Influenza B Virus Vaccine Innovation through Computational Design
Abstract
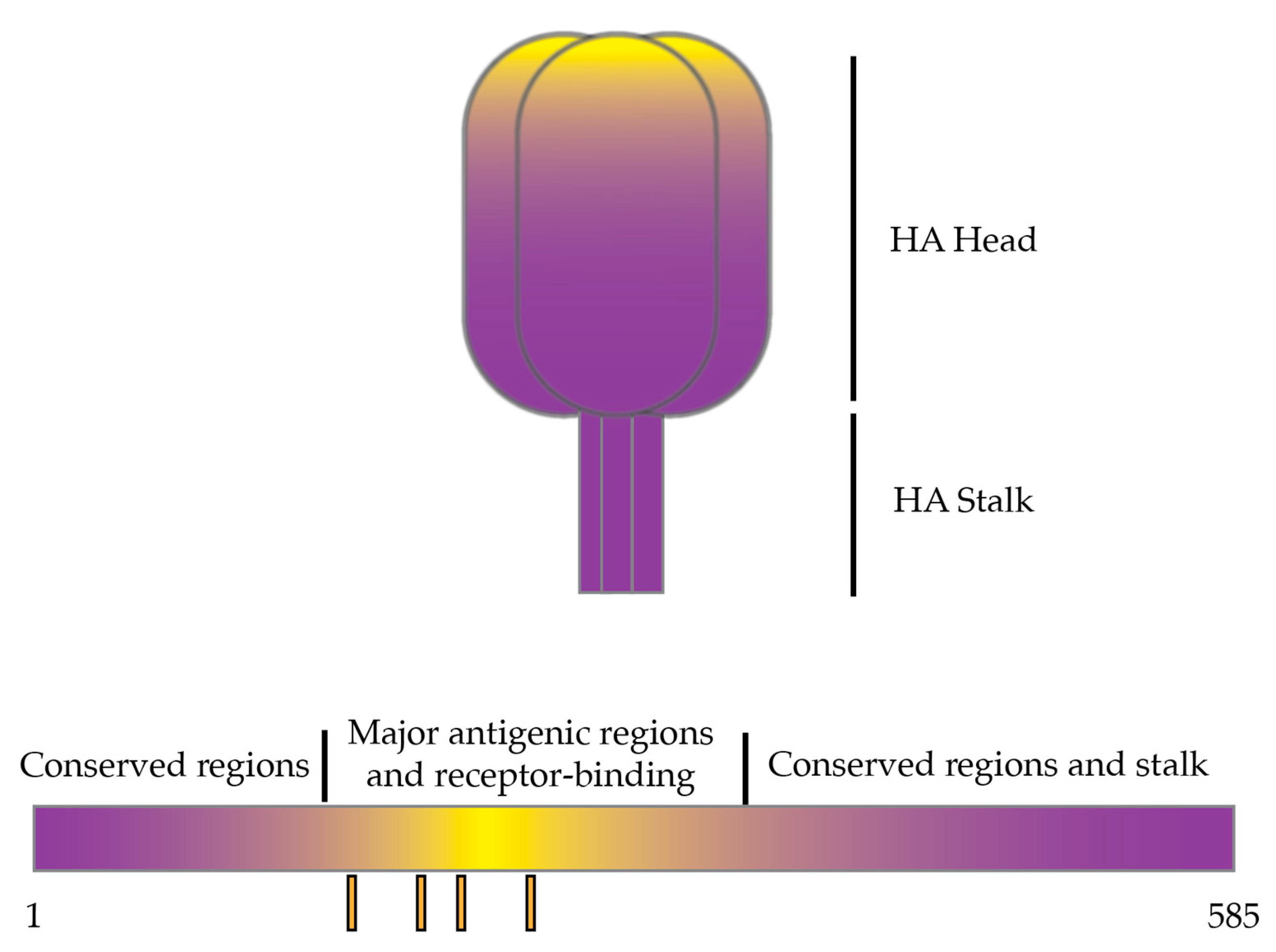
:1. Introduction
2. Evolutionary Patterns and Recent Surveillance

3. The Current State of Clinical IBV Vaccines and Trends in Immunogen Design for Pre-Clinical Vaccine Research
4. Computational Design Strategies for Influenza Vaccines
4.1. Single-Target Consensus Designs
4.2. Multivalent Computational Algorithm Designs
5. Concluding Remarks and Future Research Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cassini, A.; Colzani, E.; Pini, A.; Mangen, M.J.; Plass, D.; McDonald, S.A.; Maringhini, G.; van Lier, A.; Haagsma, J.A.; Havelaar, A.H.; et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): Results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018, 23, 17-00454. [Google Scholar] [CrossRef] [PubMed]
- Leuba, S.I.; Yaesoubi, R.; Antillon, M.; Cohen, T.; Zimmer, C. Tracking and predicting U.S. influenza activity with a real-time surveillance network. PLoS Comput. Biol. 2020, 16, e1008180. [Google Scholar] [CrossRef]
- WHO. Influenza (Seasonal). Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 1 September 2023).
