Estimating the Seroprevalence of Scrub Typhus in Nepal
Abstract
1. Introduction
2. Materials and Methods
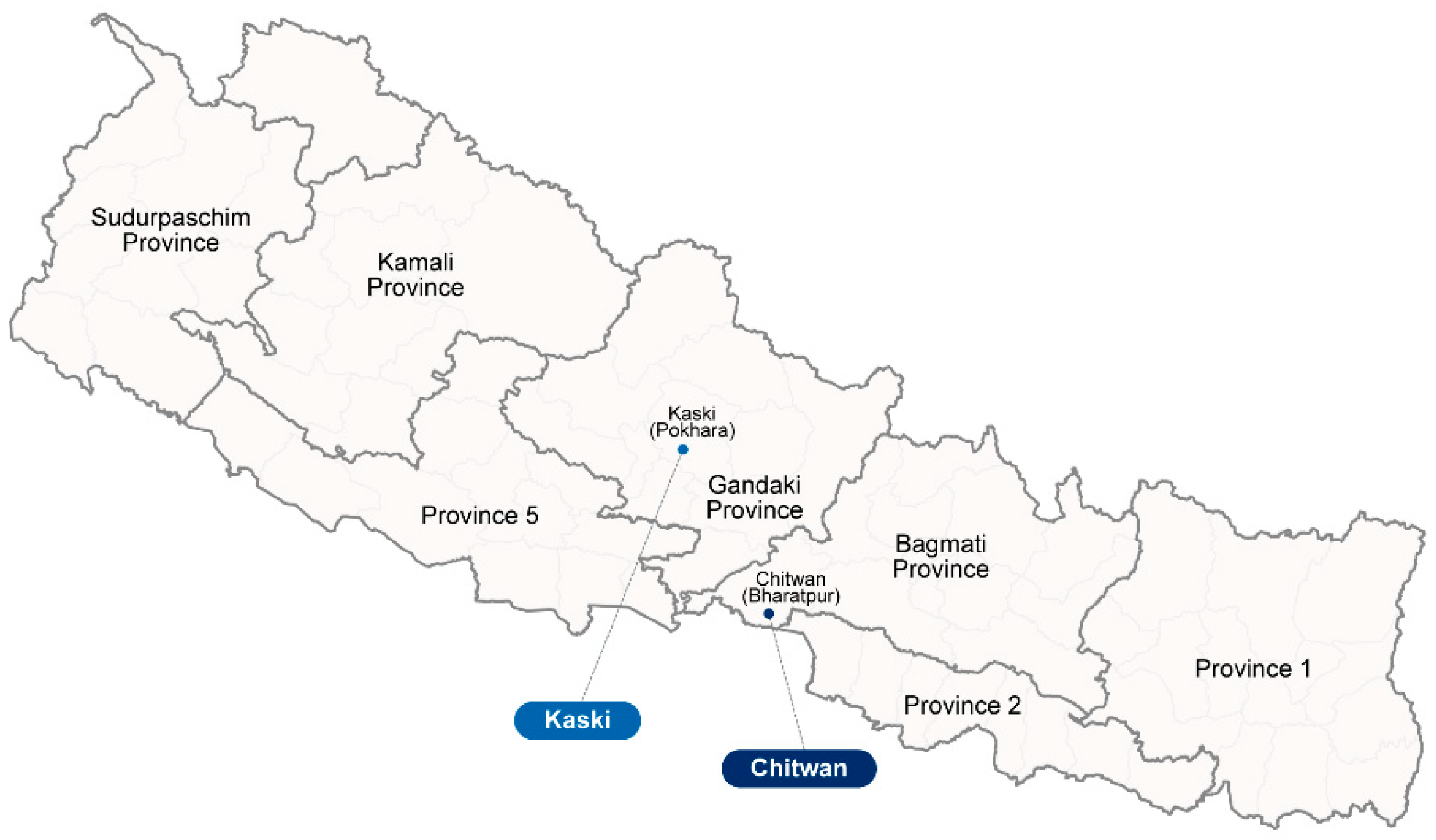
2.1. Study Sites, Patient Samples, and Ethical Approval
2.2. Enzyme-Linked Immunosorbent Assay for O. tsutsugamushi-Specific Antibodies
3. Data Interpretation and Analysis
Statistical Analysis
4. Results
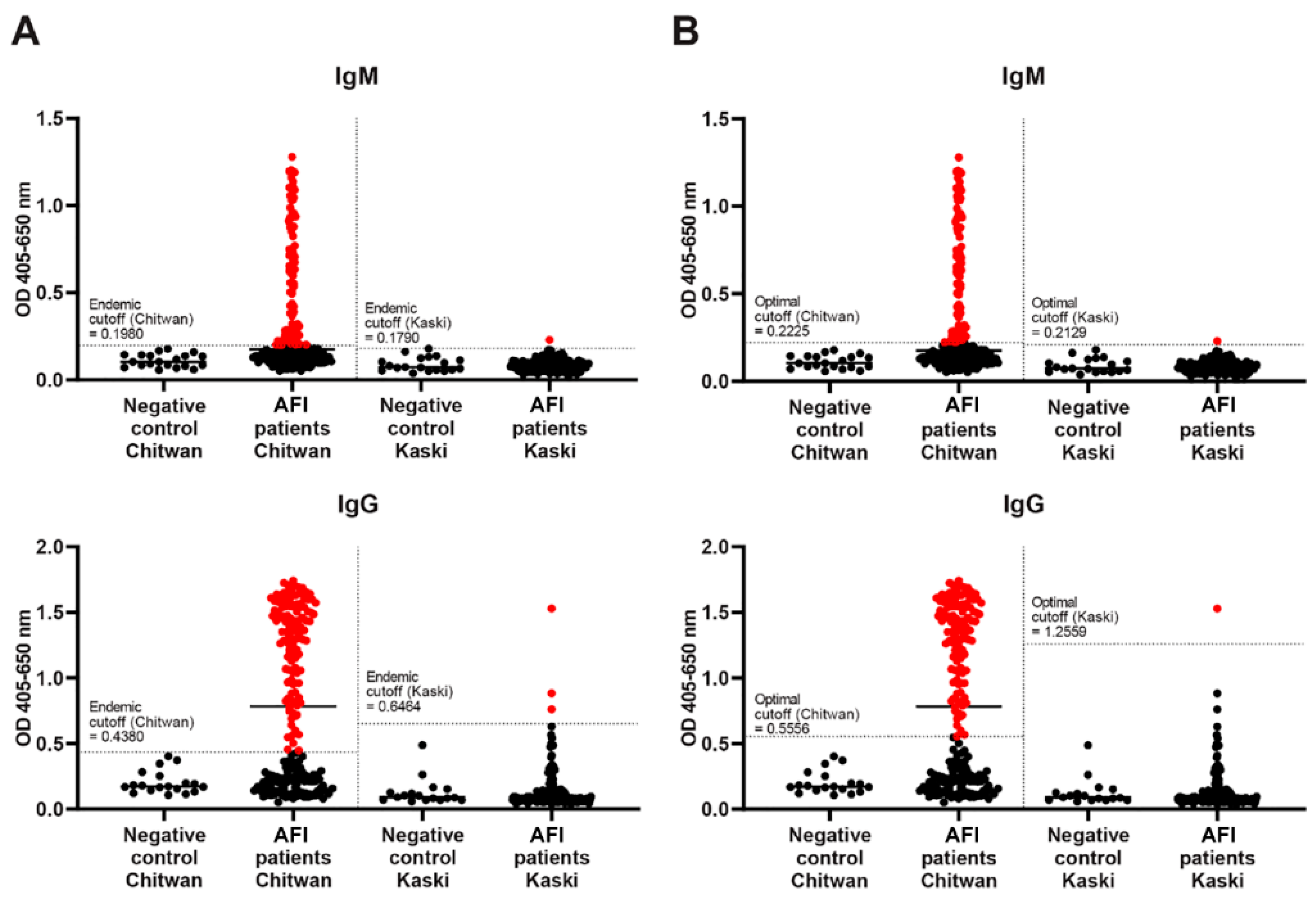
4.1. ELISA Diagnostic Cutoff
4.2. Demographic Variables of Scrub Typhus Patients
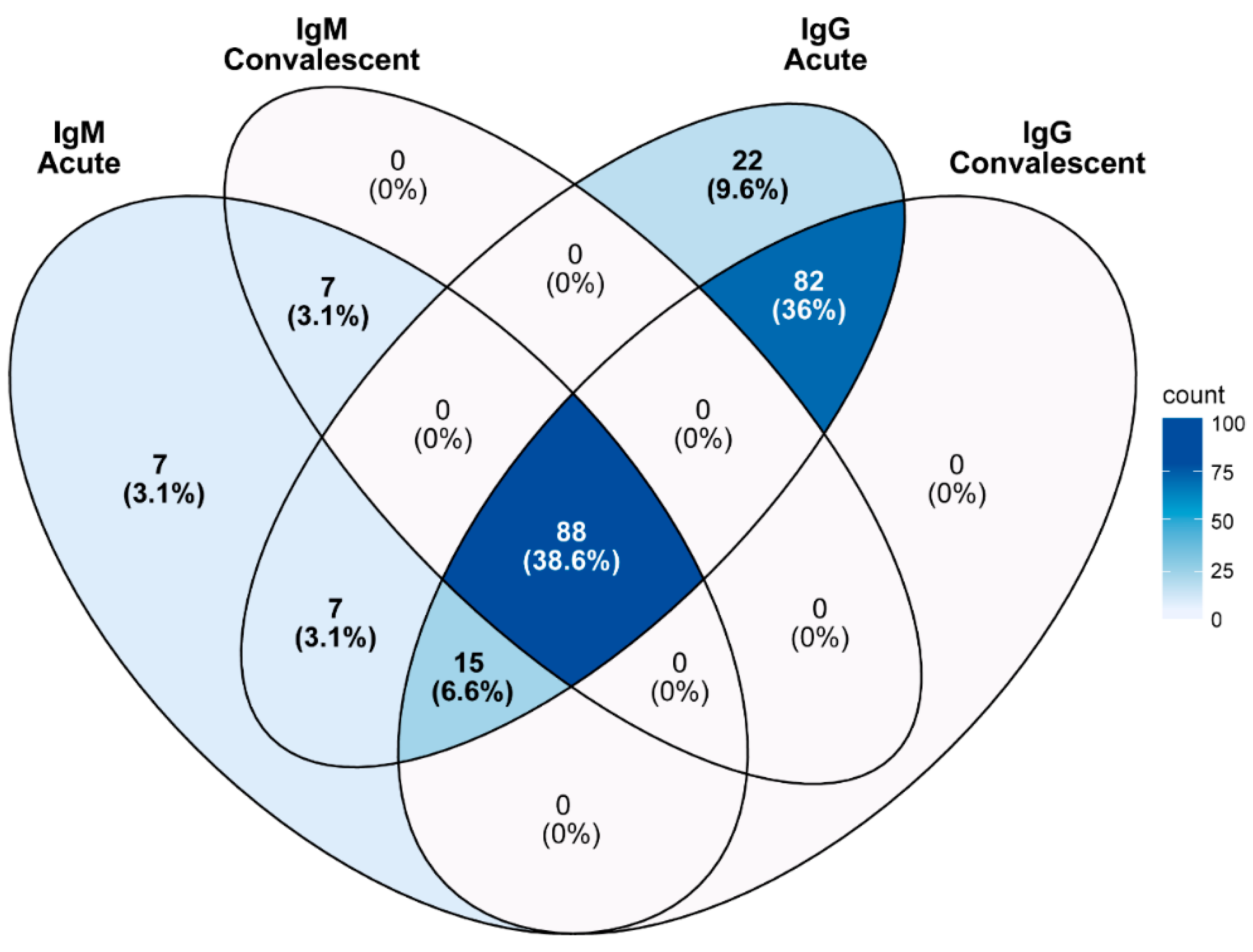
4.3. Co-Infection with Other Pathogens
4.4. Population Background for Scrub Typhus Infection
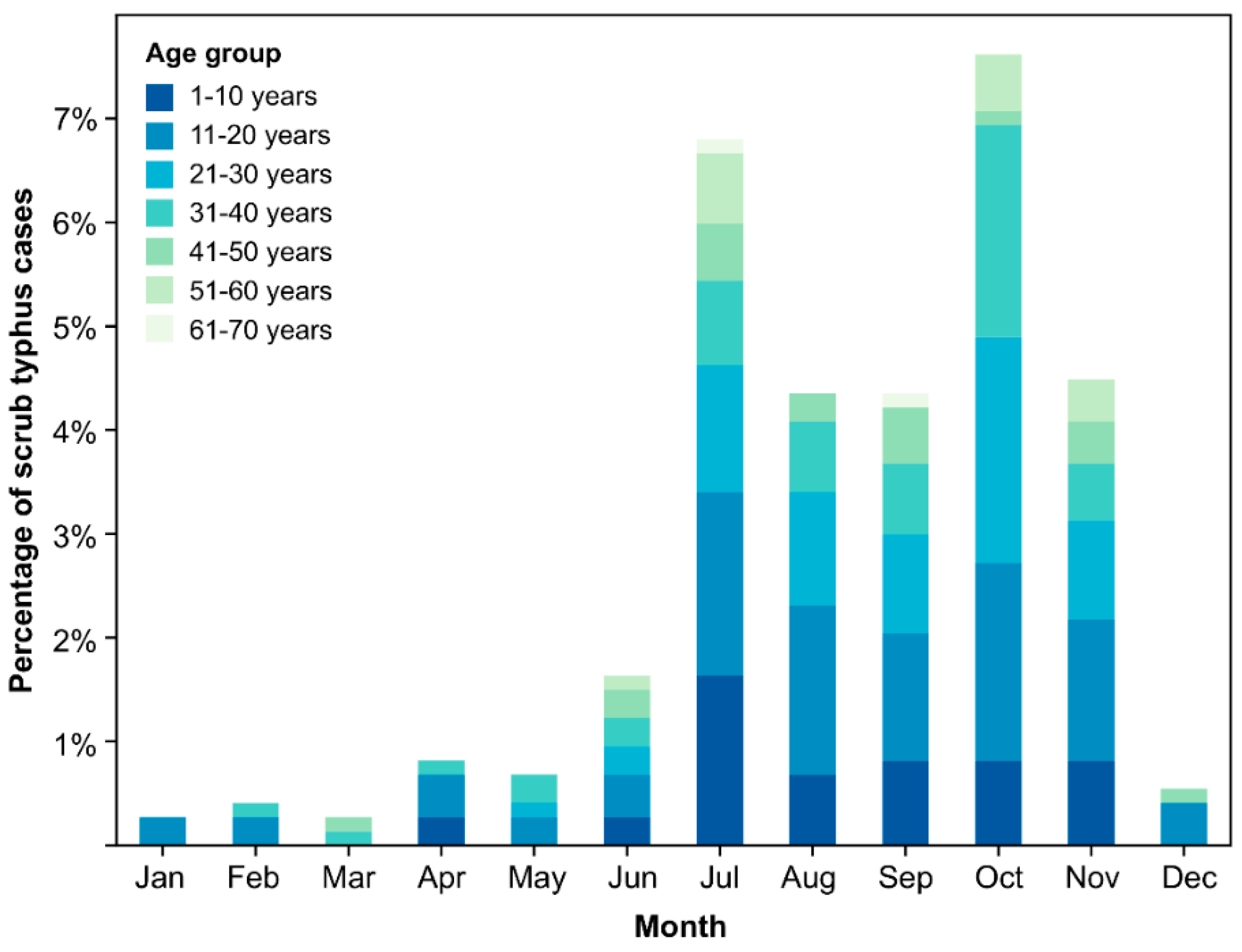
4.5. Geography, Seasonality, and Dynamics of Scrub Typhus in Nepal
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimers
References
- Ponnusamy, L.; Garshong, R.; McLean, B.S.; Wasserberg, G.; Durden, L.A.; Crossley, D.; Apperson, C.S.; Roe, R.M. Rickettsia felis and other Rickettsias species in chigger mites collected from wild rodents in North Carolina, USA. Microorganisms 2022, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Abarca, K.; Martínez-Valdebenito, C.; Angulo, J.; Jiang, J.; Farris, C.M.; Richards, A.L.; Acosta-Jamett, G.; Weitzel, T. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg. Infect. Dis. 2020, 26, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Martínez-Valdebenito, C.; Acosta-Jamett, G.; Jiang, J.; Richards, A.L.; Abarca, K. Scrub typhus in continental Chile, 2016–2018. Emerg. Infect. Dis. 2019, 25, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Masakhwe, C.; Linsuwanon, P.; Kimita, G.; Mutai, B.; Leepitakrat, S.; Yalwala, S.; Abuom, D.; Auysawasi, N.; Gilbreath, T.; Wanja, E.; et al. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J. Clin. Microbiol. 2018, 56, e01124-18. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef]
- Luce-Fedrow, A.; Lehman, M.L.; Kelly, D.J.; Mullins, K.; Maina, A.N.; Stewart, R.L.; Ge, H.; John, H.S.; Jiang, J.; Richards, A.L. A review of scrub typhus (Orientia tsutsugamushi and related organisms): Then, now, and tomorrow. Trop. Med. Infect. Dis. 2018, 3, 8. [Google Scholar] [CrossRef]
