Spatiotemporal Modeling of Aedes aegypti Risk: Enhancing Dengue Virus Control through Meteorological and Remote Sensing Data in French Guiana
Abstract
:1. Introduction
2. Materials and Methods
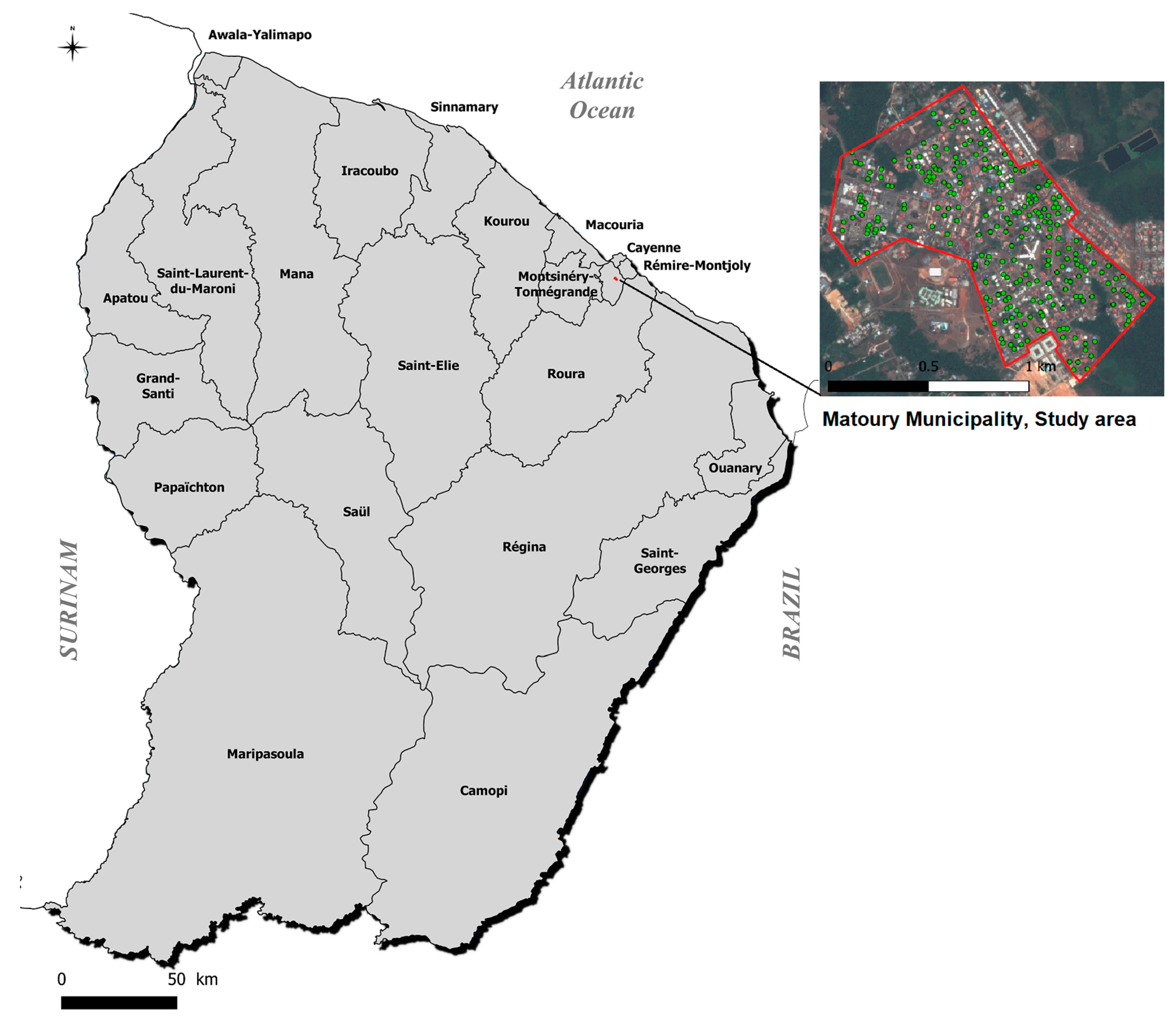
2.1. Study Area
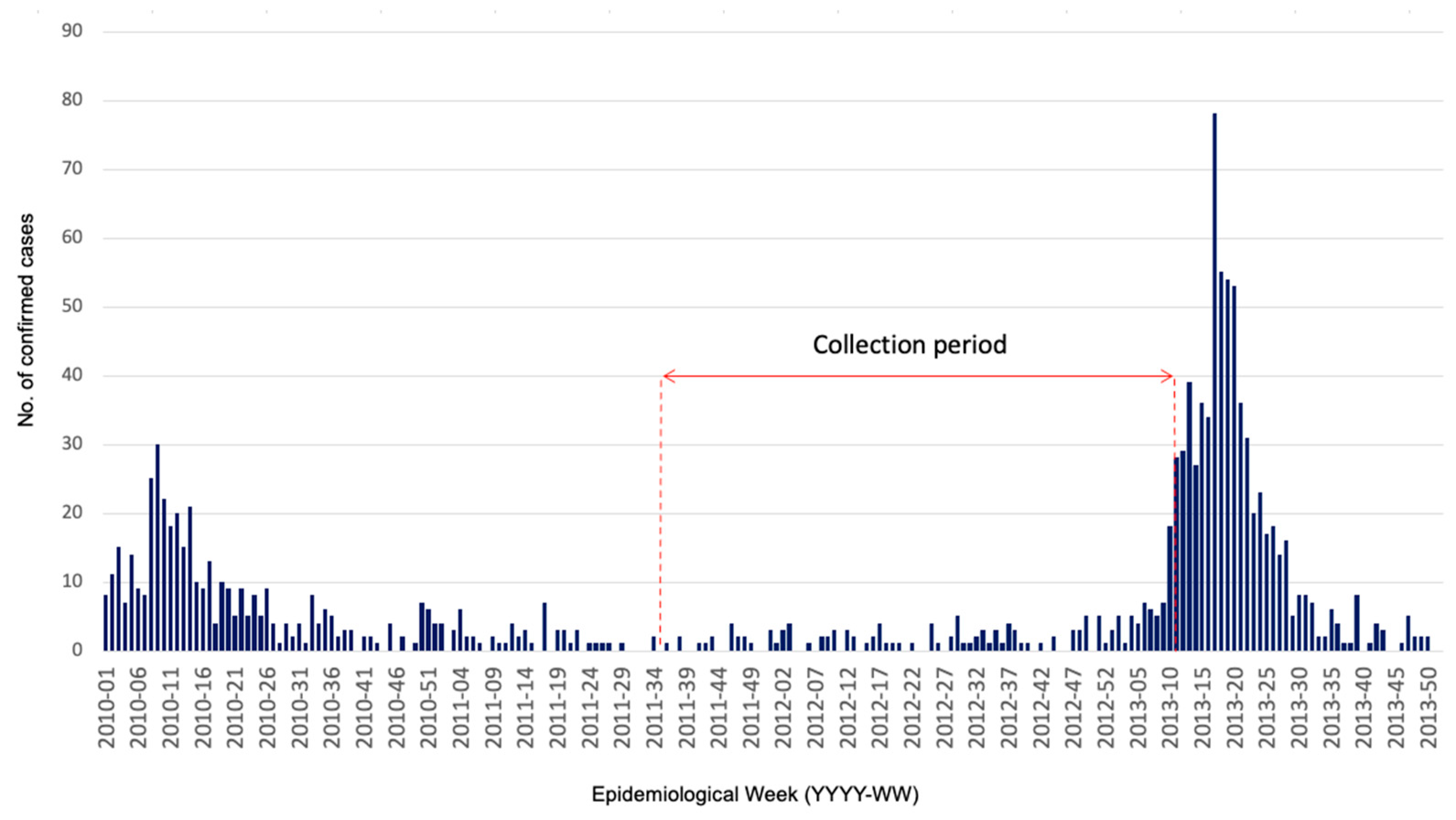
2.2. Data Collection
2.3. Statistical and Spatial Analyses
3. Results
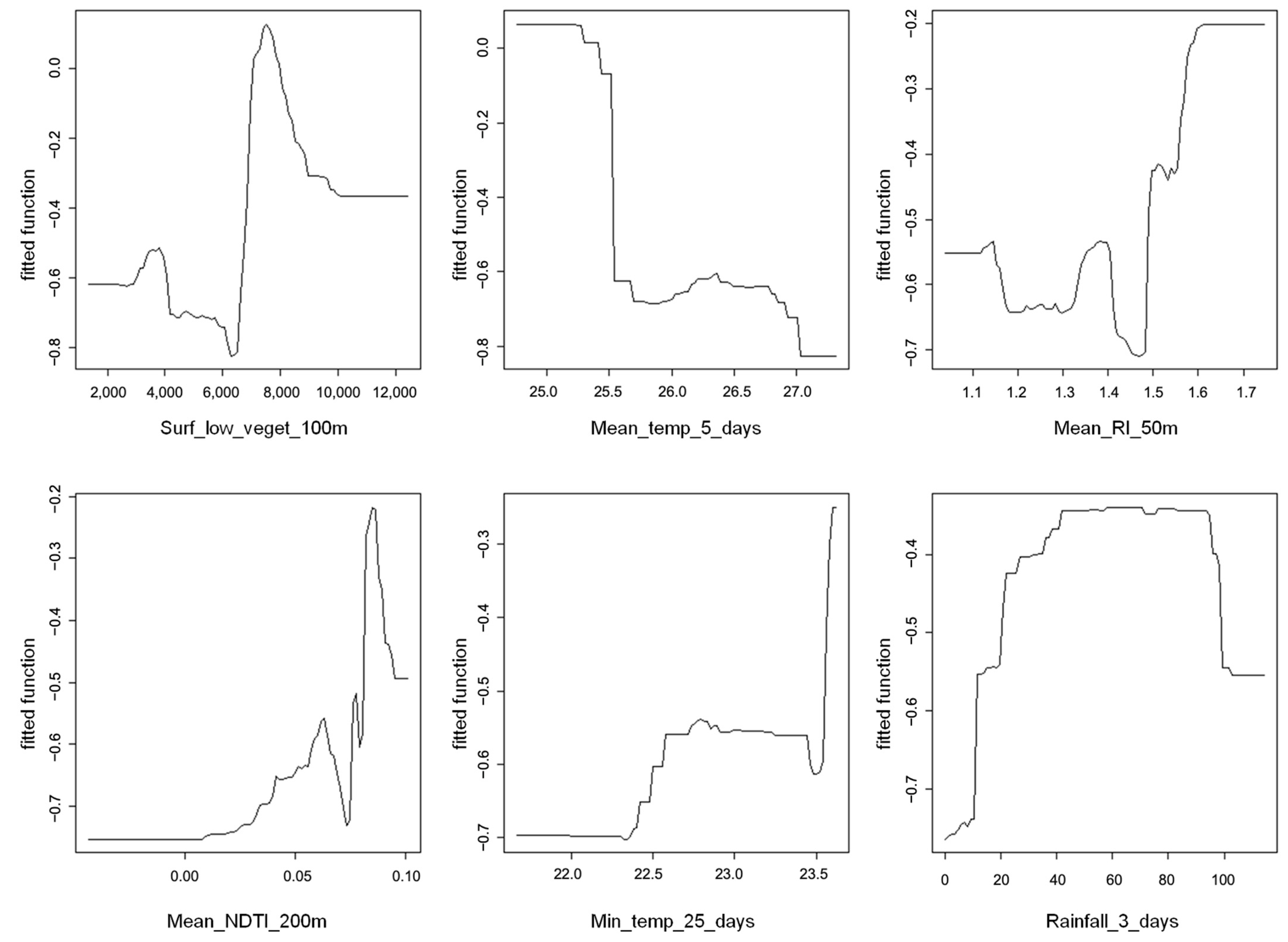
3.1. Overview of Larval Data and Modeling Approach
3.2. Larvae Risk Mapping
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Pettersson, J.H.-O.; Eldholm, V.; Seligman, S.J.; Lundkvist, Å.; Falconar, A.K.; Gaunt, M.W.; Musso, D.; Nougairède, A.; Charrel, R.; Gould, E.A.; et al. How Did Zika Virus Emerge in the Pacific Islands and Latin America? MBio 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Adde, A.; Roucou, P.; Mangeas, M.; Ardillon, V.; Desenclos, J.