Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Data Collection
2.2. Collection of Samples and Nucleic Acid Extraction
2.3. Detection of Pathogens
2.4. Statistical Analysis
3. Results
3.1. Characteristics and HPIV Detection of Overall Samples
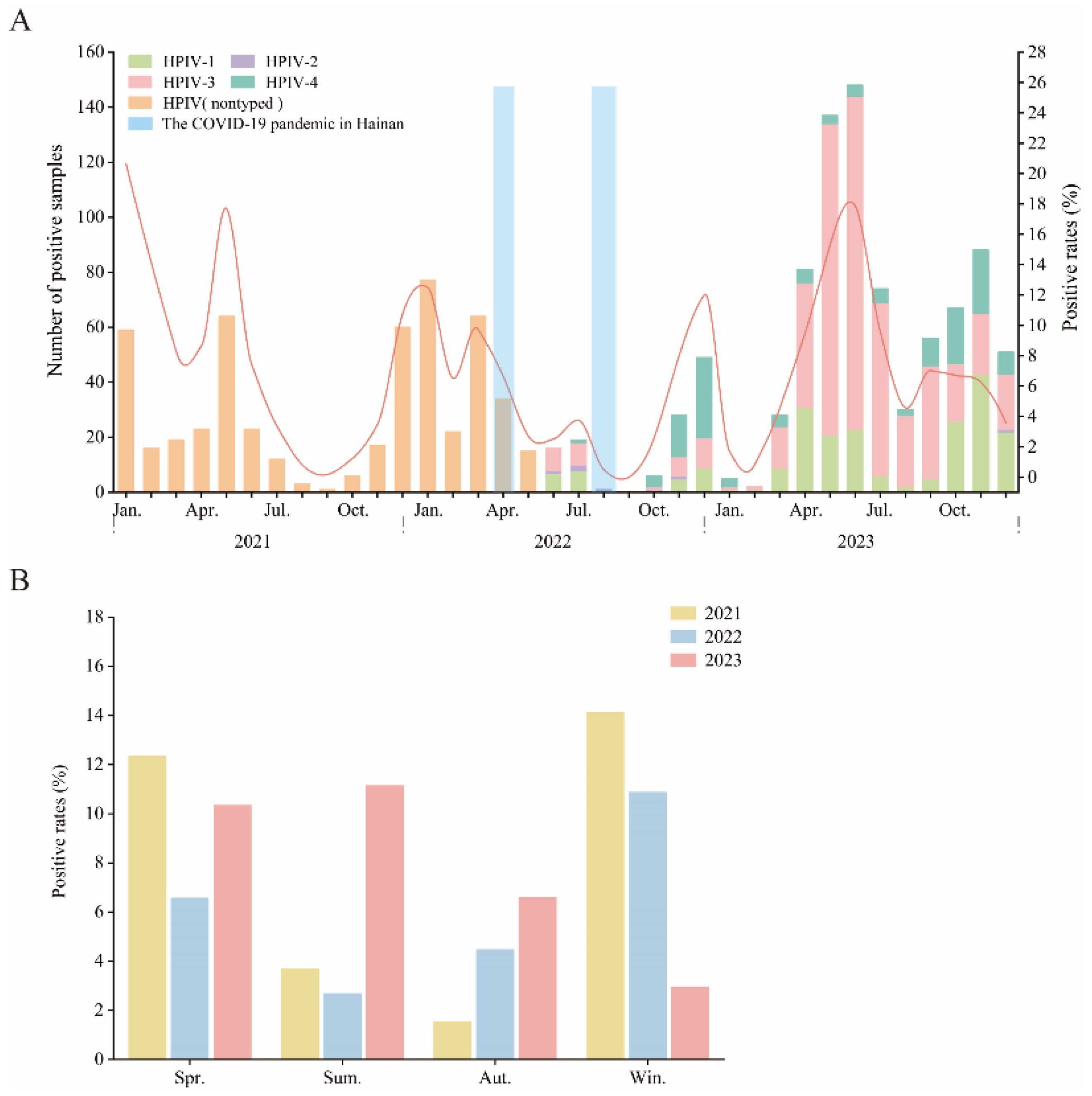
3.2. Prevalence of HPIV among Pediatric Patients in Hainan from 2021 to 2023
3.3. Epidemiology of HPIV in Pediatric Patients across Different Age Groups
3.4. Co-Infections of HPIV and Other Respiratory Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.M.; Rhee, J.E.; Lee, N.-J.; Woo, S.H.; Park, A.K.; Lee, J.; Yoo, C.K.; Kim, E.-J. Recent increase in the detection of human parainfluenza virus during the coronavirus disease-2019 pandemic in the Republic of Korea. Virol. J. 2022, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, Y.; Tang, J.; Li, N.; Yang, Y.; Guo, W.; Lin, C.; Wu, J.; Lin, Y.; Chen, Q. Clinical impact of human parainfluenza virus infections before and during the COVID-19 pandemic in Southern China. Microbes Infect. 2023, 25, 105219. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased Influenza Activity During the COVID-19 Pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Luo, M.; Li, A.; Xie, H.; Gong, C.; Du, J.; Wang, X.; Li, M.; Wang, X.; Wang, Y.; et al. Changes in the pathogenic spectrum of acute respiratory tract infections during the COVID-19 epidemic in Beijing, China: A large-scale active surveillance study. J. Infect. 2021, 83, 607–635. [Google Scholar] [CrossRef]
- Hetrich, M.K.; Oliva, J.; Wanionek, K.; Knoll, M.D.; Lamore, M.; Esteban, I.; Veguilla, V.; Dawood, F.S.; Karron, R.A. Epidemiology of Human Parainfluenza Virus Type 3 and Respiratory Syncytial Virus Infections in the Time of Coronavirus Disease 2019: Findings from a Household Cohort in Maryland. Clin. Infect. Dis. 2023, 76, 1349–1357. [Google Scholar] [CrossRef]
- Henrickson, K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003, 16, 242–264. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef]
- Pica, N.; Bouvier, N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Meer, J.W. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef]
- Hall, C.B. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001, 344, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.A.; Hall, C.B.; Iwane, M.K.; Poehling, K.A.; Edwards, K.M.; Griffin, M.R.; Staat, M.A.; Curns, A.T.; Erdman, D.D.; Szilagyi, P.G. Parainfluenza virus infection of young children: Estimates of the population-based burden of hospitalization. J. Pediatr. 