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef]
- Treanor, J.J.; El Sahly, H.; King, J.; Graham, I.; Izikson, R.; Kohberger, R.; Patriarca, P.; Cox, M. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok®) against influenza in healthy adults: A randomized, placebo-controlled trial. Vaccine 2011, 29, 7733–7739. [Google Scholar] [CrossRef]
- Wei, C.J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Corder, B.N.; Bullard, B.L.; Poland, G.A.; Weaver, E.A. A Decade in Review: A Systematic Review of Universal Influenza Vaccines in Clinical Trials during the 2010 Decade. Viruses 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Bullard, B.L.; Weaver, E.A. Strategies Targeting Hemagglutinin as a Universal Influenza Vaccine. Vaccines 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Tsybalova, L.M.; Stepanova, L.A.; Ramsay, E.S.; Vasin, A.V. Influenza B: Prospects for the Development of Cross-Protective Vaccines. Viruses 2022, 14, 1323. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.; Chen, X.; Kim, C.U. Discovery and development of GS 4104 (oseltamivir): An orally active influenza neuraminidase inhibitor. Curr. Med. Chem. 2000, 7, 663–672. [Google Scholar] [CrossRef]
- Burnham, A.J.; Baranovich, T.; Govorkova, E.A. Neuraminidase inhibitors for influenza B virus infection: Efficacy and resistance. Antiviral Res. 2013, 100, 520–534. [Google Scholar] [CrossRef]
- Koszalka, P.; Subbarao, K.; Baz, M. Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 2022, 18, e1010481. [Google Scholar] [CrossRef] [PubMed]
- Caceres, C.J.; Seibert, B.; Cargnin Faccin, F.; Cardenas-Garcia, S.; Rajao, D.S.; Perez, D.R. Influenza antivirals and animal models. FEBS Open Bio 2022, 12, 1142–1165. [Google Scholar] [CrossRef]
- Zhang, A.; Chaudhari, H.; Agung, Y.; D’Agostino, M.R.; Ang, J.C.; Tugg, Y.; Miller, M.S. Hemagglutinin stalk-binding antibodies enhance effectiveness of neuraminidase inhibitors against influenza via Fc-dependent effector functions. Cell Rep. Med. 2022, 3, 100718. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef]
- Webster, R.G.; Laver, W.G.; Air, G.M.; Schild, G.C. Molecular mechanisms of variation in influenza viruses. Nature 1982, 296, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, H.; Liu, G.D.; Xue, C.; Cao, Y. Targeting Hemagglutinin: Approaches for Broad Protection against the Influenza A Virus. Viruses 2019, 11, 405. [Google Scholar] [CrossRef]
- Wang, W.C.; Sayedahmed, E.E.; Sambhara, S.; Mittal, S.K. Progress towards the Development of a Universal Influenza Vaccine. Viruses 2022, 14, 1684. [Google Scholar] [CrossRef]
- Bloom, J.D.; Gong, L.I.; Baltimore, D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 2010, 328, 1272–1275. [Google Scholar] [CrossRef]
- Meijer, A.; Rebelo-de-Andrade, H.; Correia, V.; Besselaar, T.; Drager-Dayal, R.; Fry, A.; Gregory, V.; Gubareva, L.; Kageyama, T.; Lackenby, A.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2012–2013. Antiviral Res. 2014, 110, 31–41. [Google Scholar] [CrossRef]
- Osterhaus, A.D.; Rimmelzwaan, G.F.; Martina, B.E.; Bestebroer, T.M.; Fouchier, R.A. Influenza B virus in seals. Science 2000, 288, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.H.; Harris, P.A.; Alexander, D.J. Serological studies of influenza viruses in pigs in Great Britain 1991-2. Epidemiol. Infect. 1995, 114, 511–520. [Google Scholar] [CrossRef]
- Tsai, C.P.; Tsai, H.J. Influenza B viruses in pigs, Taiwan. Influenza Other Respir. Viruses 2019, 13, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Hastings, P.R.; Krewski, D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef]
- Su, S.; Chaves, S.S.; Perez, A.; D’Mello, T.; Kirley, P.D.; Yousey-Hindes, K.; Farley, M.M.; Harris, M.; Sharangpani, R.; Lynfield, R.; et al. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin. Infect. Dis. 2014, 59, 252–255. [Google Scholar] [CrossRef]
- Avni, T.; Babich, T.; Nir, A.; Yahav, D.; Shaked, H.; Sorek, N.; Zvi, H.B.; Bishara, J.; Atamna, A. Comparison of clinical outcomes of influenza A and B at the 2017–2018 influenza season: A cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1109–1114. [Google Scholar] [CrossRef]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antiviral Res. 2021, 185, 104970. [Google Scholar] [CrossRef]
- Shang, M.; Blanton, L.; Brammer, L.; Olsen, S.J.; Fry, A.M. Influenza-Associated Pediatric Deaths in the United States, 2010–2016. Pediatrics 2018, 141, e20172918. [Google Scholar] [CrossRef]
- Read, J.M.; Zimmer, S.; Vukotich, C., Jr.; Schweizer, M.L.; Galloway, D.; Lingle, C.; Yearwood, G.; Calderone, P.; Noble, E.; Quadelacy, T.; et al. Influenza and other respiratory viral infections associated with absence from school among schoolchildren in Pittsburgh, Pennsylvania, USA: A cohort study. BMC Infect. Dis. 2021, 21, 291. [Google Scholar] [CrossRef]