-C.; Rousset, D.; Girod, R.; Briolant, S.; Quenel, P.; Flamand, C. Predicting Dengue Fever Outbreaks in French Guiana Using Climate Indicators. PLoS Negl. Trop. Dis. 2016, 10, e0004681. [Google Scholar] [CrossRef] [PubMed]
- Flamand, C.; Fabregue, M.; Bringay, S.; Ardillon, V.; Quénel, P.; Desenclos, J.C.; Teisseire, M. Mining Local Climate Data to Assess Spatiotemporal Dengue Fever Epidemic Patterns in French Guiana. J. Am. Med. Inform. Assoc. 2014, 21, e232–e240. [Google Scholar] [CrossRef] [PubMed]
- L’Azou, M.; Taurel, A.F.; Flamand, C.; Quénel, P. Recent Epidemiological Trends of Dengue in the French Territories of the Americas (2000–2012): A Systematic Literature Review. PLoS Negl. Trop. Dis. 2014, 8, e3235. [Google Scholar] [CrossRef]
- Flamand, C.; Bailly, S.; Fritzell, C.; Berthelot, L.; Vanhomwegen, J.; Salje, H.; Paireau, J.; Matheus, S.; Enfissi, A.; Fernandes-Pellerin, S.; et al. Impact of Zika Virus Emergence in French Guiana: A Large General Population Seroprevalence Survey. J. Infect. Dis. 2019, 220, 1915–1925. [Google Scholar] [CrossRef]
