Hantavirus Pulmonary Syndrome Outbreak Anticipation by a Rapid Synchronous Increase in Rodent Abundance in the Northwestern Argentina Endemic Region: Towards an Early Warning System for Disease Based on Climate and Rodent Surveillance Data
Abstract
1. Introduction
2. Materials and Methods
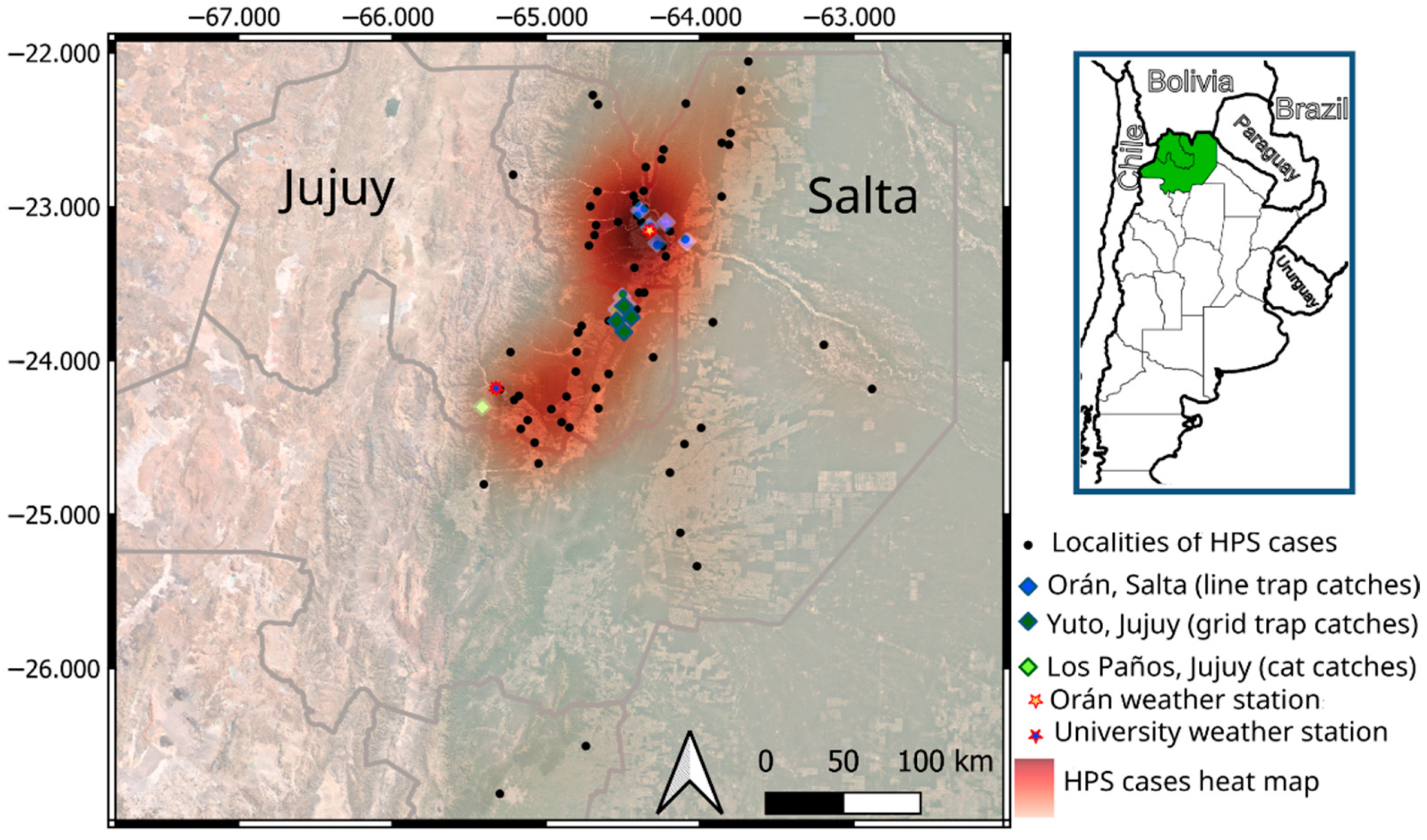
2.1. Study Area
2.2. Rodent Abundance Estimates
2.3. Orán Rodent Sampling
2.4. Yuto Rodent Sampling
2.5. Los Paños Rodent Sampling
2.6. HPS Cases
2.7. Climatic Variables
2.8. Data Analysis
3. Results
3.1. Rodent Sampling
3.2. Human Orthohantavirus Infection
3.3. Orthohantavirus Human Infections and the Lagged Rodent Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milholland, M.T.; Castro-Arellano, I.; Suzán, G.; Garcia-Peña, G.E.; Lee, T.E.; Rohde, R.E.; Aguirre, A.A.; Mills, J.N. Global diversity and distribution of hantaviruses and their hosts. EcoHealth 2018, 15, 163–208. [Google Scholar] [CrossRef] [PubMed]
- Ferres, M.; Vial, P.; Marco, C.; Yañez, L.; Godoy, P.; Castillo, C.; Hjelle, B.; Delgado, I.; Lee, S.; Mertz, G.J. Prospective evaluation of household contacts of persons with hantavirucardiopulmonary syndrome in Chile. J. Infect. Dis. 2007, 195, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.P.; Di Paola, N.; Alonso, D.O.; Pérez-Sautu, U.; Bellomo, C.M.; Iglesias, A.A.; Coelho, R.M.; López, B.; Periolo, N.; Larson, P.A.; et al. “Super-Spreaders” and person-to-person transmission of Andes Virus in Argentina. N. Engl. J. Med. 2020, 383, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21, E6–E16. [Google Scholar] [CrossRef]
- Chen, R.-X.; Gong, H.-Y.; Wang, X.; Sun, M.-H.; Ji, Y.-F.; Tan, S.-M.; Chen, J.-M.; Shao, J.-W.; Liao, M. Zoonotic Hantaviridae with Global Public Health Significance. Viruses 2023, 15, 1705. [Google Scholar] [CrossRef]
- Alonso, D.O.; Iglesias, A.; Coelho, R.; Periolo, N.; Bruno, A.; Córdoba, M.T.; Filomarino, N.; Quipildor, M.; Biondo, E.; Fortunato, E.; et al. Epidemiological description, case-fatality rate, and trends of Hantavirus Pulmonary Syndrome: 9 years of surveillance in Argentina. J. Med. Virol. 2019, 91, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Vial, P.A.; Ferrés, M.; Vial, C.; Klingström, J.; Ahlm, C.; López, R.; Le Corre, N.; Mertz, G.J. Hantavirus in humans: A review of clinical aspects and management. Lancet. Infect. Dis. 2023, 23, e371–e382. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Brunt, J.W.; Moore, D.I.; Ernest, S. The hantavirus epidemic in the Southwest: Rodent population dynamics and the implications for transmission of hantavirus-associated adult respiratory distress syndrome (HARDS) in the Four Corners region. In Report to the Federal Centers for Disease Control and Prevention; Sevilleta Long-Term Ecological Research Program, Department of Biology, University of New Mexico: Albuquerque, NM, USA, 1993; pp. 1–45. [Google Scholar]