- Moniuszko, H.; Wojnarowski, K.; Cholewińska, P. Not only Leptotrombidium spp. an annotated checklist of chigger mites (Actinotrichida: Trombiculidae) associated with bacterial pathogens. Pathogens 2022, 11, 1084. [Google Scholar] [CrossRef]
- Taylor, A.J.; Paris, D.H.; Newton, P.N. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl. Trop. Dis. 2015, 9, e0003971. [Google Scholar] [CrossRef]
- Paris, D.H.; Shelite, T.R.; Day, N.P.; Walker, D.H. Unresolved problems related to scrub typhus: A seriously neglected life-threatening disease. Am. J. Trop. Med. Hyg. 2013, 89, 301–307. [Google Scholar] [CrossRef]
- Dey, S. Devastating disaster: A case study of Nepal earthquake and its impact on human beings. IOSR JHSS 2015, 20, 28–34. [Google Scholar]
- Adhikari, B.; Mishra, S.R.; Marahatta, S.B.; Kaehler, N.; Paudel, K.; Adhikari, J.; Raut, S. Earthquakes, fuel crisis, power outages, and health care in Nepal: Implications for the future. Disaster Med. Public Health Prep. 2017, 11, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.P.; Shakya, G.; Adhikari, S.; Rijal, N.; Acharya, J.; Maharjan, L.; Marasini, B.R. Scrub Typhus: An Emerging Neglected Tropical Disease in Nepal. J. Nepal Health Res. Counc. 2016, 14, 122–127. [Google Scholar] [PubMed]
- Dhimal, M.; Dumre, S.P.; Sharma, G.N.; Khanal, P.; Ranabhat, K.; Shah, L.P.; Lal, B.K.; Jha, R.; Upadhyaya, B.P.; Acharya, B.; et al. An outbreak investigation of scrub typhus in Nepal: Confirmation of local transmission. BMC Infect. Dis. 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Ruang-Areerate, T.; Jeamwattanalert, P.; Rodkvamtook, W.; Richards, A.L.; Sunyakumthorn, P.; Gaywee, J. Genotype diversity and distribution of Orientia tsutsugamushi causing scrub typhus in Thailand. J. Clin. Microbiol. 2011, 49, 2584–2589. [Google Scholar] [CrossRef]
- Gaywee, J.; Sunyakumthorn, P.; Rodkvamtook, W.; Ruang-areerate, T.; Mason, C.J.; Sirisopana, N. Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg. Infect. Dis. 2007, 13, 671–673. [Google Scholar] [CrossRef]
