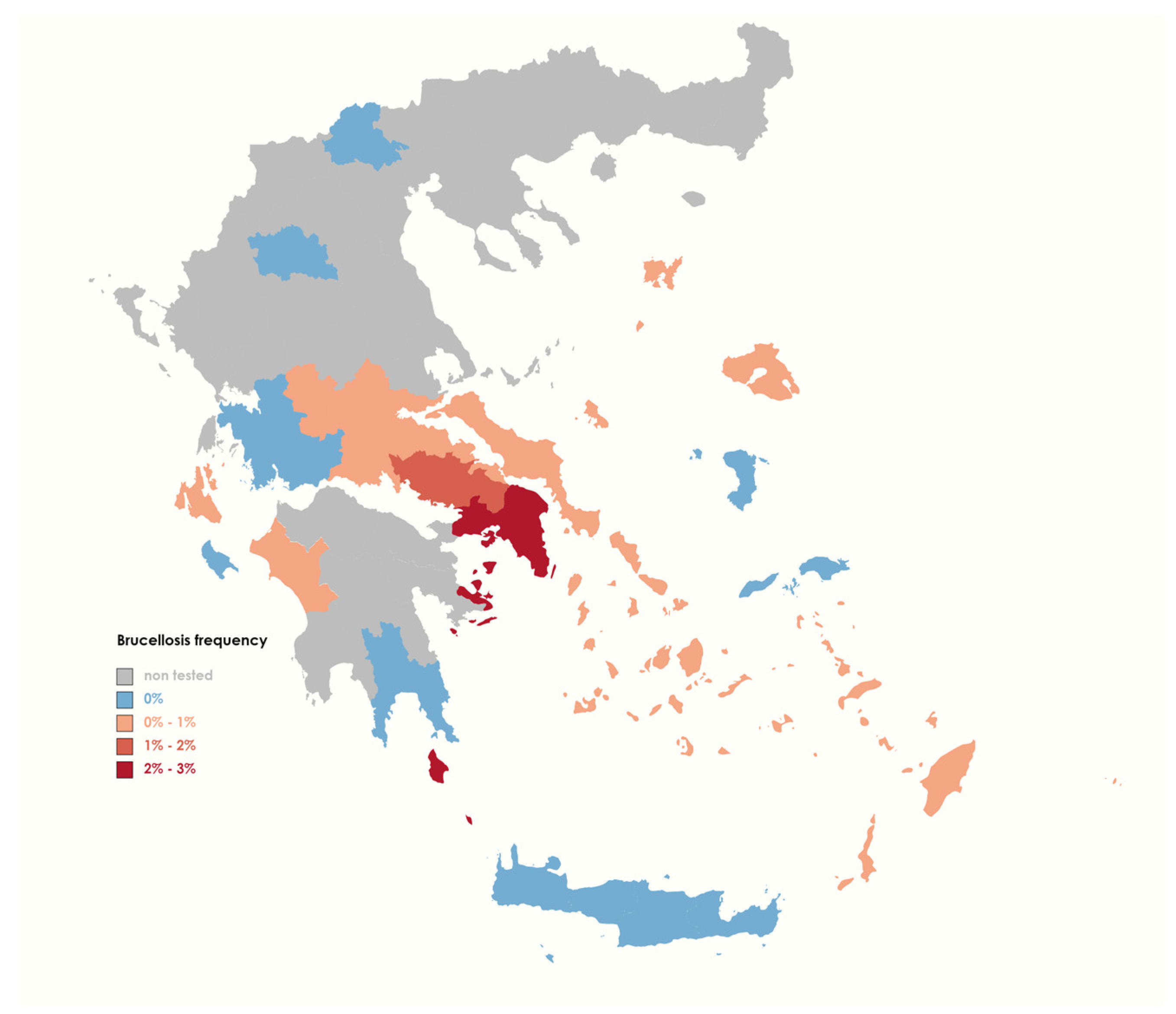
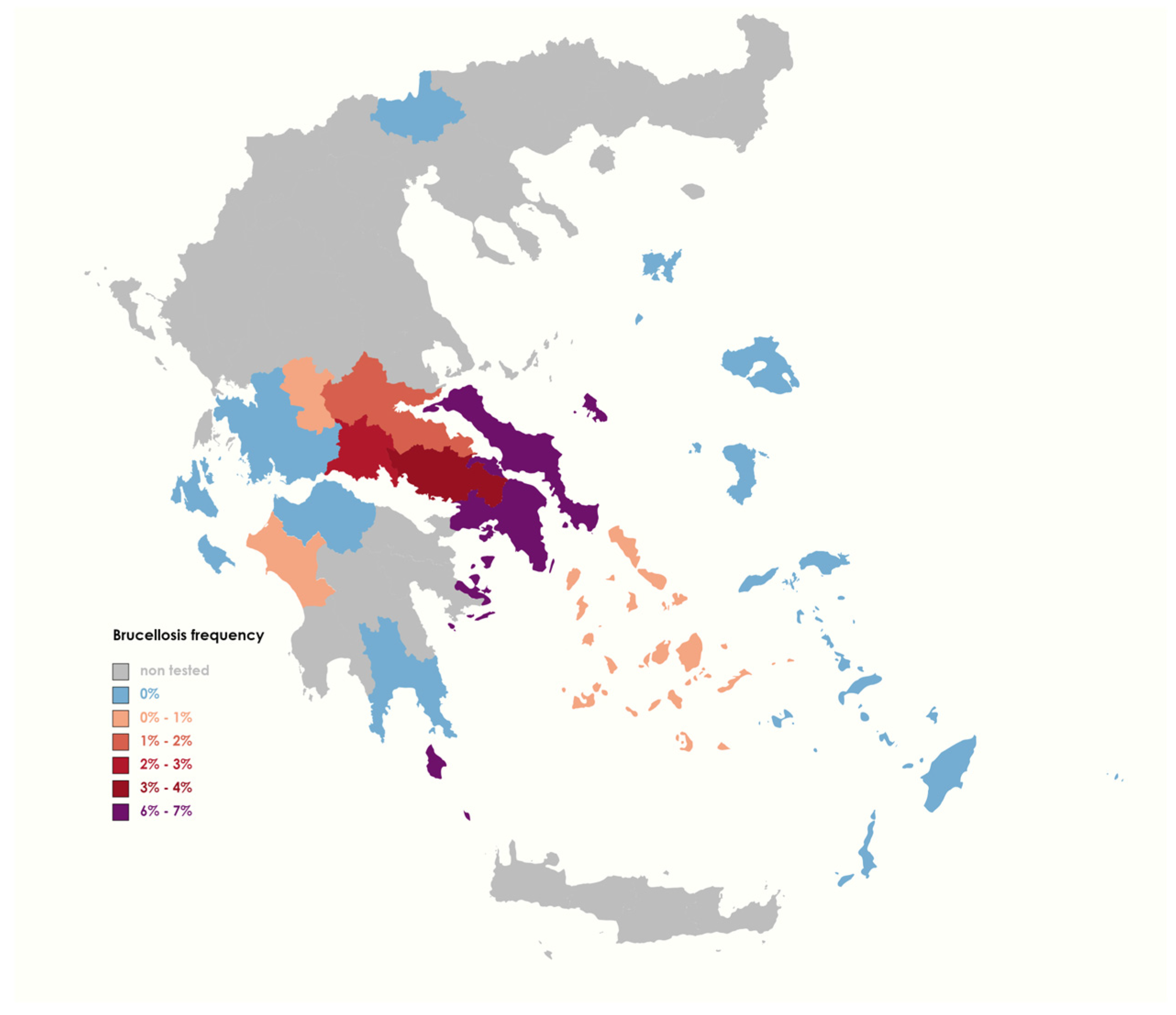
| Region | Tested | Positive | Positivity (%) |
|---|---|---|---|
| Aetolia–Acarnania | 195 | 0 | 0 |
| Attica | 24,771 | 557 | 2.25 |
| Boeotia | 5317 | 63 | 1.18 |
| Cephalonia | 115,234 | 15 | 0.01 |
| Chania | 25,388 | 0 | 0 |
| Chios | 8588 | 0 | 0 |
| Cyclades | 133,591 | 2 | 0.001 |
| Dodekanese | 42,633 | 128 | 0.3 |
| Elis | 129 | 1 | 0.78 |
| Euboea | 22,787 | 98 | 0.43 |
| Evrytania | 5910 | 1 | 0.02 |
| Grevena | 49 | 0 | 0 |
| Heraklion | 34,637 | 0 | 0 |
| Laconia | 37 | 0 | 0 |
| Lasithi | 8240 | 0 | 0 |
| Lesbos | 26,618 | 32 | 0.12 |
| Pella | 60 | 0 | 0 |
| Phocis | 796 | 1 | 0.13 |
| Pthiotis | 33,813 | 202 | 0.01 |
| Rethymno | 150,952 | 0 | 0 |
| Samos and Ikaria | 12,055 | 0 | 0 |
| Zakynthos | 2399 | 0 | 0 |
| Total | 654,199 | 1100 | 0.17 |
| Region | Tested | Positive | Positivity (%) |
|---|---|---|---|
| Achaea | 116 | 0 | 0 |
| Aetolia–Acarnania | 297 | 0 | 0 |
| Attica | 12,914 | 900 | 6.97 |