- Yazici Özkaya, P.; Turanli, E.E.; Metin, H.; Aydın Uysal, A.; Çiçek, C.; Karapinar, B. Severe influenza virus infection in children admitted to the PICU: Comparison of influenza A and influenza B virus infection. J. Med. Virol. 2022, 94, 575–581. [Google Scholar] [CrossRef]
- Dai, Z.; Fan, K.; Zhang, L.; Yang, M.; Yu, Q.; Liu, L.; Leung, L. Risk factors for influenza B virus-associated pneumonia in adults. Am. J. Infect. Control 2020, 48, 194–198. [Google Scholar] [CrossRef]
- Borchering, R.K.; Gunning, C.E.; Gokhale, D.V.; Weedop, K.B.; Saeidpour, A.; Brett, T.S.; Rohani, P. Anomalous influenza seasonality in the United States and the emergence of novel influenza B viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2012327118. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased Influenza Activity During the COVID-19 Pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1305–1309. [Google Scholar] [CrossRef]
- Qi, Y.; Shaman, J.; Pei, S. Quantifying the Impact of COVID-19 Nonpharmaceutical Interventions on Influenza Transmission in the United States. J. Infect. Dis. 2021, 224, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Achangwa, C.; Park, H.; Ryu, S.; Lee, M.-S. Collateral Impact of Public Health and Social Measures on Respiratory Virus Activity during the COVID-19 Pandemic 2020–2021. Viruses 2022, 14, 1071. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kawashima, R. Disappearance and Re-Emergence of Influenza during the COVID-19 Pandemic: Association with Infection Control Measures. Viruses 2023, 15, 223. [Google Scholar] [CrossRef]
- Kuitunen, I.; Artama, M.; Haapanen, M.; Renko, M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J. Med. Virol. 2021, 93, 6063–6067. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, M.; Cao, L.; Su, L.; Lu, L.; Dong, N.; Jia, R.; Zhu, X.; Xu, J. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol. J. 2021, 18, 159. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Sikazwe, C.T.; Minney-Smith, C.A.; Ernst, T.; Moore, H.C.; Nicol, M.P.; Smith, D.W.; Levy, A.; Blyth, C.C. An Unusual Resurgence of Human Metapneumovirus in Western Australia Following the Reduction of Non-Pharmaceutical Interventions to Prevent SARS-CoV-2 Transmission. Viruses 2022, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Caini, S.; Del Riccio, M.; van Waarden, W.; Meijer, A. Has influenza B/Yamagata become extinct and what implications might this have for quadrivalent influenza vaccines? Euro Surveill. 2022, 27, 2200753. [Google Scholar] [CrossRef]
- Francis, T., Jr. A New Type of Virus from Epidemic Influenza. Science 1940, 92, 405–408. [Google Scholar] [CrossRef]
- Rota, P.A.; Hemphill, M.L.; Whistler, T.; Regnery, H.L.; Kendal, A.P. Antigenic and genetic characterization of the haemagglutinins of recent cocirculating strains of influenza B virus. J. Gen. Virol. 1992, 73 Pt 10, 2737–2742. [Google Scholar] [CrossRef]
- Rosu, M.E.; Lexmond, P.; Bestebroer, T.M.; Hauser, B.M.; Smith, D.J.; Herfst, S.; Fouchier, R.A.M. Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc. Natl. Acad. Sci. USA 2022, 119, e2211616119. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lindstrom, S.E.; Shaw, M.W.; Smith, C.B.; Hall, H.E.; Mungall, B.A.; Subbarao, K.; Cox, N.J.; Klimov, A. Reassortment and evolution of current human influenza A and B viruses. Virus Res. 2004, 103, 55–60. [Google Scholar] [CrossRef]
- Lin, Y.P.; Gregory, V.; Bennett, M.; Hay, A. Recent changes among human influenza viruses. Virus Res. 2004, 103, 47–52. [Google Scholar] [CrossRef]
- Vijaykrishna, D.; Holmes, E.C.; Joseph, U.; Fourment, M.; Su, Y.C.; Halpin, R.; Lee, R.T.; Deng, Y.M.; Gunalan, V.; Lin, X.; et al. The contrasting phylodynamics of human influenza B viruses. eLife 2015, 4, e05055. [Google Scholar] [CrossRef]
- Langat, P.; Raghwani, J.; Dudas, G.; Bowden, T.A.; Edwards, S.; Gall, A.; Bedford, T.; Rambaut, A.; Daniels, R.S.; Russell, C.A.; et al. Genome-wide evolutionary dynamics of influenza B viruses on a global scale. PLoS Pathog. 2017, 13, e1006749. [Google Scholar] [CrossRef]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.E.; Lee, H.K.; et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Prud’homme, I.; Weber, J.M. Evolution of the hemagglutinin gene of influenza B virus was driven by both positive and negative selection pressures. Virus Genes 1997, 14, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, F.; Lu, M.; Tian, X.; Ma, J. Crystal structure of unliganded influenza B virus hemagglutinin. J. Virol. 2008, 82, 3011–3020. [Google Scholar] [CrossRef]
- Sun, W.; Kang, D.S.; Zheng, A.; Liu, S.T.H.; Broecker, F.; Simon, V.; Krammer, F.; Palese, P. Antibody Responses toward the Major Antigenic Sites of Influenza B Virus Hemagglutinin in Mice, Ferrets, and Humans. J. Virol. 2019, 93, e01673-18. [Google Scholar] [CrossRef]