- Flamand, C.; Fritzell, C.; Prince, C.; Abboud, P.; Ardillon, V.; Carvalho, L.; Demar, M.; Boukhari, R.; Papaix-Puech, M.; Elenga, N.; et al. Epidemiological Assessment of the Severity of Dengue Epidemics in French Guiana. PLoS ONE 2017, 12, e0172267. [Google Scholar] [CrossRef]
- Fritzell, C.; Raude, J.; Adde, A.; Dusfour, I.; Quenel, P.; Flamand, C. Knowledge, Attitude and Practices of Vector-Borne Disease Prevention during the Emergence of a New Arbovirus: Implications for the Control of Chikungunya Virus in French Guiana. PLoS Negl. Trop. Dis. 2016, 10, e0005081. [Google Scholar] [CrossRef]
- Flamand, C.; Fritzell, C.; Matheus, S.; Dueymes, M.; Carles, G.; Favre, A.; Enfissi, A.; Adde, A.; Demar, M.; Kazanji, M.; et al. The Proportion of Asymptomatic Infections and Spectrum of Disease among Pregnant Women Infected by Zika Virus: Systematic Monitoring in French Guiana, 2016. Euro Surveill. 2017, 22, 17-00102. [Google Scholar] [CrossRef]
- Bailly, S.; Rousset, D.; Fritzell, C.; Hozé, N.; Ben Achour, S.; Berthelot, L.; Enfissi, A.; Vanhomwegen, J.; Salje, H.; Fernandes-Pellerin, S.; et al. Spatial Distribution and Burden of Emerging Arboviruses in French Guiana. Viruses 2021, 13, 1299. [Google Scholar] [CrossRef]
- Nacher, M.; Douine, M.; Gaillet, M.; Flamand, C.; Rousset, D.; Rousseau, C.; Mahdaoui, C.; Carroll, S.; Valdes, A.; Passard, N.; et al. Simultaneous Dengue and COVID-19 Epidemics: Difficult Days Ahead? PLoS Negl. Trop. Dis. 2020, 14, e0008426. [Google Scholar] [CrossRef] [PubMed]
- Santé Publique France Situation Épidémiologique de la Dengue en Guyane. Point au 6 mai 2021. Available online: https://www.santepubliquefrance.fr/regions/guyane/documents/bulletin-regional/2021/situation-epidemiologique-de-la-dengue-en-guyane.-point-au-6-mai-2021 (accessed on 22 April 2023).