- Yates, T.L.; Mills, J.N.; Parmenter, C.A.; Ksiazek, T.G.; Parmenter, R.; Castle, J.R.V.; Calisher, C.H.; Nichol, S.T.; Abbott, K.D.; Young, J.C.; et al. The ecology and evolutionary history of an emergent disease: Hantavirus pulmonary syndrome. BioScience 2002, 52, 989–998. [Google Scholar] [CrossRef]
- Luis, A.D.; Douglass, R.J.; Mills, J.N.; Bjørnstad, O.N. Environmental fluctuations lead to predictability in Sin Nombre hanta virus outbreaks. Ecology 2015, 96, 1691–1701. [Google Scholar] [CrossRef]
- Pacifici, M.; Santini, L.; Di Marco, M.; Baisero, D.; Francucci, L.; Grottolo Marasini, G.; Visconti, P.; Rondinini, C. Generation length for mammals. Nat. Conserv. 2013, 5, 89–94. [Google Scholar] [CrossRef]
- Wilson, D.E.; Mittermeier, R.A.; Lacher, T.E. Handbook of the Mammals of the World; Rodents II. Lynx Edicions: Barcelona, Spain, 2017; Volume 7. [Google Scholar]
- Martinez, V.P.; Bellomo, C.M.; Cacace, M.L.; Suárez, P.; Bogni, L.; Paula, J. Hantavirus pulmonary syndrome in Argentina, 1995–2008. Emerg. Infect. Dis. 2010, 12, 1853–1860. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Schmaljohn, C.S. A Brief History of Bunyaviral Family Hantaviridae. Diseases 2023, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Cavia, R.; Carbajo, A.E.; Bellomo, C.; Gonzalez-Capria, S.; Padula, P. Spatial and temporal analysis of the distribution of hantavirus pulmonary syndrome in Buenos Aires Province, and its relation to rodent distribution, agricultural and demographic variables. Trop. Med. Int. Health 2004, 9, 508–519. [Google Scholar] [CrossRef]
- Andreo, V.; Neteler, M.; Rocchini, D.; Provensal, C.; Levis, S.; Porcasi, X.; Rizzoli, A.; Lanfri, M.; Scavuzzo, M.; Pini, N. Estimating Hantavirus risk in southern Argentina: A GIS-based approach combining human cases and host distribution. Viruses 2014, 6, 201–222. [Google Scholar] [CrossRef]
- Vadell, M.V.; GomezVillafañe, I. Environmental variables associated with hantavirus reservoirs and other small rodent Hantavirus pulmonary syndrome risk in Entre Rios, Argentina species in two national parks in the Paraná Delta, Argentina: Implications for disease prevention. EcoHealth 2016, 13, 248–260. [Google Scholar] [CrossRef]
- Andreo, V.; Preovensal, M.C.; Levis, S.; Pini, N.; Enría, D.; Polop, J. Summer–autumn distribution and abundance of the hantavirus host, Oligoryzomys longicaudatus, in northwestern Chubut, Argentina. J. Mammal. 2012, 93, 1559–1568. [Google Scholar] [CrossRef]