- Fulton, B.O.; Sun, W.; Heaton, N.S.; Palese, P. The Influenza B Virus Hemagglutinin Head Domain Is Less Tolerant to Transposon Mutagenesis than That of the Influenza A Virus. J. Virol. 2018, 92, e00754-18. [Google Scholar] [CrossRef]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef]
- Valesano, A.L.; Fitzsimmons, W.J.; McCrone, J.T.; Petrie, J.G.; Monto, A.S.; Martin, E.T.; Lauring, A.S. Influenza B Viruses Exhibit Lower Within-Host Diversity than Influenza A Viruses in Human Hosts. J. Virol. 2020, 94, e01710-19. [Google Scholar] [CrossRef]
- Nobusawa, E.; Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 2006, 80, 3675–3678. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.; Riley, S.; Barr, I.G.; Broor, S.; Chadha, M.; Cox, N.J.; Daniels, R.S.; Gunasekaran, C.P.; Hurt, A.C.; Kelso, A.; et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 2015, 523, 217–220. [Google Scholar] [CrossRef]
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: Results of the Global Influenza B Study. Influenza Other Respir. Viruses 2015, 9 (Suppl. 1), 3–12. [Google Scholar] [CrossRef]
- Caini, S.; Kusznierz, G.; Garate, V.V.; Wangchuk, S.; Thapa, B.; de Paula Júnior, F.J.; Ferreira de Almeida, W.A.; Njouom, R.; Fasce, R.A.; Bustos, P.; et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS ONE 2019, 14, e0222381. [Google Scholar] [CrossRef]
- Campbell, A.P.; Ogokeh, C.; Weinberg, G.A.; Boom, J.A.; Englund, J.A.; Williams, J.V.; Halasa, N.B.; Selvarangan, R.; Staat, M.A.; Klein, E.J.; et al. Effect of Vaccination on Preventing Influenza-Associated Hospitalizations among Children during a Severe Season Associated with B/Victoria Viruses, 2019–2020. Clin. Infect. Dis. 2021, 73, e947–e954. [Google Scholar] [CrossRef]
- Miron, V.D.; Bănică, L.; Săndulescu, O.; Paraschiv, S.; Surleac, M.; Florea, D.; Vlaicu, O.; Milu, P.; Streinu-Cercel, A.; Bilașco, A.; et al. Clinical and molecular epidemiology of influenza viruses from Romanian patients hospitalized during the 2019/20 season. PLoS ONE 2021, 16, e0258798. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, K.; Mansanguan, C.; Schuerch, D.; Boonyuen, U.; Lerdsamran, H.; Jiamsomboon, K.; Sae Wang, F.; Huntrup, A.; Prasertsopon, J.; Kosoltanapiwat, N.; et al. Molecular Characterization of Seasonal Influenza A and B from Hospitalized Patients in Thailand in 2018–2019. Viruses 2021, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Heider, A.; Wedde, M.; Dürrwald, R.; Wolff, T.; Schweiger, B. Molecular characterization and evolution dynamics of influenza B viruses circulating in Germany from season 1996/1997 to 2019/2020. Virus Res. 2022, 322, 198926. [Google Scholar] [CrossRef] [PubMed]
- US Centers for Disease Control. FluView Interactive; National, Regional, and State Level Outpatient Illness and Viral Surveillance. Available online: https://gis.cdc.gov/grasp/fluview/fluportaldashboard.html (accessed on 5 June 2024).
- Koutsakos, M.; Wheatley, A.K.; Laurie, K.; Kent, S.J.; Rockman, S. Influenza lineage extinction during the COVID-19 pandemic? Nat. Rev. Microbiol. 2021, 19, 741–742. [Google Scholar] [CrossRef]
- Monto, A.S.; Zambon, M.; Weir, J.P. The End of B/Yamagata Influenza Transmission—Transitioning from Quadrivalent Vaccines. N. Engl. J. Med. 2024, 390, 1256–1258. [Google Scholar] [CrossRef]
- Koutsakos, M.; Rockman, S.; Krammer, F. Is eradication of influenza B viruses possible? Lancet Infect. Dis. 2024, 24, 451–453. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Masaro, C.; Kwindt, T.L.; Mak, A.; Petric, M.; Li, Y.; Sebastian, R.; Chong, M.; Tam, T.; De Serres, G. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: Results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007, 25, 2842–2851. [Google Scholar] [CrossRef]
- Jackson, L.A.; Gaglani, M.J.; Keyserling, H.L.; Balser, J.; Bouveret, N.; Fries, L.; Treanor, J.J. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: A randomized, placebo-controlled trial over two influenza seasons. BMC Infect. Dis. 2010, 10, 71. [Google Scholar] [CrossRef]
- Lo, Y.C.; Chuang, J.H.; Kuo, H.W.; Huang, W.T.; Hsu, Y.F.; Liu, M.T.; Chen, C.H.; Huang, H.H.; Chang, C.H.; Chou, J.H.; et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–2012 season. PLoS ONE 2013, 8, e58222. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.D.; Siqueira, M.M.; Brown, D.; Lopes, J.O.; Costa, B.C.D.; Gama, E.L.; Aguiar-Oliveira, M.L. Vaccine Mismatches, Viral Circulation, and Clinical Severity Patterns of Influenza B Victoria and Yamagata Infections in Brazil over the Decade 2010–2020: A Statistical and Phylogeny-Trait Analyses. Viruses 2022, 14, 1477. [Google Scholar] [CrossRef] [PubMed]
- FDA, U.S. Fluzone, Fluzone High-Dose and Fluzone Intradermal. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/fluzone-fluzone-high-dose-and-fluzone-intradermal (accessed on 13 January 2024).