- Dusfour, I.; Zorrilla, P.; Guidez, A.; Issaly, J.; Girod, R.; Guillaumot, L.; Robello, C.; Strode, C. Deltamethrin Resistance Mechanisms in Aedes Aegypti Populations from Three French Overseas Territories Worldwide. PLoS Neglected Trop. Dis. 2015, 9, e0004226. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, Y.; Chaney, S.C.; Guidez, A.; Habchi-Hanriot, N.; Talaga, S.; Wang, L.; Dusfour, I. Successes and Failures of Sixty Years of Vector Control in French Guiana: What Is the next Step? Mem. Inst. Oswaldo Cruz 2018, 113, e170398. [Google Scholar] [CrossRef] [PubMed]
- MacCormack-Gelles, B.; Lima Neto, A.S.; Sousa, G.S.; do Nascimento, O.J.; Castro, M.C. Evaluation of the Usefulness of Aedes Aegypti Rapid Larval Surveys to Anticipate Seasonal Dengue Transmission between 2012–2015 in Fortaleza, Brazil. Acta Trop. 2020, 205, 105391. [Google Scholar] [CrossRef]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the Relationship between Vector Indices and Dengue Transmission: A Systematic Review of the Evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.V.; Doan, H.T.; Phan, T.T.; Minh, N.N. Ecological Factors Associated with Dengue Fever in a Central Highlands Province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef]
- Sanchez, L.; Vanlerberghe, V.; Alfonso, L.; del Marquetti, M.C.; Guzman, M.G.; Bisset, J.; van der Stuyft, P. Aedes Aegypti Larval Indices and Risk for Dengue Epidemics. Emerg. Infect. Dis. 2006, 12, 800–806. [Google Scholar] [CrossRef]
- Liyanage, P.; Tozan, Y.; Tissera, H.A.; Overgaard, H.J.; Rocklöv, J. Assessing the Associations between Aedes Larval Indices and Dengue Risk in Kalutara District, Sri Lanka: A Hierarchical Time Series Analysis from 2010 to 2019. Parasit Vectors 2022, 15, 277. [Google Scholar] [CrossRef]
- de Thoisy, B.; Duron, O.; Epelboin, L.; Musset, L.; Quénel, P.; Roche, B.; Binetruy, F.; Briolant, S.; Carvalho, L.; Chavy, A.; et al. Ecology, Evolution, and Epidemiology of Zoonotic and Vector-Borne Infectious Diseases in French Guiana: Transdisciplinarity Does Matter to Tackle New Emerging Threats. Infect. Genet. Evol. 2021, 93, 104916. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Lozano-Fuentes, S. Use of Mapping and Spatial and Space-Time Modeling Approaches in Operational Control of Aedes Aegypti and Dengue. PLoS Negl. Trop. Dis. 2009, 3, e411. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pilot, E.; Rehbock, C.; Gontariuk, M.; Doreleijers, S.; Wang, L.; Krafft, T.; Martens, P.; Liu, Q. Land Use and Land Cover Change and Its Impacts on Dengue Dynamics in China: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009879. [Google Scholar] [CrossRef] [PubMed]
- Machault, V.; Yébakima, A.; Etienne, M.; Vignolles, C.; Palany, P.; Tourre, Y.M.; Guérécheau, M.; Lacaux, J.-P. Mapping Entomological Dengue Risk Levels in Martinique Using High-Resolution Remote-Sensing Environmental Data. ISPRS Int. J. Geo-Inf. 2014, 3, 1352–1371. [Google Scholar] [CrossRef]
- Sarfraz, M.S.; Tripathi, N.K.; Tipdecho, T.; Thongbu, T.; Kerdthong, P.; Souris, M. Analyzing the Spatio-Temporal Relationship between Dengue Vector Larval Density and Land-Use Using Factor Analysis and Spatial Ring Mapping. BMC Public Health 2012, 12, 1–19. [Google Scholar] [CrossRef]
- Lana, R.M.; Carneiro, T.G.S.; Honório, N.A.; Codeço, C.T. Seasonal and Nonseasonal Dynamics of Aedes Aegypti in Rio de Janeiro, Brazil: Fitting Mathematical Models to Trap Data. Acta Trop. 2014, 129, 25–32. [Google Scholar] [CrossRef]
- de Melo, D.P.O.; Scherrer, L.R.; Eiras, Á.E. Dengue Fever Occurrence and Vector Detection by Larval Survey, Ovitrap and MosquiTRAP: A Space-Time Clusters Analysis. PLoS ONE 2012, 7, e42125. [Google Scholar] [CrossRef]
- Flamand, C.; Quenel, P.; Ardillon, V.; Carvalho, L.; Bringay, S.; Teisseire, M. The Epidemiologic Surveillance of Dengue-Fever in French Guiana: When Achievements Trigger Higher Goals. Stud. Health Technol. Inform. 2011, 169, 629–633. [Google Scholar]
- Hanf, M.; Friedman, E.; Basurko, C.; Roger, A.; Bruncher, P.; Dussart, P.; Flamand, C.; Carles, G.; Buekens, P.; Breart, G.; et al. Dengue Epidemics and Adverse Obstetrical Outcomes in French Guiana: A Semi-Ecological Study. Trop. Med. Int. Health 2014, 19, 153–158. [Google Scholar] [CrossRef]