- Muschetto, E.; Cueto, G.R.; Cavia, R.; Padula, J.P.; Suárez, O.V. Long-termstudy of a Hantavirus resevoir population in an urban protected area, Argentina. EcoHealth 2018, 15, 804–814. [Google Scholar] [CrossRef]
- Ferro, I.; Bellomo, C.M.; López, W.; Coelho, R.; Alonso, D.; Bruno, A.; Córdoba, F.E.; Martinez, V.P. Hantavirus pulmonary syndrome outbreaks associated with climate variability in Northwestern Argentina, 1997–2017. PLoS Negl. Trop. Dis. 2020, 14, e0008786. [Google Scholar] [CrossRef]
- López, W.R.; Altamiranda-Saavedra, M.; Kehl, S.D.; Ferro, I.; Bellomo, C.; Martínez, V.P.; Simoy, M.I.; Gil, J.F. Modeling potential risk areas of Orthohantavirus transmission in Northwestern Argentina using an ecological niche approach. BMC Public Health 2023, 23, 1236. [Google Scholar] [CrossRef]
- Coman, B.J.; Brunner, H. Food habits of the feral house cat in Victoria. J. Wildl. Manage. 1972, 36, 848–853. [Google Scholar] [CrossRef]
- George, W.G. Domestic cats as density independent hunters and ‘surplus’ killers. Carn. Genet. Newsl. 1978, 3, 282–287. [Google Scholar]
- Barratt, D.G. Predation by House Cats, Felis catus (L.), in Canberra, Australia. I. Prey Composition and Preference. Wildl. Res. 1997, 24, 263–277. [Google Scholar] [CrossRef]
- Kays, R.W.; DeWan, A.A. Ecological impact of inside/outside house cats around suburban nature preserve. Anim. Conserv. 2004, 7, 273–283. [Google Scholar] [CrossRef]
- Thomas, R.L.; Fellowes, M.D.; Baker, P.J. Spatio-Temporal Variation in Predation by Urban Domestic Cats (Felis catus) and the Acceptability of Possible Management Actions in the UK. PLoS ONE 2012, 7, e49369. [Google Scholar] [CrossRef]
- Castañeda, I.; Forin-Wiart, M.-A.; Pisanu, B.; de Bouillane de Lacoste, N. Spatiotemporal and Individual Patterns of Domestic Cat (Felis catus) Hunting Behaviour in France. Animals 2023, 13, 3507. [Google Scholar] [CrossRef]
- Meek, P.D. Food Items Brought Home by Domestic Cats Felis catus (L.) Living in Booderee National Park, Jervis Bay. Proc. Linn. Soc. N. S. W. 1998, 120, 43–48. [Google Scholar]
- Woods, M.; McDonald, R.A.; Harris, S. Predation of Wildlife by Domestic Cats Felis catus in Great Britain. Mammal Rev. 2003, 33, 174–188. [Google Scholar] [CrossRef]
- Mori, E.; Menchetti, M.; Camporesi, A.; Cavigioli, L.; Tabarelli de Fatis, K.; Girardello, M. License to Kill? Domestic Cats Affect a Wide Range of Native Fauna in a Highly Biodiverse Mediterranean Country. Front. Ecol. Evol. 2019, 7, 477. [Google Scholar] [CrossRef]
- Trapletti, A.; Hornik, K. tseries: Time Series Analysis and Computational Finance. 2020. Available online: https://CRAN.R-project.org/package=tseries (accessed on 20 February 2023).