- Zimmerman, R.K.; Nowalk, M.P.; Chung, J.; Jackson, M.L.; Jackson, L.A.; Petrie, J.G.; Monto, A.S.; McLean, H.Q.; Belongia, E.A.; Gaglani, M.; et al. 2014–2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin. Infect. Dis. 2016, 63, 1564–1573. [Google Scholar] [CrossRef]
- Flannery, B.; Kondor, R.J.G.; Chung, J.R.; Gaglani, M.; Reis, M.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; et al. Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018–2019 Season. J. Infect. Dis. 2019, 221, 8–15. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kondor, R.J.G.; Chung, J.R.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019–2020. Clin. Infect. Dis. 2021, 73, e4244–e4250. [Google Scholar] [CrossRef]
- Castrucci, M.R. Factors affecting immune responses to the influenza vaccine. Hum. Vaccines Immunother. 2018, 14, 637–646. [Google Scholar] [CrossRef]
- Rajaram, S.; Wojcik, R.; Moore, C.; Ortiz de Lejarazu, R.; de Lusignan, S.; Montomoli, E.; Rossi, A.; Pérez-Rubio, A.; Trilla, A.; Baldo, V.; et al. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 2020, 38, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Seong, B.L. The Quest for a Truly Universal Influenza Vaccine. Front. Cell Infect. Microbiol. 2019, 9, 344. [Google Scholar] [CrossRef]
- Essink, B.J.; Heeringa, M.; Jeanfreau, R.J.; Finn, D.; Matassa, V.; Edelman, J.; Hohenboken, M.; Molrine, D. Safety and Immunogenicity of Cell-Based Quadrivalent Influenza Vaccine: A Randomized Trial. Pediatrics 2022, 150, e2022057509. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hsieh, S.M.; Hwang, S.J.; Liu, C.S.; Li, X.; Fournier, M.; Yeh, T.Y.; Yin, J.K.; Samson, S.I. Immunogenicity and safety of high-dose quadrivalent influenza vaccine in older adults in Taiwan: A phase III, randomized, multi-center study. Vaccine 2022, 40, 6450–6454. [Google Scholar] [CrossRef]
- Vanni, T.; da Graça Salomão, M.; Viscondi, J.Y.K.; Braga, P.E.; da Silva, A.; de Oliveira Piorelli, R.; do Prado Santos, J.; Gattás, V.L.; Lucchesi, M.B.B.; de Oliveira, M.M.M.; et al. A randomized, double-blind, non-inferiority trial comparing the immunogenicity and safety of two seasonal inactivated influenza vaccines in adults. Vaccine 2023, 41, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.G.; Levine, M.Z.; McClure, D.L.; King, J.P.; Flannery, B.; Nguyen, H.Q.; Belongia, E.A. Antibody response to sequential vaccination with cell culture, recombinant, or egg-based influenza vaccines among U.S. adults. Hum. Vaccin Immunother. 2024, 20, 2370087. [Google Scholar] [CrossRef]
- Heaton, N.S.; Sachs, D.; Chen, C.J.; Hai, R.; Palese, P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20248–20253. [Google Scholar] [CrossRef]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef]
- Ermler, M.E.; Kirkpatrick, E.; Sun, W.; Hai, R.; Amanat, F.; Chromikova, V.; Palese, P.; Krammer, F. Chimeric Hemagglutinin Constructs Induce Broad Protection against Influenza B Virus Challenge in the Mouse Model. J. Virol. 2017, 91, e00286-17. [Google Scholar] [CrossRef]
- Sun, W.; Kirkpatrick, E.; Ermler, M.; Nachbagauer, R.; Broecker, F.; Krammer, F.; Palese, P. Development of Influenza B Universal Vaccine Candidates Using the “Mosaic” Hemagglutinin Approach. J. Virol. 2019, 93, e00333-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Strohmeier, S.; González-Domínguez, I.; Tan, J.; Simon, V.; Krammer, F.; García-Sastre, A.; Palese, P.; Sun, W. Mosaic Hemagglutinin-Based Whole Inactivated Virus Vaccines Induce Broad Protection Against Influenza B Virus Challenge in Mice. Front. Immunol. 2021, 12, 746447. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, W.; Wang, Y.; Deng, L.; Ma, Y.; Dong, C.; Gonzalez, G.X.; Kim, J.; Wei, L.; Kang, S.M.; et al. Layered protein nanoparticles containing influenza B HA stalk induced sustained cross-protection against viruses spanning both viral lineages. Biomaterials 2022, 287, 121664. [Google Scholar] [CrossRef]
- Cardenas-Garcia, S.; Caceres, C.J.; Rajao, D.; Perez, D.R. Reverse genetics for influenza B viruses and recent advances in vaccine development. Curr. Opin. Virol. 2020, 44, 191–202. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Pardi, N.; Carreño, J.M.; O’Dell, G.; Tan, J.; Bajusz, C.; Muramatsu, H.; Rijnink, W.; Strohmeier, S.; Loganathan, M.; Bielak, D.; et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 2022, 13, 4677. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6, e02556. [Google Scholar] [CrossRef]
- McMahon, M.; Kirkpatrick, E.; Stadlbauer, D.; Strohmeier, S.; Bouvier, N.M.; Krammer, F. Mucosal Immunity against Neuraminidase Prevents Influenza B Virus Transmission in Guinea Pigs. mBio 2019, 10, e00560-19. [Google Scholar] [CrossRef]
- Portela Catani, J.P.; Ysenbaert, T.; Smet, A.; Vuylsteke, M.; Vogel, T.U.; Saelens, X. Anti-neuraminidase and anti-hemagglutinin immune serum can confer inter-lineage cross protection against recent influenza B. PLoS ONE 2023, 18, e0280825. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.T.; Wheatley, A.K.; Kent, S.J.; Koutsakos, M. Influenza B virus neuraminidase: A potential target for next-generation vaccines? Expert Rev. Vaccines 2024, 23, 39–48. [Google Scholar] [CrossRef]