| Boeotia | 22,487 | 861 | 3.8 |
| Cephalonia | 311 | 0 | 0 |
| Chios | 177 | 0 | 0 |
| Cyclades | 10,552 | 3 | 0.03 |
| Dodekanese | 2329 | 0 | 0 |
| Euboea | 1972 | 129 | 6.54 |
| Evrytania | 750 | 1 | 0.13 |
| Kilkis | 35 | 0 | 0 |
| Laconia | 13 | 0 | 0 |
| Lesbos | 117 | 0 | 0 |
| Phokis | 760 | 21 | 2.76 |
| Pthiotis | 3938 | 53 | 1.35 |
| Samos and Ikaria | 443 | 0 | 0 |
| Zakynthos | 5 | 0 | 0 |
| Total | 57,216 | 1968 | 3.4 |
| Primer Set | Forward Seq 5′-3′ | Reverse Seq 5′-3′ | Gene Locus | Repeat | NOR Sample A | NOR Sample B |
|---|---|---|---|---|---|---|
| Bruce 04 | CTGACGAAGGGAAGGCAATAAG | CGATCTGGAGATTATCGGGAAG | BMEI1496 | AGGGCAGT | 2 | 2 |
| Bruce 07 | GCTGACGGGGAAGAACATCTAT | ACCCTTTTTCAGTCAAGGCAAA | BMEI1204 | GAATAGGG | 4 | 4 |
| Bruce 09 | GCGGATTCGTTCTTCAGTTATC | GGGAGTATGTTTTGGTTGTACATAG | BMEI0570 | AGGGCAGT | 2 | 2 |
| Bruce 16 | ACGGGAGTTTTTGTTGCTCAAT | GGCCATGTTTCCGTTGATTTAT | BMEII0523 | TAAGGGAG | 3 | 3 |
| Bruce 18 | TATGTTAGGGCAATAGGGCAGT | GATGGTTGAGAGCATTGTGAAG | BMEII0326 | AGTAAGGG | 2 | 2 |
| Bruce 30 | TGACCGCAAAACCATATCCTTC | TATGTGCAGAGCTTCATGTTCG | downstream of BMEI1453 | AGGGCAGT | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanouil, M.; Vourvidis, D.; Kyrma, A.; Makka, S.; Horefti, E.; Angelakis, E. Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains. Pathogens 2024, 13, 720. https://doi.org/10.3390/pathogens13090720
Emmanouil M, Vourvidis D, Kyrma A, Makka S, Horefti E, Angelakis E. Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains. Pathogens. 2024; 13(9):720. https://doi.org/10.3390/pathogens13090720
Chicago/Turabian StyleEmmanouil, Mary, Dimitrios Vourvidis, Anna Kyrma, Sofia Makka, Elina Horefti, and Emmanouil Angelakis. 2024. "Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains" Pathogens 13, no. 9: 720. https://doi.org/10.3390/pathogens13090720
APA StyleEmmanouil, M., Vourvidis, D., Kyrma, A., Makka, S., Horefti, E., & Angelakis, E. (2024). Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains. Pathogens, 13(9), 720. https://doi.org/10.3390/pathogens13090720