- Chao, C.C.; Huber, E.S.; Porter, T.B.; Zhang, Z.; Ching, W.M. Analysis of the cross-reactivity of various 56 kDa recombinant protein antigens with serum samples collected after Orientia tsutsugamushi infection by ELISA. Am. J. Trop. Med. Hyg. 2011, 84, 967–972. [Google Scholar] [CrossRef]
- Chao, C.-C.; Zhang, Z.; Belinskaya, T.; Thipmontree, W.; Tantibhedyangkul, W.; Silpasakorn, S.; Wongsawat, E.; Suputtamongkol, Y.; Ching, W.-M. An ELISA assay using a combination of recombinant proteins from multiple strains of Orientia tsutsugamushi offers an accurate diagnosis for scrub typhus. BMC Infect. Dis. 2017, 17, 413. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhang, Z.; Huber, E.; Mutumanje, E.; Chao, C.C.; Ching, W.M. Kinetics and magnitude of antibody responses against the conserved 47-kilodalton antigen and the variable 56-kilodalton antigen in scrub typhus patients. Clin. Vaccine Immunol. 2011, 18, 1021–1027. [Google Scholar] [CrossRef]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Phanichkrivalkosil, M.; Tanganuchitcharnchai, A.; Jintaworn, S.; Kantipong, P.; Laongnualpanich, A.; Chierakul, W.; Paris, D.H.; Richards, A.L.; Wangrangsimakul, T.; Day, N.P.J.; et al. Determination of optimal diagnostic cut-offs for the naval medical research center scrub typhus IgM ELISA in Chiang Rai, Thailand. Am. J. Trop. Med. Hyg. 2019, 100, 1134–1140. [Google Scholar] [CrossRef]
- Katoh, S.; Cuong, N.C.; Hamaguchi, S.; Thuy, P.T.; Cuong, D.D.; Anh, L.K.; Anh, N.T.H.; Anh, D.D.; Sando, E.; Suzuki, M.; et al. Challenges in diagnosing scrub typhus among hospitalized patients with undifferentiated fever at a national tertiary hospital in northern Vietnam. PLoS Negl. Trop. Dis. 2019, 13, e0007928. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Parajuli, K.; Tshokey, T.; Stenos, J.; Sherchand, J.B. Diagnostic evaluation of IgM ELISA and IgM Immunofluorescence assay for the diagnosis of acute scrub typhus in central Nepal. BMC Infect. Dis. 2020, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Lim, C.; Tanganuchitcharnchai, A.; Jintaworn, S.; Kantipong, P.; Richards, A.L.; Paris, D.H.; Limmathurotsakul, D.; Day, N.P.J. Optimal cutoff and accuracy of an IgM enzyme-linked immunosorbent assay for diagnosis of acute scrub typhus in northern Thailand: An alternative reference method to the IgM immunofluorescence assay. J. Clin. Microbiol. 2016, 54, 1472–1478. [Google Scholar] [CrossRef]
- Gupta, N.; Chaudhry, R.; Thakur, C.K. Determination of cutoff of ELISA and immunofluorescence assay for scrub typhus. J. Glob. Infect. Dis. 2016, 8, 97–99. [Google Scholar]
- Blacksell, S.D.; Tanganuchitcharnchai, A.; Nawtaisong, P.; Kantipong, P.; Laongnualpanich, A.; Day, N.P.J.; Paris, D.H. Diagnostic accuracy of the inbios scrub typhus detect enzyme-linked immunoassay for the detection of IgM antibodies in northern Thailand. Clin. Vaccine Immunol. 2015, 23, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Hamal, P.; Chaudhary, N.K.; Sapkota, L.B.; Singh, J.P. Burden of scrub typhus among patients with acute febrile illness attending tertiary care hospital in Chitwan, Nepal. BMJ Open 2020, 10, e034727. [Google Scholar] [CrossRef]
- Gautam, R.; Parajuli, K.; Sherchand, J.B. Epidemiology, risk factors and seasonal variation of scrub typhus fever in central Nepal. Trop. Med. Infect. Dis. 2019, 4, 27. [Google Scholar] [CrossRef]
- Thompson, C.N.; Blacksell, S.D.; Paris, D.H.; Arjyal, A.; Karkey, A.; Dongol, S.; Giri, A.; Dolecek, C.; Day, N.; Baker, S.; et al. Undifferentiated febrile illness in Kathmandu, Nepal. Am. J. Trop. Med. Hyg. 2015, 92, 875–878. [Google Scholar] [CrossRef]
- Pokhrel, A.; Rayamajhee, B.; Khadka, S.; Thapa, S.; Kapali, S.; Pun, S.B.; Banjara, M.R.; Joshi, P.; Lekhak, B.; Rijal, K.R. Seroprevalence and clinical features of scrub typhus among febrile patients attending a referral hospital in Kathmandu, Nepal. Trop. Med. Infect. Dis. 2021, 6, 78. [Google Scholar] [CrossRef]

| Diagnosis | Serology Result | % Positivity |