- McMahon, M.; Tan, J.; O’Dell, G.; Kirkpatrick Roubidoux, E.; Strohmeier, S.; Krammer, F. Immunity induced by vaccination with recombinant influenza B virus neuraminidase protein breaks viral transmission chains in guinea pigs in an exposure intensity-dependent manner. J. Virol. 2023, 97, e0105723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ross, T.M. Anti-neuraminidase immunity in the combat against influenza. Expert Rev. Vaccines 2024, 23, 474–484. [Google Scholar] [CrossRef]
- Wu, N.C.; Ellebedy, A.H. Targeting neuraminidase: The next frontier for broadly protective influenza vaccines. Trends Immunol. 2024, 45, 11–19. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef]
- Freyn, A.W.; Ramos da Silva, J.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; de Souza Ferreira, L.C.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, J.O.; Chang, J. Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus. Clin. Exp. Vaccine Res. 2019, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, E.A.; Chang, J. A “Prime and Deploy” Strategy for Universal Influenza Vaccine Targeting Nucleoprotein Induces Lung-Resident Memory CD8 T cells. Immune Netw. 2021, 21, e28. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, T.; Yoshida, R.; Sugimoto, C.; Takada, A.; Kajino, K. Cross-Protective Peptide Vaccine against Influenza A Viruses Developed in HLA-A*2402 Human Immunity Model. PLoS ONE 2011, 6, e24626. [Google Scholar] [CrossRef]
- Crowe, S.R.; Miller, S.C.; Shenyo, R.M.; Woodland, D.L. Vaccination with an acidic polymerase epitope of influenza virus elicits a potent antiviral T cell response but delayed clearance of an influenza virus challenge. J. Immunol. 2005, 174, 696–701. [Google Scholar] [CrossRef]
- Carascal, M.B.; Pavon, R.D.N.; Rivera, W.L. Recent Progress in Recombinant Influenza Vaccine Development toward Heterosubtypic Immune Response. Front. Immunol. 2022, 13, 878943. [Google Scholar] [CrossRef] [PubMed]
- Gravel, C.; Muralidharan, A.; Duran, A.; Zetner, A.; Pfeifle, A.; Zhang, W.; Hashem, A.; Tamming, L.; Farnsworth, A.; Loemba, H.; et al. Synthetic vaccine affords full protection to mice against lethal challenge of influenza B virus of both genetic lineages. iScience 2021, 24, 103328. [Google Scholar] [CrossRef]
- Weaver, E.A.; Rubrum, A.M.; Webby, R.J.; Barry, M.A. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS ONE 2011, 6, e18314. [Google Scholar] [CrossRef]
- Webby, R.J.; Weaver, E.A. Centralized Consensus Hemagglutinin Genes Induce Protective Immunity against H1, H3 and H5 Influenza Viruses. PLoS ONE 2015, 10, e0140702. [Google Scholar] [CrossRef]
- Lingel, A.; Bullard, B.L.; Weaver, E.A. Efficacy of an Adenoviral Vectored Multivalent Centralized Influenza Vaccine. Sci. Rep. 2017, 7, 14912. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.M.; Bullard, B.L.; Pekarek, M.J.; Weaver, E.A. Adenoviral-Vectored Centralized Consensus Hemagglutinin Vaccine Provides Broad Protection against H2 Influenza a Virus. Vaccines 2022, 10, 926. [Google Scholar] [CrossRef]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Giles, B.M.; Crevar, C.J.; Carter, D.M.; Bissel, S.J.; Schultz-Cherry, S.; Wiley, C.A.; Ross, T.M. A Computationally Optimized Hemagglutinin Virus-Like Particle Vaccine Elicits Broadly Reactive Antibodies that Protect Nonhuman Primates from H5N1 Infection. J. Infect. Dis. 2012, 205, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U.; et al. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef]
- Wong, T.M.; Allen, J.D.; Bebin-Blackwell, A.G.; Carter, D.M.; Alefantis, T.; DiNapoli, J.; Kleanthous, H.; Ross, T.M. Computationally Optimized Broadly Reactive Hemagglutinin Elicits Hemagglutination Inhibition Antibodies against a Panel of H3N2 Influenza Virus Cocirculating Variants. J. Virol. 2017, 91, e01581-17. [Google Scholar] [CrossRef]
- Allen, J.D.; Ray, S.; Ross, T.M. Split inactivated COBRA vaccine elicits protective antibodies against H1N1 and H3N2 influenza viruses. PLoS ONE 2018, 13, e0204284. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Owino, S.O.; Suzuki-Williams, L.P.; Crevar, C.J.; Carter, D.M.; Ross, T.M. Computationally optimized broadly reactive vaccine based upon swine H1N1 influenza hemagglutinin sequences protects against both swine and human isolated viruses. Hum. Vaccin. Immunother. 2019, 15, 2013–2029. [Google Scholar] [CrossRef]
- Reneer, Z.B.; Jamieson, P.J.; Skarlupka, A.L.; Huang, Y.; Ross, T.M. Computationally Optimized Broadly Reactive H2 HA Influenza Vaccines Elicited Broadly Cross-Reactive Antibodies and Protected Mice from Viral Challenges. J. Virol. 2020, 95, e01526-20. [Google Scholar] [CrossRef] [PubMed]
- Bertran, K.; Kassa, A.; Criado, M.F.; Nuñez, I.A.; Lee, D.H.; Killmaster, L.; e Silva, M.S.; Ross, T.M.; Mebatsion, T.; Pritchard, N.; et al. Efficacy of recombinant Marek’s disease virus vectored vaccines with computationally optimized broadly reactive antigen (COBRA) hemagglutinin insert against genetically diverse H5 high pathogenicity avian influenza viruses. Vaccine 2021, 39, 1933–1942. [Google Scholar] [CrossRef]
- Reneer, Z.B.; Skarlupka, A.L.; Jamieson, P.J.; Ross, T.M. Broadly Reactive H2 Hemagglutinin Vaccines Elicit Cross-Reactive Antibodies in Ferrets Preimmune to Seasonal Influenza A Viruses. mSphere 2021, 6, e00052-21. [Google Scholar] [CrossRef]