- Hyndman, R.J.; Khandakar, Y. Automatic time series forecasting: The forecast package for R. J. Stat. Softw. 2008, 27, 1–22. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi Model Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Tian and Stenseth, 2019; Tian, H.; Stenseth, N.C. The ecological dynamics of hantavirus diseases: From environmental variability to disease prevention largely based on data from China. PLoS Negl. Trop. Dis. 2019, 13, e0006901. [Google Scholar] [CrossRef]
- Douglas, K.O.; Payne, K.; Sabino-Santos, G., Jr.; Agard, J. Influence of Climatic Factors on Human Hantavirus Infections in Latin America and the Caribbean: Systematic Review. Pathogens 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Cassinelli, F.; Ferro, I.; Coelho, R.; Kehl, S.; López, W. Orthohantavirus en el noroeste de Argentina: Seroprevalencia en roedores de Jujuy y primer registro de Euryoryzomys legatus con serología positiva para SPH. In Proceedings of the Jornadas Argentinas de Mastozoología, San Salvador de Jujuy, Argentina, 28 November 2023. [Google Scholar]
- Jonsson, C.B.; Figueiredo, L.T.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Jara, J.P.; Muñoz-Quezada, M.T.; Córdova-Lepe, F.; Silva-Guzmán, A. Mathematical Model of the Spread of Hantavirus Infection. Pathogens 2023, 12, 1147. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Ostfeld, R. Biodiversity and the Dilution Effect in Disease Ecology. Ecology 2001, 82, 609–619. [Google Scholar] [CrossRef]
- Colombo, V.C.; Brignone, J.; Sen, C.; Previtali, M.A.; Martin, M.L.; Levis, S.; Monje, L.; Gonzalez-Ittig, R.; Beldomenico, P.M. Orthohantavirus genotype Lechiguanas in Oligoryzomys nigripes (Rodentia: Cricetidae): New evidence of host-switching. Acta tropica 2019, 191, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Tang, Y.W.; Kan, L.Y.; Tsai, T.F. Cats- source of protection or infection? A case-control study of hemorrhagic fever with renal syndrome. Am. J. Epidemiol. 1987, 126, 942–948. [Google Scholar] [CrossRef]
- Malecki, T.M.; Jillson, G.P.; Thilsted, J.P.; Elrod, J.; Torrez-Martinez, N.; Hjelle, B. Serologic survey for hantavirus infection in domestic animals and coyotes from New Mexico and northeastern Arizona. J. Am. Vet. Med. Assoc. 1998, 212, 970–973. [Google Scholar] [CrossRef]
- Dobly, A.; Cochez, C.; Goossens, E.; De Bosschere, H.; Hansen, P.; Roels, S.; Heyman, P. Sero-epidemiological study of the presence of hantaviruses in domestic dogs and cats from Belgium. Res. Vet. Sci. 2012, 92, 221–224. [Google Scholar] [CrossRef]
- Nowotny, N.; Weissenboeck, H.; Aberle, S.; Hinterdorfer, F. Hantavirus infection in the domestic cat. JAMA 1994, 272, 1100. [Google Scholar] [CrossRef]
- Mills, J.; Schmidt, K.; Ellis, B.; Calderón, G.; Enría, D.A.; Ksiazek, T.G. A Longitudinal Study of Hantavirus Infection in Three Sympatric Reservoir Species in Agroecosystems on the Argentine Pampa. Vector Borne Zoonotic Dis. 2007, 7, 229–240. [Google Scholar] [CrossRef]