|---|---|---|
| Scrub typhus | Suspected cases: single titer of IgG or IgM or both ≥400 | 21.5% (163) |
| Confirmed cases: 4-fold increase in IgG or IgM titer in paired samples | 8.0% (61) | |
| Confirmed cases: 56tsa PCR positive only | 1.4% (11) | |
| Confirmed cases: Positive in both serology and PCR | 0.6% (4) | |
| Unknown | Negative by all criteria | 68.5% (520) |
| Variable | Febrile Cases (n = 759) | Scrub Typhus Cases | p-Value | Odds Ratio | 95% Confidence Interval | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases (n = 759) a | Female (n = 345) a | Male (n = 414) a | ||||||||
| No. | % | No. | % | No. | % | |||||
| Scrub Typhus Case | ||||||||||
| Positive | 239 | 239 | (31.5%) | 125 | (36.2%) | 114 | (27.5%) | 0.013 * | 1.495 | 1.099–2.034 |
| Negative | 520 | 520 | (68.5%) | 220 | (63.8%) | 300 | (72.5%) | 1 | ||
| Age Group b | ||||||||||
| 1–10 years | 177 | 39 | (5.1%) | 17 | (4.9%) | 22 | (5.3%) | 1 | 0.773 | 0.045–13.268 |
| 11–20 | 241 | 73 | (9.6%) | 33 | (9.6%) | 40 | (9.7%) | 1 | 0.825 | 0.049–13.702 |
| 21–30 | 168 | 50 | (6.6%) | 27 | (7.8%) | 23 | (5.6%) | 1 | 1.174 | 0.069–19.835 |
| 31–40 | 91 | 42 | (5.5%) | 30 | (8.7%) | 12 | (2.9%) | 1 | 2.5 | 0.144–43.287 |
| 41–50 | 41 | 18 | (2.4%) | 10 | (2.9%) | 8 | (1.9%) | 1 | 1.25 | 0.067–23.261 |
| 51–60 | 26 | 13 | (1.7%) | 6 | (1.7%) | 7 | (1.7%) | 1 | 0.857 | 0.044–16.850 |
| 61–70 | 11 | 2 | (0.3%) | 1 | (0.3%) | 1 | (0.2%) | 1 | 1 | 0.020–50.400 |
| 71 and above | 4 | 2 | (0.3%) | 1 | (0.3%) | 1 | (0.2%) | 1 | ||
| Location b | ||||||||||
| Chitwan | 449 | 219 | (28.9%) | 116 | (33.6%) | 103 | (24.9%) | 0.653 | 1.376 | 0.548–3.454 |
| Kaski | 310 | 20 | (2.6%) | 9 | (2.6%) | 11 | (2.7%) | 1 | ||
| Seasonal Variation b | ||||||||||
| 2009–2010 | ||||||||||
| January | 20 | 3 | (0.4%) | 1 | (0.3%) | 2 | (0.5%) | 1 | ||
| February | 21 | 3 | (0.4%) | 2 | (0.6%) | 1 | (0.2%) | 1 | 4 | 0.134–119.237 |
| March | 30 | 2 | (0.3%) | 0 | (0%) | 2 | (0.5%) | 1 | ||
| April | 48 | 6 | (0.8%) | 1 | (0.3%) | 5 | (1.2%) | 1 | 4 | 0.016–10.017 |