- Eckshtain-Levi, M.; Batty, C.J.; Lifshits, L.M.; McCammitt, B.; Moore, K.M.; Amouzougan, E.A.; Stiepel, R.T.; Duggan, E.; Ross, T.M.; Bachelder, E.M.; et al. Metal-Organic Coordination Polymer for Delivery of a Subunit Broadly Acting Influenza Vaccine. ACS Appl. Mater. Interfaces 2022, 14, 28548–28558. [Google Scholar] [CrossRef]
- Skarlupka, A.L.; Zhang, X.; Blas-Machado, U.; Sumner, S.F.; Ross, T.M. Multi-Influenza HA Subtype Protection of Ferrets Vaccinated with an N1 COBRA-Based Neuraminidase. Viruses 2023, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.F.; Kassa, A.; Bertran, K.; Kwon, J.H.; e Silva, M.S.; Killmaster, L.; Ross, T.M.; Mebatsion, T.; Swayne, D.E. Efficacy of multivalent recombinant herpesvirus of turkey vaccines against high pathogenicity avian influenza, infectious bursal disease, and Newcastle disease viruses. Vaccine 2023, 41, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Gandhapudi, S.K.; Shi, H.; Ward, M.R.; Bush, J.P.; Avdiushko, M.; Sundarapandiyan, K.; Wood, L.V.; Dorrani, M.; Fatima, A.; Dervan, J.; et al. Recombinant Protein Vaccines Formulated with Enantio-Specific Cationic Lipid R-DOTAP Induce Protective Cellular and Antibody-Mediated Immune Responses in Mice. Viruses 2023, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Carlock, M.A.; Ross, T.M. A computationally optimized broadly reactive hemagglutinin vaccine elicits neutralizing antibodies against influenza B viruses from both lineages. Sci. Rep. 2023, 13, 15911. [Google Scholar] [CrossRef]
- Kamlangdee, A.; Kingstad-Bakke, B.; Anderson, T.K.; Goldberg, T.L.; Osorio, J.E. Broad protection against avian influenza virus by using a modified vaccinia Ankara virus expressing a mosaic hemagglutinin gene. J. Virol. 2014, 88, 13300–13309. [Google Scholar] [CrossRef]
- Florek, K.R.; Kamlangdee, A.; Mutschler, J.P.; Kingstad-Bakke, B.; Schultz-Darken, N.; Broman, K.W.; Osorio, J.E.; Friedrich, T.C. A modified vaccinia Ankara vaccine vector expressing a mosaic H5 hemagglutinin reduces viral shedding in rhesus macaques. PLoS ONE 2017, 12, e0181738. [Google Scholar] [CrossRef]
- Corder, B.N.; Bullard, B.L.; DeBeauchamp, J.L.; Ilyushina, N.A.; Webby, R.J.; Weaver, E.A. Influenza H1 Mosaic Hemagglutinin Vaccine Induces Broad Immunity and Protection in Mice. Vaccines 2019, 7, 195. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; DeBeauchamp, J.; Rubrum, A.; Korber, B.; Webby, R.J.; Weaver, E.A. Epigraph hemagglutinin vaccine induces broad cross-reactive immunity against swine H3 influenza virus. Nat. Commun. 2021, 12, 1203. [Google Scholar] [CrossRef]
- Bullard, B.L.; DeBeauchamp, J.; Pekarek, M.J.; Petro-Turnquist, E.; Vogel, P.; Webby, R.J.; Weaver, E.A. An epitope-optimized human H3N2 influenza vaccine induces broadly protective immunity in mice and ferrets. NPJ Vaccines 2022, 7, 65. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.; Pekarek, M.; Jeanjaquet, N.; Wooledge, C.; Steffen, D.; Vu, H.; Weaver, E.A. Adenoviral-vectored epigraph vaccine elicits robust, durable, and protective immunity against H3 influenza A virus in swine. Front. Immunol. 2023, 14, 1143451. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.; Corder Kampfe, B.; Gadeken, A.; Pekarek, M.J.; Weaver, E.A. Multivalent Epigraph Hemagglutinin Vaccine Protects against Influenza B Virus in Mice. Pathogens 2024, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Perkins, S.; Theiler, J.; Bhattacharya, T.; Yusim, K.; Funkhouser, R.; Kuiken, C.; Haynes, B.; Letvin, N.L.; Walker, B.D.; et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 2007, 13, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; O’Brien, K.L.; Simmons, N.L.; King, S.L.; Abbink, P.; Maxfield, L.F.; Sun, Y.H.; La Porte, A.; Riggs, A.M.; Lynch, D.M.; et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat. Med. 2010, 16, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Liao, H.X.; Zhang, R.; Muldoon, M.; Watson, S.; Fischer, W.; Theiler, J.; Szinger, J.; Balachandran, H.; Buzby, A.; et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat. Med. 2010, 16, 324–328. [Google Scholar] [CrossRef]
- Theiler, J.; Yoon, H.; Yusim, K.; Picker, L.J.; Fruh, K.; Korber, B. Epigraph: A Vaccine Design Tool Applied to an HIV Therapeutic Vaccine and a Pan-Filovirus Vaccine. Sci. Rep. 2016, 6, 33987. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.N.; Wee, E.G.; He, S.; Audet, J.; Tierney, K.; Moyo, N.; Hannoun, Z.; Crook, A.; Baines, A.; Korber, B.; et al. Complete protection of the BALB/c and C57BL/6J mice against Ebola and Marburg virus lethal challenges by pan-filovirus T-cell epigraph vaccine. PLoS Pathog. 2019, 15, e1007564. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Robertson, C.A.; Noss, M.J.; Blatter, M.M.; Biedenbender, R.; Decker, M.D. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013, 31, 770–776. [Google Scholar] [CrossRef]
- Treanor, J.T.; Albano, F.R.; Sawlwin, D.C.; Graves Jones, A.; Airey, J.; Formica, N.; Matassa, V.; Leong, J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study. Vaccine 2017, 35, 1856–1864. [Google Scholar] [CrossRef]