- Hallet, E.; Flamand, C.; Rousset, D.; Bonifay, T.; Fritzell, C.; Matheus, S.; Dueymes, M.; Ntab, B.; Nacher, M. ZIKA Virus Infection in Pregnant Women in French Guiana: More Precarious-More at Risk. PLoS Negl. Trop. Dis. 2020, 14, e0008193. [Google Scholar] [CrossRef]
- Flamand, C.; Fritzell, C.; Obale, P.; Quenel, P.; Raude, J. The Role of Risk Proximity in the Beliefs and Behaviors Related to Mosquito-Borne Diseases: The Case of Chikungunya in French Guiana. Am. J. Trop. Med. Hyg. 2017, 97, 344–355. [Google Scholar] [CrossRef]
- Bonifay, T.; Le Turnier, P.; Epelboin, Y.; Carvalho, L.; De Thoisy, B.; Djossou, F.; Duchemin, J.-B.; Dussart, P.; Enfissi, A.; Lavergne, A.; et al. Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses 2023, 15, 1268. [Google Scholar] [CrossRef]
- Aceituno, P. On the Functioning of the Southern Oscillation in the South American Sector. Part I: Surface Climate. Mon. Wea. Rev. 1988, 116, 505–524. [Google Scholar] [CrossRef]
- Moron, V.; Bigot, S.; Roucou, P. Rainfall Variability in Subequatorial America and Africa and Relationships with the Main Sea-Surface Temperature Modes (1951–1990). Oceanogr. Lit. Rev. 1996, 8, 771. [Google Scholar] [CrossRef]
- Vezzani, D.; Albicócco, A.P. The Effect of Shade on the Container Index and Pupal Productivity of the Mosquitoes Aedes Aegypti and Culex Pipiens Breeding in Artificial Containers. Med. Vet. Entomol. 2009, 23, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Greene, K.L.; Lounibos, L.P. Leaf Species Identity and Combination Affect Performance and Oviposition Choice of Two Container Mosquito Species. Ecol. Entomol. 2009, 34, 447–456. [Google Scholar] [CrossRef]
- Tun-Lin, W.; Burkot, T.R.; Kay, B.H. Effects of Temperature and Larval Diet on Development Rates and Survival of the Dengue Vector Aedes Aegypti in North Queensland, Australia. Med. Vet. Entomol. 2000, 14, 31–37. [Google Scholar] [CrossRef]
- Yan, J.; Kibech, R.; Stone, C.M. Differential Effects of Larval and Adult Nutrition on Female Survival, Fecundity, and Size of the Yellow Fever Mosquito, Aedes Aegypti. Front. Zool. 2021, 18, 10. [Google Scholar] [CrossRef]