| May | 57 | 5 | (0.7%) | 3 | (0.9%) | 2 | (0.5%) | 1 | 3 | 0.15–59.893 |
| June | 44 | 12 | (1.6%) | 7 | (2%) | 5 | (1.2%) | 0.897 | 2.8 | 0.196–40.059 |
| July | 134 | 50 | (6.6%) | 22 | (6.4%) | 28 | (6.8%) | 1 | 1.571 | 0.134–18.478 |
| August | 97 | 32 | (4.2%) | 14 | (4.1%) | 18 | (4.3%) | 1 | 1.556 | 0.128–18.951 |
| September | 82 | 32 | (4.2%) | 17 | (4.9%) | 15 | (3.6%) | 0.959 | 2.267 | 0.186–27.583 |
| October | 99 | 56 | (7.4%) | 34 | (9.9%) | 22 | (5.3%) | 0.736 | 3.091 | 0.264–36.169 |
| November | 77 | 34 | (4.5%) | 21 | (6.1%) | 13 | (3.1%) | 0.728 | 3.231 | 0.266–39.287 |
| December | 50 | 4 | (0.5%) | 3 | (0.9%) | 1 | (0.2%) | 0.741 | 6 | 0.221–162.541 |
| Variable | Chitwan | Kaski | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Febrile Cases (n = 449) | Scrub-Typhus-Positive Cases (n = 219) | Febrile Cases (n = 310) | Scrub-Typhus-Positive Cases (n = 20) | ||||
| Sex | |||||||
| Male | 247 | 103 | (22.9%) | 167 | 11 | (3.5%) | <0.0001 * |
| Female | 202 | 116 | (25.8%) | 143 | 9 | (2.9%) | <0.0001 * |
| Age Group | |||||||
| 1–10 Years | 98 | 38 | (8.5%) | 79 | 1 | (0.3%) | <0.0001 * |
| 11–20 | 137 | 62 | (13.8%) | 104 | 11 | (3.5%) | <0.0001 * |
| 21–30 | 102 | 46 | (10.2%) | 66 | 4 | (1.3%) | <0.0001 * |
| 31–40 | 64 | 41 | (9.1%) | 27 | 1 | (0.3%) | <0.0001 * |
| 41–50 | 25 | 17 | (3.8%) | 16 | 1 | (0.3%) | 0.0004 * |
| 51–60 | 14 | 12 | (2.7%) | 12 | 1 | (0.3%) | 0.0004 * |
| 61–70 | 6 | 2 | (0.4%) | 5 | 0 | (0%) | 0.5207 |
| 71 and above | 3 | 1 | (0.2%) | 1 | 1 | (0.3%) | 1 |
| Seasonal Variation | |||||||
| 2009–2010 | |||||||
| January | 5 | 1 | (0.2%) | 15 | 2 | (0.6%) | 1 |
| February | 6 | 1 | (0.2%) | 15 | 2 | (0.6%) | 1 |
| March | 7 | 1 | (0.2%) | 23 | 1 | (0.3%) | 0.9540 |
| April | 19 | 5 | (1.1%) | 29 | 1 | (0.3%) | 0.0579 |
| May | 22 | 3 | (0.7%) | 35 | 2 | (0.6%) | 0.5834 |
| June | 18 | 11 | (2.4%) | 26 | 1 | (0.3%) | 0.0001 * |
| July | 97 | 44 | (9.8%) | 37 | 6 | (1.9%) | 0.0035 * |
| August | 60 | 32 | (7.1%) | 37 | 0 | <0.0001 * | |