2009, 154, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Deloria-Knoll, M.; A Madhi, S.; Cohen, C.; Arguelles, V.L.; Basnet, S.; Bassat, Q.; Brooks, W.A.; Echavarria, M.; et al. Global burden of acute lower respiratory infection associated with human parainfluenza virus in children younger than 5 years for 2018: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e1077–e1087. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.P.; Shah, P.K.; Azzi, J.M.; Chemaly, R.F. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: A systematic review. Cancer Lett. 2016, 370, 358–364. [Google Scholar] [CrossRef]
- Falsey, A. Current management of parainfluenza pneumonitis in immunocompromised patients: A review. Infect. Drug Resist. 2012, 5, 121–127. [Google Scholar] [CrossRef]
- Rafeek, R.A.M.; Divarathna, M.V.M.; Noordeen, F. A review on disease burden and epidemiology of childhood parainfluenza virus infections in Asian countries. Rev. Med. Virol. 2021, 31, e2164. [Google Scholar] [CrossRef]
- Zhong, P.; Zhang, H.; Chen, X.; Lv, F. Clinical characteristics of the lower respiratory tract infection caused by a single infection or coinfection of the human parainfluenza virus in children. J. Med. Virol. 2019, 91, 1625–1632. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Li, K.S.M.; Chau, K.-Y.; So, L.-Y.; Lee, R.A.; Lau, Y.-L.; Chan, K.-H.; Lim, W.W.L.; Woo, P.C.Y.; Yuen, K.-Y. Clinical and molecular epidemiology of human parainfluenza virus 4 infections in hong kong: Subtype 4B as common as subtype 4A. J. Clin. Microbiol. 2009, 47, 1549–1552. [Google Scholar] [CrossRef][Green Version]
- de Zwart, A.; Riezebos-Brilman, A.; Lunter, G.; Vonk, J.; Glanville, A.R.; Gottlieb, J.; Permpalung, N.; Kerstjens, H.; Alffenaar, J.-W.; Verschuuren, E. Respiratory Syncytial Virus, Human Metapneumovirus, and Parainfluenza Virus Infections in Lung Transplant Recipients: A Systematic Review of Outcomes and Treatment Strategies. Clin. Infect. Dis. 2022, 74, 2252–2260. [Google Scholar] [CrossRef]
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554. [Google Scholar] [CrossRef]
- Liang, W.N.; Yao, J.H.; Wu, J.; Liu, X.; Liu, J.; Zhou, L.; Chen, C.; Wang, G.F.; Wu, Z.Y.; Yang, W.Z.; et al. Experience and thinking on the normalization stage of prevention and control of COVID-19 in China. Zhonghua Yi Xue Za Zhi 2021, 101, E001. [Google Scholar] [CrossRef]