- Valdell, M.V.; Bellomo, C.; San Martin, A.; Padula, P.; GomezVillafañe, I. Hantavirus ecology in rodent populations in three protected areas of Argentina. Trop. Med. Int. Health 2011, 16, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Polop, F.J.; Provensal, M.C.; Pini, N.; Levis, S.; Priotto, J.W.; Enría, D.; Calderón, G.E.; Costa, F.; Polop, J.J. Temporal and spatial host abundance and prevalence of Andes Hantavirus in southern Argentina. EcoHealth 2017, 7, 176–184. [Google Scholar] [CrossRef] [PubMed]


| Year | Month | HPS Cases | Orán Rainfall | Orán Trap Success | Orán Species Richness | Yuto Trap Success | Yuto Species Richness | Paños Hunted Rodents | Paños Species Richness |
|---|---|---|---|---|---|---|---|---|---|
| 2022 | 1 | 0 | 103.5 | 26.1 | 3 | ||||
| 2022 | 2 | 2 | 118.1 | 7.5 | 5 | ||||
| 2022 | 3 | 1 | 74.0 | 11.4 | 5 | 1 | 1 | ||
| 2022 | 4 | 4 | 91.7 | 33.5 | 5 | 6.5 | 5 | 2 | 2 |
| 2022 | 5 | 4 | 0.8 | 3 | 2 | ||||
| 2022 | 6 | 5 | 0.1 | 1.5 | 2 | 1 | 1 | ||
| 2022 | 7 | 2 | 4.0 | 4 | 3 | ||||
| 2022 | 8 | 0 | 1.5 | 1.5 | 2 | 0 | 0 | ||
| 2022 | 9 | 2 | 14.1 | 5 | 3 | ||||
| 2022 | 10 | 7 | 10.4 | 2.69 | 4 | 0.25 | 1 | 1 | 1 |
| 2022 | 11 | 7 | 6.3 | 1.01 | 2 | 0 | 0 | ||
| 2022 | 12 | 8 | 63.8 | 0.75 | 3 | 0 | 0 | ||
| 2023 | 1 | 0 | 117.5 | 0 | 0 | ||||
| 2023 | 2 | 2 | 67.5 | 0 | 0 | 0 | 0 | ||
| 2023 | 3 | 1 | 172.5 | 2.26 | 3 | 0 | 0 | ||
| 2023 | 4 | 1 | 144.5 | 0.25 | 1 | 1 | 3 | ||
| 2023 | 5 | 1 | 7.8 | 10.02 | 2 | 3 | 2 | ||
| 2023 | 6 | 0 | 0 | 7.75 | 3 | 2 | 3 | ||
| 2023 | 7 | 2 | 0.1 | 32.3 | 8 | 9 | 3 | ||
| 2023 | 8 | 0 | 0 | 35.3 | 6 | 12.75 | 3 | 7 | 3 |
| 2023 | 9 | 4 | 1.7 | 8 | 1 | ||||
| 2023 | 10 | 11 | 12.8 | 2.25 | 3 | 2 | 1 | ||
| 2023 | 11 | 13 | 80.7 | 14.4 | 5 | 2 | 2 | ||
| 2023 | 12 | 1 | 173.6 | 1.25 | 2 | 1 | 1 |
| Models | Model Selection | AICc | Ljung–Box Test | R2adj | p-Value |
|---|---|---|---|---|---|
| HPS~Lagged Rainfall | Rainfall 8 months before, AR2 | 47.4 | Q* = 1.29, p-value = 0.73 | 0.69 | >0.01 |
| Rainfall 8 months before, MA2 | 48.1 | Q* = 4.12, p-value = 0.24 | 0.71 | >0.01 | |
| Rainfall 7 months before | 49.2 | Q* = 4.14, p-value = 0.24 | 0.34 | 0.01 | |
| Rainfall 8 months before | 49.6 | Q* = 5.47, p-value = 0.14 | 0.32 | 0.01 | |
| Rainfall 8 months before, MA1 | 50.1 | Q* = 1.79, p-value = 0.52 | 0.59 | >0.01 | |
| HPS~Lagged Orán Rodent Abundance | Trap-success 3 months before | 47.5 | Q* = 1.42, p-value = 0.69 | 0.41 | >0.01 |
| Trap-success 4 months before, MA1 | 48.6 | Q* = 2.76, p-value = 0.43 | 0.58 | >0.01 | |
| Trap-success 3 months before, MA1 | 50.9 | Q* = 1.48, p-value = 0.68 | 0.42 | 0.01 | |
| Trap-success 4 months before | 50.9 | Q* = 3.13, p-value = 0.37 | 0.26 | 0.02 | |
| Trap-success 3 months before, AR1 | 51 | Q* = 1.81, p-value = 0.61 | 0.41 | >0.01 | |
| HPS~Lagged Yuto Rodent Abundance | Trap-success 3 months before | 48.6 | Q* = 0.72, p-value = 0.86 | 0.36 | >0.01 |
| Trap-success 4 months before, MA1 | 51.4 | Q* = 0.95, p-value = 0.81 | 0.40 | >0.01 | |
| Trap-success 3 months before, AR1 | 51.6 | Q* = 0.99, p-value = 0.80 | 0.39 | >0.01 | |