- Gaglani, M.; Vasudevan, A.; Raiyani, C.; Murthy, K.; Chen, W.; Reis, M.; Belongia, E.A.; McLean, H.Q.; Jackson, M.L.; Jackson, L.A.; et al. Effectiveness of Trivalent and Quadrivalent Inactivated Vaccines Against Influenza B in the United States, 2011–2012 to 2016–2017. Clin. Infect. Dis. 2021, 72, 1147–1157. [Google Scholar] [CrossRef]
- Sautto, G.A.; Kirchenbaum, G.A.; Ecker, J.W.; Bebin-Blackwell, A.G.; Pierce, S.R.; Ross, T.M. Elicitation of Broadly Protective Antibodies following Infection with Influenza Viruses Expressing H1N1 Computationally Optimized Broadly Reactive Hemagglutinin Antigens. Immunohorizons 2018, 2, 226–237. [Google Scholar] [CrossRef]
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Rando, H.M.; Lordan, R.; Kolla, L.; Sell, E.; Lee, A.J.; Wellhausen, N.; Naik, A.; Kamil, J.P.; Gitter, A.; Greene, C.S. The Coming of Age of Nucleic Acid Vaccines during COVID-19. mSystems 2023, 8, e0092822. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
| Immunogen and Design | Influenza Target(s) | Vaccine Delivery Platform | Model | Study Highlights | References |
|---|---|---|---|---|---|
| Consensus IBV HA2 | IBV | Recombinant Adenovirus | Mouse | Intranasal prime-boost delivery induced robust IgG antibody class-switching and protected mice from lethal challenge with B/Vic. Robust ADCC induced through vaccination. Fusion peptide and transmembrane domain critical for protection from lethal challenge. | [109] |
| Centralized consensus HA | H1, H2, H3, H5 IAV | Recombinant Adenovirus | Mouse | Immunization protected mice from multiple heterologous lethal challenge in a dose-dependent manner. Robust induction of IFN-γ+ splenocytes to greater levels than wild-type HA vaccines. Antibodies mediate HAI response against divergent strains within subtype. Multivalency led to increased neutralizing antibodies and IFN-γ+ splenocytes compared to commercial vaccines. Serotype switching of adenovirus vector leads to more robust HAI antibody responses in mice. | [110,111,112,113] |
| Computationally optimized, broadly reactive antigens (COBRA) | H1, H2, H3, H5, N1 IAV, IBV | Virus-like particle Recombinant/conjugated protein Split-inactivated virus particle Recombinant Marek’s disease virus/turkey Herpesvirus vector Cationic lipid nanoparticle | Mouse Ferret Non-human Primate Chicken | COBRA-designed final consensus layer outperforms clade-/subtype-specific vaccine designs in homologous and heterologous antibody production while maintaining critical protein structures. HAI antibodies induced against multiple clades of viruses using homologous subtype COBRA immunization. Heterologous immunogen boosting or cocktail immunization led to increased cross-reactivity and robustness of HAI antibodies and protection from challenge compared to wild-type immunogens. Swine H1 COBRA induces HAI antibodies against both swine and human H1 strains. Nanoparticle delivery of COBRA immunization boosts T-cell response compared to VLP delivery. | [114,115,116,117,118,119,120,121,122,123,124,125,126,127] |
| Mosaic algorithm HA | H1, H5 IAV | Modified Vaccinia Ankara (MVA) Plasmid DNA Recombinant Adenovirus | Mouse Non-human Primate | MVA delivery of H5 Mosaic protected mice from divergent homosubtypic challenge mediated through decreased lung inflammation and viral replication durable up to 6 months post-vaccination. T cells stimulated through H5 Mosaic vaccination possessed cross-reactivity with H1 C-terminus. Rhesus macaques vaccinated with H5 Mosaic produced antibodies against both H5 and H1 subtypes, which mediated protection through both HAI and ADCC activity post-challenge. H1 Mosaic vaccination induced broad cross-reactive antibody responses better than wild-type HA and commercial comparator vaccination. Vaccination with H1 Mosaic protected mice in dose-dependent manner. | [128,129,130] |
| Epigraph algorithm HA | H3 IAV, IBV | Recombinant Adenovirus | Mouse Swine Ferret | Epigraph vaccination induced durable cross-reactive HAI antibodies and IFN-γ+ T-cell responses in BALB/c mice and pigs. Epigraph vaccination protected mice from lethal and non-lethal human H3 challenge. Protection afforded by Epigraph vaccination led to lower infectious viruses in the lungs of pigs 6-months post-vaccination. Both CD4+ and CD8+ T cells play a role in protection from lethal challenge in mice, with some evidence of non-neutralizing antibodies mediating protection. Individual immunogens differentially contributed to immune response to human H3 Epigraph vaccination. Cross-reactive immune responses were observed in ferrets along with protection from clinical and microscopic disease pathology after challenge. | [131,132,133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekarek, M.J.; Weaver, E.A. Influenza B Virus Vaccine Innovation through Computational Design. Pathogens 2024, 13, 755. https://doi.org/10.3390/pathogens13090755
Pekarek MJ, Weaver EA. Influenza B Virus Vaccine Innovation through Computational Design. Pathogens. 2024; 13(9):755. https://doi.org/10.3390/pathogens13090755
Chicago/Turabian StylePekarek, Matthew J., and Eric A. Weaver. 2024. "Influenza B Virus Vaccine Innovation through Computational Design" Pathogens 13, no. 9: 755. https://doi.org/10.3390/pathogens13090755