- Zhou, L.; Nie, K.; Zhao, H.; Zhao, X.; Ye, B.; Wang, J.; Chen, C.; Wang, H.; Di, J.; Li, J.; et al. Eleven COVID-19 Outbreaks with Local Transmissions Caused by the Imported SARS-CoV-2 Delta VOC—China, July–August, 2021. China CDC Wkly. 2021, 3, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Sinha, I.; Barr, I.G.; Zambon, M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Eurosurveillance 2021, 26, 2100186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Zhang, S.; Wang, Y. An estimation and development model of tourism resource values at the township scale on Hainan Island, China. PLoS ONE 2022, 17, e0262837. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Xu, Z.; Zhu, P.; Huang, B.; Li, K.; Xu, Y.; Zhang, Z.; Wu, Y.; Di, B. Evaluation of a multiplex PCR assay for detection of respiratory viruses and Mycoplasma pneumoniae in oropharyngeal swab samples from outpatients. J. Clin. Lab. Anal. 2020, 34, e23032. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, J.; Li, X.; Jongh, C.E.v.d.G.-D.; Wang, W.; He, X.; Xu, Z.; Yang, Y.; de Groot, R.; de Jonge, M.I.; et al. Broad range detection of viral and bacterial pathogens in bronchoalveolar lavage fluid of children to identify the cause of lower respiratory tract infections. BMC Infect. Dis. 2021, 21, 152. [Google Scholar] [CrossRef]
- Li, S.; Tong, J.; Li, H.; Mao, C.; Shen, W.; Lei, Y.; Hu, P. L. pneumophila Infection Diagnosed by tNGS in a Lady with Lymphadenopathy. Infect. Drug Resist. 2023, 16, 4435–4442. [Google Scholar] [CrossRef]
- Xiao, M.; Banu, A.; Jia, Y.; Chang, M.; Wang, G.; An, J.; Huang, Y.; Hu, X.; Tang, C.; Li, Z.; et al. Circulation pattern and genetic variation of rhinovirus infection among hospitalized children on Hainan Island, before and after the dynamic zero-COVID policy, from 2021 to 2023. J. Med. Virol. 2024, 96, e29755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef]
- Mai, W.; Ren, Y.; Tian, X.; Al-Mahdi, A.Y.; Peng, R.; An, J.; Lin, Q.; Hu, X.; Wang, G.; Sun, C.; et al. Comparison of common human respiratory pathogens among hospitalized children aged ≤ 6 years in Hainan Island, China, during spring and early summer in 2019–2021. J. Med. Virol. 2023, 95, e28692. [Google Scholar] [CrossRef]
- Li, L.; Jia, R.; Zhang, Y.; Sun, H.; Ma, J. Changes of parainfluenza virus infection in children before and after the COVID-19 pandemic in Henan, China. J. Infect. 2023, 86, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.J.B.D.; O’mathúna, D.P.; von Groote, T.C.; Abdulazeem, H.M.; Weerasekara, I.; Marusic, A.; Puljak, L.; Civile, V.T.; Zakarija-Grkovic, I.; Pericic, T.P.; et al. Coronavirus disease (COVID-19) pandemic: An overview of systematic reviews. BMC Infect. Dis. 2021, 21, 525. [Google Scholar] [CrossRef]