| September | 66 | 31 | (6.9%) | 16 | 1 | (0.3%) | 0.0067 * |
| October | 81 | 54 | (12%) | 18 | 2 | (0.6%) | 0.0001 * |
| November | 58 | 33 | (7.3%) | 19 | 1 | (0.3%) | 0.0002 * |
| December | 10 | 3 | (0.7%) | 40 | 1 | (0.3%) | 0.0267 * |
| Patient Characteristic | Cases (n = 228) | Primary Infection (n = 102) | Secondary Infection (n = 126) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 25 ± 15.2 | 23.1 ± 14.2 | 26.6 ± 15.8 | 0.0873 |
| Sex | Female 53.5% (122) | Female 61.8% (63) | Male 53.2% (67) | 0.0318 * |
| ONSET-ENRLD | 6.3 ± 3.2 | 7.9 ± 3.1 | 5 ± 2.5 | <0.0001 * |
| ENRLD-FU | 29 ± 19.8 | 27.1 ± 15.6 | 30.5 ± 22.6 | 0.1985 |
| ONSET-FU | 35.3 ± 20.1 | 35 ± 16.4 | 35.5 ± 22.7 | 0.8400 |
| Body temperature (°C) | 39.2 ± 3.1 | 39.3 ± 1.3 | 39.1 ± 4 | 0.5720 |
| Pulse /min | 98.5 ± 11.8 | 99.3 ± 13 | 97.9 ± 10.7 | 0.3550 |
| Bp (D) | 98.2 ± 13.6 | 96.7 ± 13.2 | 99.5 ± 13.8 | 0.1146 |
| Bp (S) | 61.9 ± 10.3 | 61.1 ± 9.7 | 62.5 ± 10.8 | 0.2899 |
| Respiratory rate/min | 21 ± 3.2 | 21.7 ± 4.4 | 20.5 ± 1.7 | 0.0014 * |
| Fever | 226 (99.1%) | 102 (100%) | 124 (98.4%) | 0.3797 |
| Headache | 193 (84.6%) | 89 (87.3%) | 104 (82.5%) | 0.5054 |
| Malaise | 187 (82%) | 85 (83.3%) | 102 (81%) | 0.6931 |
| Anorexia | 183 (80.3%) | 84 (82.4%) | 99 (78.6%) | 0.4384 |
| Fatigue | 179 (78.5%) | 83 (81.4%) | 96 (76.2%) | 0.3396 |
| Muscle Aches | 178 (78.1%) | 79 (77.5%) | 99 (78.6%) | 0.4333 |
| Chills | 166 (72.8%) | 80 (78.4%) | 86 (68.3%) | 0.0665 |
| Retro-orbital Pain | 116 (50.9%) | 57 (55.9%) | 59 (46.8%) | 0.1084 |
| Joint Pain | 105 (46.1%) | 47 (46.1%) | 58 (46%) | 0.6385 |
| Nausea | 99 (43.4%) | 43 (42.2%) | 56 (44.4%) | 0.7815 |
| Cough | 89 (39%) | 40 (39.2%) | 49 (38.9%) | 0.9092 |
| Vomiting | 87 (38.2%) | 41 (40.2%) | 46 (36.5%) | 0.3614 |
| Abdominal cramps | 76 (33.3%) | 38 (37.3%) | 38 (30.2%) | 0.1833 |
| Chest pain | 52 (22.8%) | 29 (28.4%) | 23 (18.3%) | 0.0530 |
| Sore throat | 52 (22.8%) | 22 (21.6%) | 30 (23.8%) | 0.6843 |
| Rhinorrhea | 42 (18.4%) | 19 (18.6%) | 23 (18.3%) | 0.7434 |