| Trap-success 2 months before | 52.1 | Q* = 3.01, p-value = 0.38 | 0.16 | 0.06 | |
| Trap-success 4 months before | 52.8 | Q* = 1.17, p-value = 0.75 | 0.17 | 0.06 | |
| HPS~Lagged Paños Rodent Abundance | Hunted rodents 3 months before | 48.8 | Q* = 1.25, p-value = 0.73 | 0.36 | >0.01 |
| Hunted rodents 2 months before | 49.2 | Q* = 0.72, p-value = 0.86 | 0.34 | 0.01 | |
| Hunted rodents 3 months before, MA1 | 51.9 | Q* = 1.16, p-value = 0.76 | 0.38 | >0.01 | |
| Hunted rodents 3 months before, AR1 | 52 | Q* = 1.44, p-value = 0.69 | 0.37 | >0.01 | |
| Hunted rodents 2 months before, MA1 | 52.6 | Q* = 0.70, p-value = 0.87 | 0.34 | 0.01 |
| Selected Models | Coefficient | Estimated | Standard Error |
|---|---|---|---|
| HPS~Rainfall 8 month before, AR2 | Rainfall t-8 | 0.89 | 0.13 |
| AR1 | −0.03 | 0.25 | |
| AR2 | −0.81 | 0.15 | |
| HPS~Orán trap-success 3 months before | Rodent Abundance t-3 | 0.74 | 0.20 |
| HPS~Yuto trap-success 3 months before | Rodent Abundance t-3 | 0.69 | 0.20 |
| HPS~Paños hunted rodents 3 months before | Rodent Abundance t-3 | 0.61 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, I.; Lopez, W.; Cassinelli, F.; Aguirre, S.; Cuyckens, G.A.E.; Kehl, S.; Abán-Moreyra, D.; Castillo, P.; Bellomo, C.; Gil, J.; et al. Hantavirus Pulmonary Syndrome Outbreak Anticipation by a Rapid Synchronous Increase in Rodent Abundance in the Northwestern Argentina Endemic Region: Towards an Early Warning System for Disease Based on Climate and Rodent Surveillance Data. Pathogens 2024, 13, 753. https://doi.org/10.3390/pathogens13090753
Ferro I, Lopez W, Cassinelli F, Aguirre S, Cuyckens GAE, Kehl S, Abán-Moreyra D, Castillo P, Bellomo C, Gil J, et al. Hantavirus Pulmonary Syndrome Outbreak Anticipation by a Rapid Synchronous Increase in Rodent Abundance in the Northwestern Argentina Endemic Region: Towards an Early Warning System for Disease Based on Climate and Rodent Surveillance Data. Pathogens. 2024; 13(9):753. https://doi.org/10.3390/pathogens13090753
Chicago/Turabian StyleFerro, Ignacio, Walter Lopez, Flavia Cassinelli, Sara Aguirre, Griet A. E. Cuyckens, Sebastián Kehl, Daira Abán-Moreyra, Paola Castillo, Carla Bellomo, José Gil, and et al. 2024. "Hantavirus Pulmonary Syndrome Outbreak Anticipation by a Rapid Synchronous Increase in Rodent Abundance in the Northwestern Argentina Endemic Region: Towards an Early Warning System for Disease Based on Climate and Rodent Surveillance Data" Pathogens 13, no. 9: 753. https://doi.org/10.3390/pathogens13090753
APA StyleFerro, I., Lopez, W., Cassinelli, F., Aguirre, S., Cuyckens, G. A. E., Kehl, S., Abán-Moreyra, D., Castillo, P., Bellomo, C., Gil, J., & Martinez, V. P. (2024). Hantavirus Pulmonary Syndrome Outbreak Anticipation by a Rapid Synchronous Increase in Rodent Abundance in the Northwestern Argentina Endemic Region: Towards an Early Warning System for Disease Based on Climate and Rodent Surveillance Data. Pathogens, 13(9), 753. https://doi.org/10.3390/pathogens13090753