- Kim, Y.K.; Song, S.H.; Ahn, B.; Lee, J.K.; Choi, J.H.; Choi, S.-H.; Yun, K.W.; Choi, E.H. Shift in Clinical Epidemiology of Human Parainfluenza Virus Type 3 and Respiratory Syncytial Virus B Infections in Korean Children Before and During the COVID-19 Pandemic: A Multicenter Retrospective Study. J. Korean Med. Sci. 2022, 37, e215. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Kadambari, S.; Goldacre, R.; Morris, E.; Goldacre, M.J.; Pollard, A.J. Indirect effects of the covid-19 pandemic on childhood infection in England: Population based observational study. BMJ 2022, 376, e067519. [Google Scholar] [CrossRef]
- Gooskens, J.; van der Ploeg, V.; Sukhai, R.N.; Vossen, A.C.; Claas, E.C.; Kroes, A.C. Clinical evaluation of viral acute respiratory tract infections in children presenting to the emergency department of a tertiary referral hospital in the Netherlands. BMC Pediatr. 2014, 14, 297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Zheng, Y.; Deng, J.; Wang, W.; Liu, P.; Yang, F.; Jiang, H. Prevalence of respiratory viruses among children hospitalized from respiratory infections in Shenzhen, China. Virol. J. 2016, 13, 39. [Google Scholar] [CrossRef]
- Bicer, S.; Giray, T.; Çöl, D.; Erdağ, G.Ç.; Vitrinel, A.; Gürol, Y.; Çelik, G.; Kaspar, Ç.; Küçük, Ö. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital. J. Pediatr. 2013, 39, 22. [Google Scholar] [CrossRef]
- Xiao, N.; Duan, Z.; Xie, Z.; Zhong, L.; Zeng, S.; Huang, H.; Gao, H.; Zhang, B. Human parainfluenza virus types 1–4 in hospitalized children with acute lower respiratory infections in China. J. Med. Virol. 2016, 88, 2085–2091. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, M.; Zhou, J.; Wang, Y.; Hao, C.; Ji, W.; Zhang, X.; Gu, W.; Shao, X. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infect. Dis. 2017, 17, 787. [Google Scholar] [CrossRef]
- Wu, K.-W.; Wang, S.-M.; Shen, C.-F.; Ho, T.-S.; Wang, J.-R.; Liu, C.-C. Clinical and epidemiological characteristics of human parainfluenza virus infections of children in southern Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 749–755. [Google Scholar] [CrossRef]
- Amatya, N.; Paudel, G.; Saud, B.; Wagle, S.; Shrestha, V.; Adhikari, B. Prevalence of Moraxella catarrhalis as a Nasal Flora among Healthy Kindergarten Children in Bhaktapur, Nepal. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 3989781. [Google Scholar] [CrossRef] [PubMed]
- Scotta, M.C.; Chakr, V.C.B.G.; de Moura, A.; Becker, R.G.; de Souza, A.P.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef]
- Richard, N.; Komurian-Pradel, F.; Javouhey, E.; Perret, M.; Rajoharison, A.; Bagnaud, A.; Billaud, G.; Vernet, G.; Lina, B.; Floret, D.; et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr. Infect. Dis. J. 2008, 27, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol. Infect. 2015, 143, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cilla, G.; Oñate, E.; Perez-Yarza, E.G.; Montes, M.; Vicente, D.; Perez-Trallero, E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008, 80, 1843–1849. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]