| Diarrhea | 17 (7.5%) | 5 (4.9%) | 12 (9.5%) | 0.4180 |
| Coryza | 13 (5.7%) | 6 (5.9%) | 7 (5.6%) | 0.6204 |
| Conjunctivitis | 12 (5.3%) | 7 (6.9%) | 5 (4%) | 0.8415 |
| Rash | 12 (5.3%) | 7 (6.9%) | 5 (4%) | 0.1156 |
| Loss of consciousness | 9 (3.9%) | 1 (1%) | 8 (6.3%) | 0.0396 * |
| Wheezing | 8 (3.5%) | 7 (6.9%) | 1 (0.8%) | 0.0207 * |
| Shortness of breath | 7 (3.1%) | 4 (3.9%) | 3 (2.4%) | 0.3281 |
| Seizures | 6 (2.6%) | 2 (2%) | 4 (3.2%) | 0.9237 |
| Jaundice | 4 (1.8%) | 2 (2%) | 2 (1.6%) | 0.8769 |
| Confusion | 2 (0.9%) | 0 | 2 (1.6%) | 0.1954 |
| Stiff neck | 2 (0.9%) | 1 (1%) | 1 (0.8%) | 0.3613 |
| Eschar lesion | 1 (0.4%) | 0 | 1 (0.8%) | 0.3748 |
| Other | 1 (0.4%) | 0 | 1 (0.8%) | 0.3765 |
| Bleeding | 0 (0%) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linsuwanon, P.; Auysawasdi, N.; Chao, C.-C.; Rodkvamtook, W.; Shrestha, B.; Bajracharya, S.; Shrestha, J.; Wongwairot, S.; Limsuwan, C.; Lindroth, E.; et al. Estimating the Seroprevalence of Scrub Typhus in Nepal. Pathogens 2024, 13, 736. https://doi.org/10.3390/pathogens13090736
Linsuwanon P, Auysawasdi N, Chao C-C, Rodkvamtook W, Shrestha B, Bajracharya S, Shrestha J, Wongwairot S, Limsuwan C, Lindroth E, et al. Estimating the Seroprevalence of Scrub Typhus in Nepal. Pathogens. 2024; 13(9):736. https://doi.org/10.3390/pathogens13090736
Chicago/Turabian StyleLinsuwanon, Piyada, Nutthanun Auysawasdi, Chien-Chung Chao, Wuttikon Rodkvamtook, Binob Shrestha, Samita Bajracharya, Jasmin Shrestha, Sirima Wongwairot, Chawin Limsuwan, Erica Lindroth, and et al. 2024. "Estimating the Seroprevalence of Scrub Typhus in Nepal" Pathogens 13, no. 9: 736. https://doi.org/10.3390/pathogens13090736
APA StyleLinsuwanon, P., Auysawasdi, N., Chao, C.-C., Rodkvamtook, W., Shrestha, B., Bajracharya, S., Shrestha, J., Wongwairot, S., Limsuwan, C., Lindroth, E., Mann, A., Davidson, S., Wanja, E., & Shrestha, S. K. (2024). Estimating the Seroprevalence of Scrub Typhus in Nepal. Pathogens, 13(9), 736. https://doi.org/10.3390/pathogens13090736