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization Is a Main Driver for the Larval Ecology of Aedes Mosquitoes in Arbovirus-Endemic Settings in South-Eastern Côte d’Ivoire. PLoS Neglected Trop. Dis. 2017, 11, e0005751. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Moreno, M.; Fuller, D.O.; Cardenas, G.; Petrie, W.D.; Beier, J.C. Urbanization Favors the Proliferation of Aedes Aegypti and Culex Quinquefasciatus in Urban Areas of Miami-Dade County, Florida. Sci. Rep. 2021, 11, 22989. [Google Scholar] [CrossRef]
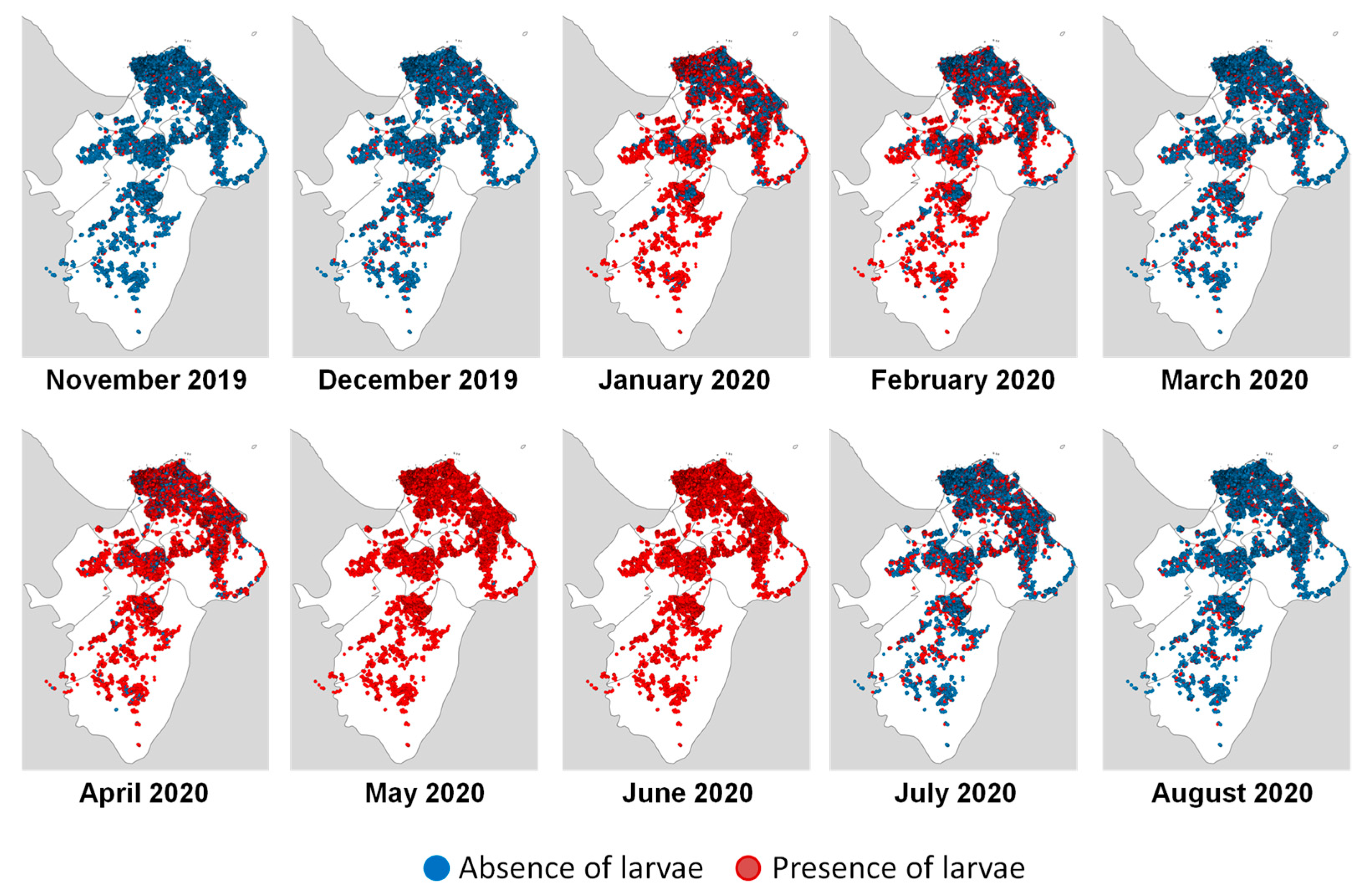
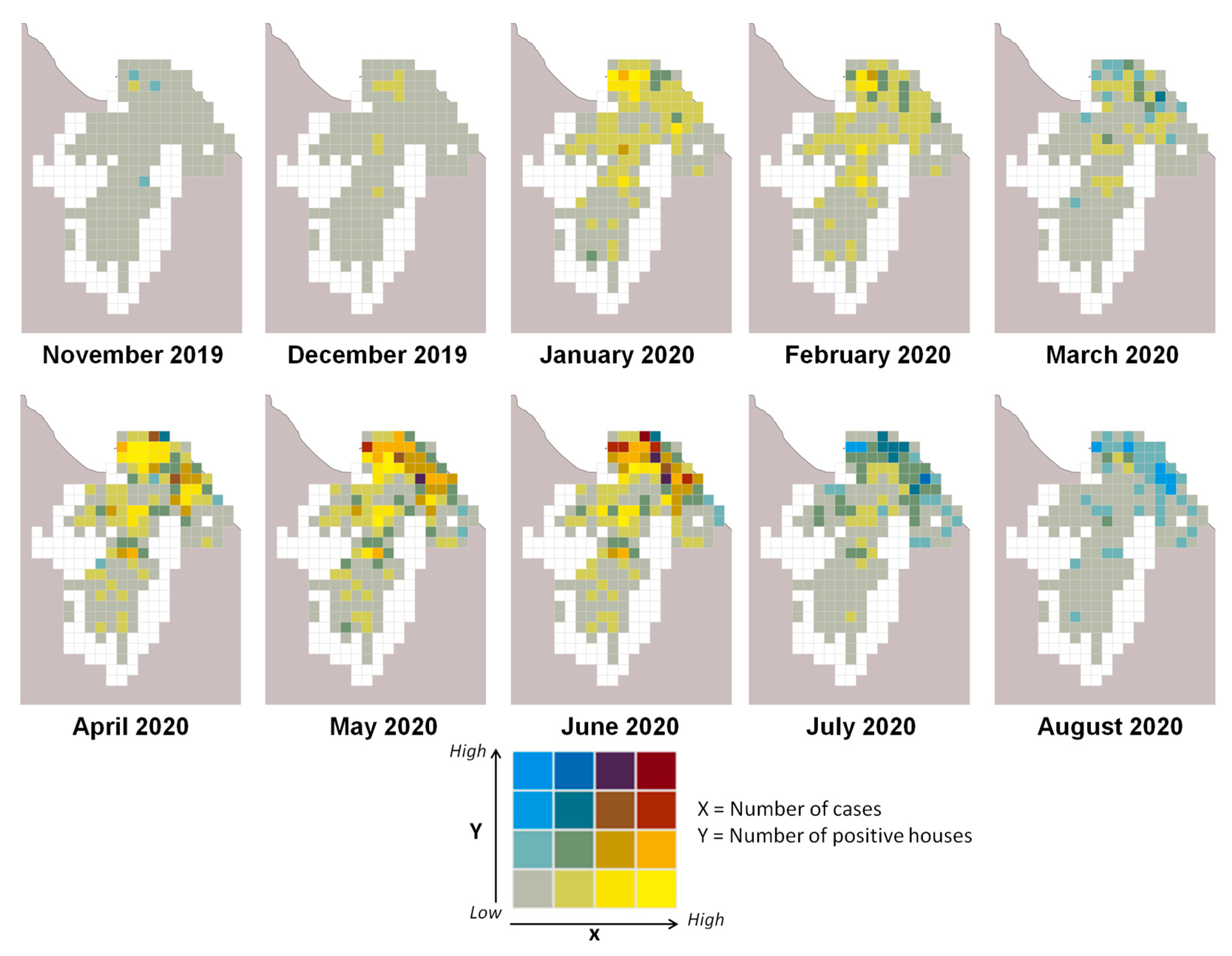
| Number of Positive Cases | Number of Positive Houses | Proportion of Predicted Positive Houses (%) | Correlation Rate | |
|---|---|---|---|---|
| November 2019 | 3 | 1125 | 3.8 | 0.2 |
| December 2019 | 0 | 2339 | 7.9 | - |
| January 2020 | 8 | 15,796 | 58.6 | 0.3 |
| February 2020 | 11 | 10,730 | 38.6 | 0.2 |
| March 2020 | 29 | 3707 | 9.0 | 0.2 |
| April 2020 | 79 | 20,874 | 72.8 | 0.3 |
| May 2020 | 121 | 25,610 | 99.6 | 0.4 |
| June 2020 | 156 | 25,608 | 99.6 | 0.6 |
| July 2020 | 168 | 6890 | 24.6 | 0.5 |
| August 2020 | 122 | 2033 | 6.6 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, S.; Machault, V.; Beneteau, S.; Palany, P.; Fritzell, C.; Girod, R.; Lacaux, J.-P.; Quénel, P.; Flamand, C. Spatiotemporal Modeling of Aedes aegypti Risk: Enhancing Dengue Virus Control through Meteorological and Remote Sensing Data in French Guiana. Pathogens 2024, 13, 738. https://doi.org/10.3390/pathogens13090738
Bailly S, Machault V, Beneteau S, Palany P, Fritzell C, Girod R, Lacaux J-P, Quénel P, Flamand C. Spatiotemporal Modeling of Aedes aegypti Risk: Enhancing Dengue Virus Control through Meteorological and Remote Sensing Data in French Guiana. Pathogens. 2024; 13(9):738. https://doi.org/10.3390/pathogens13090738
Chicago/Turabian StyleBailly, Sarah, Vanessa Machault, Samuel Beneteau, Philippe Palany, Camille Fritzell, Romain Girod, Jean-Pierre Lacaux, Philippe Quénel, and Claude Flamand. 2024. "Spatiotemporal Modeling of Aedes aegypti Risk: Enhancing Dengue Virus Control through Meteorological and Remote Sensing Data in French Guiana" Pathogens 13, no. 9: 738. https://doi.org/10.3390/pathogens13090738