| Characteristic | Total (n = 19,339) | 2021 (n = 4419) | 2022 (n = 5188) | 2023 (n = 9732) | |
|---|---|---|---|---|---|
| Gender | Male | 915/11,909 (7.68%) | 191/2809 (6.80%) | 221/3149 (7.02%) | 503/5951 (8.45%) |
| Female | 480/7430 (6.46%) | 112/1610 (6.96%) | 110/2039 (5.39%) | 258/3781 (6.82%) | |
| χ2 | 10.224 | 0.039 | 5.460 | 8.509 | |
| p | 0.001 | 0.843 | 0.019 | 0.004 | |
| Age | 0–1 | 506/5910 (8.56%) | 116/1818 (6.38%) | 115/1408 (8.17%) | 275/2684 (10.25%) |
| 1–3 | 485/4989 (9.72%) | 107/1306 (8.19%) | 121/1292 (9.37%) | 257/2391 (10.75%) | |
| 3–7 | 362/5990 (6.04%) | 77/1056 (7.29%) | 88/1794 (4.91%) | 197/3140 (6.27%) | |
| 7–18 | 42/2450 (1.71%) | 3/239 (1.26%) | 7/694 (1.01%) | 32/1517 (2.11%) | |
| χ2 | 185.885 | 16.351 | 66.865 | 129.409 | |
| p | 0.000 | 0.001 | 0.000 | 0.000 | |
| Season | Spring | 463/4937 (9.38%) | 106/858(12.35%) | 113/1722 (6.56%) | 224/2357 (10.35%) |
| Summer | 325/4629 (7.02%) | 38/1033 (3.68%) | 36/1344 (2.68%) | 251/2252 (11.15%) | |
| Autumn | 266/5491 (4.84%) | 24/1571 (1.53%) | 34/761 (4.47%) | 208/3159 (6.58%) | |
| Winter | 341/4282 (7.96%) | 135/957 (14.11%) | 148/1361 (10.87%) | 58/1964 (2.95%) | |
| χ2 | 84.470 | 205.555 | 81.607 | 126.749 | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Banu, A.; Zeng, X.; Shi, S.; Peng, R.; Chen, S.; Ge, N.; Tang, C.; Huang, Y.; Wang, G.; et al. Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021–2023. Pathogens 2024, 13, 740. https://doi.org/10.3390/pathogens13090740
Xiao M, Banu A, Zeng X, Shi S, Peng R, Chen S, Ge N, Tang C, Huang Y, Wang G, et al. Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021–2023. Pathogens. 2024; 13(9):740. https://doi.org/10.3390/pathogens13090740
Chicago/Turabian StyleXiao, Meifang, Afreen Banu, Xiangyue Zeng, Shengjie Shi, Ruoyan Peng, Siqi Chen, Nan Ge, Cheng Tang, Yi Huang, Gaoyu Wang, and et al. 2024. "Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021–2023" Pathogens 13, no. 9: 740. https://doi.org/10.3390/pathogens13090740
APA StyleXiao, M., Banu, A., Zeng, X., Shi, S., Peng, R., Chen, S., Ge, N., Tang, C., Huang, Y., Wang, G., Hu, X., Cui, X., Chan, J. F.-W., Yin, F., & Chang, M. (2024). Epidemiology of Human Parainfluenza Virus Infections among Pediatric Patients in Hainan Island, China, 2021–2023. Pathogens, 13(9), 740. https://doi.org/10.3390/pathogens13090740







