Abstract
The monitoring of stranded marine mammals represents a strategic method to assess their health, conservation status, and ecological role in the marine ecosystem. Networks worldwide track stranding events for the passive monitoring of mortality patterns, emerging and reemerging pathogens, climate change, and environmental degradation from a One Health perspective. This study summarizes pathogen prevalence data from the Italian Stranding Network (ISN) derived from post-mortem investigations on cetaceans found dead stranded along the Italian coastline between 2015 and 2020. The decomposition of the carcasses and logistics limited the post-mortem examination to 585 individuals, out of 1236 single-stranding reports. The most relevant pathogens identified were Cetacean Morbillivirus, Herpesvirus, Brucella spp., and Toxoplasma gondii, whose roles as environmental stressors are well known, despite their real impact still needing to be investigated in depth. Statistical analysis showed that age and sex seem to be positively related to the presence of pathogens. This study represents the first step in harmonizing post-mortem investigations, which is crucial for evidence-based conservation efforts. Implementing diagnostic and forensic frameworks could offer an indirect insight into the systematic monitoring of diseases to improve the identification of regional and temporal hotspots in which to target specific mitigation, management, and conservation strategies.
1. Introduction
Marine mammal stranding events can provide insights into the monitoring of the health and conservation status of free-ranging animals and into assessing the ecological role of these species in the marine ecosystem [1]. The monitoring of the mortality of sentinel species represents a strategic method to assess changes in mortality patterns, emerging and reemerging pathogens (EREPs), climate change, and environmental degradation from a One Health perspective [2,3]. Understanding the ecology of infectious diseases of multiple marine taxa, which share marine resources and pathogens, allows for better predictions of the origin of terrestrial dispersal and the risks to human and marine animal health [4]. Consequently, policies on marine mammals recognize the need for standardized approaches in post-mortem investigations to understand the anthropogenic pressure these species face.
Numerous networks have been established worldwide to monitor stranding events and build a functional stranding network for the passive monitoring of cetacean mortality. Additionally, considering that many marine mammal species are highly mobile, migratory, and not resident in national waters, a common and transboundary effort should be implemented, including data sharing and the harmonization of procedures. In 2016, under the Agreement on the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and Contiguous Atlantic Area (ACCOBAMS) Resolution n° 10, Italy established a stranding network responsible for collecting and analyzing data and samples from marine mammals found stranded along the entire Italian coastline (Inter-Ministerial Decree between the Italian Ministries for Health and for the Environment of 24 May 2015). This stranding network includes the regional public veterinary laboratories (Istituti Zooprofilattici Sperimentali-IIZZSS) coordinated by one reference center (Italian National Reference Center for Diagnostic Investigations on Stranded Marine Mammals-Cre.Di.Ma), universities, and museums, supported by the Coast Guard, the local authorities, and non-governmental organizations for the regular monitoring of cetacean strandings along Italian shores to report stranding events and examine carcasses routinely to obtain useful information.
After 2016, with the creation of the Italian Stranding Network (ISN), the goal of merging the results of post-mortem investigations was challenging due to existing differences among the different laboratories. To investigate the causes of death of stranded cetaceans, it is important to standardize procedures and harmonize the interpretation of post-mortem evidence. A first analysis of the data from post-mortem investigations carried out on cetaceans stranded along the Italian coastline was reported by Di Guardo et al. [5] at the 58th International Whaling Commission (IWC) Scientific Committee, summarizing the results obtained from necropsies carried out on 111 cetaceans stranded along the Italian coastline between 1995 and 2005. In this document, natural conditions accounted for 75.65% of causes of death (CODs), while human-induced mortality represented 24.35% of the diagnoses, with collisions with vessels, interaction with fishing activities, and direct killing being the most represented CODs (respectively, 11.35%, 8.97%, and 2.56%). This information was based on limited geographical and temporal coverage merging the relevant results of very few laboratories.
Based on this premise, as a first step to harmonize post-mortem investigations, the present study summarizes data on the prevalence of natural pathogens obtained from the ISN by examining cetaceans found stranded along the Italian coastline between 2015 and 2020.
2. Materials and Methods
2.1. Study Area
Cetaceans included in this study were found stranded dead over the 7500 km of Italian coastline. Italy is considered the 14th country in the world for the length of its coastlines and has developed a network of Marine Protected Areas (MPAs), Natura 2000 sites, Important Marine Mammal Areas (IMMAs), and Specially Protected Areas of Mediterranean Importance (SPAMIs), protecting 13% of its national waters (data from the Ministry of Environment and Energy Security). Along this extensive coastline, diverse landscapes and rich marine ecosystems thrive within a variety of habitats, from sandy beaches and rocky cliffs to coastal wetlands and underwater seascapes, often making stranding responses challenging.
2.2. Post-Mortem Investigations
Every year, the ISN responds to the stranding events reported by the Coast Guard based on the logistics and functionality of the regional stranding network. As a result, C.Re.Di.Ma. collects all the regional data obtained from cetacean carcasses and subsequent post-mortem investigations in a single annual report to submit an overview of the national situation to the Italian Government.
Post-mortem investigations were conducted on 585 cetaceans (Table S1) of different species stranded along the Italian coastline, out of 1236 strandings in the investigated period. The 585 specimens under investigation belonged to 3 prominent families, namely 551 Delphinidae, 21 Physeteridae, 7 Balaenopteridae, 4 Ziphiidae, and 1 Kogiidae. For one stranding it was not possible to determine the species (Table 1). Specifically, all 8 species regularly present in the Mediterranean Sea [6] were reported and, additionally, 1 occasional (Pseudorca crassidens), and 1 vagrant (Kogia sima) species were recorded.

Table 1.
Number and species of cetacean carcasses investigated.
All carcasses were examined according to standardized protocols [7,8,9], including the assessment of biological data (morphometrics, species, age class, sex, reproductive status), the 5 decomposition codes of the carcass (DCC: animal alive 1, freshly dead 2, moderate decomposition 3, advanced decomposition 4, mummified carcass or skeleton 5), and the nutritional condition code (NCC: good 1, moderate 2, poor 3). Tissues for virologic, microbiological, parasitological, and microscopic examinations were sampled depending on the DCC and the suspected lesions, and submitted to different laboratories as described by Giorda et al. [10]. Tissues routinely sampled were brain, lung, liver, spleen, kidney, intestine, skeletal muscle, and lymph nodes (prescapular, lung-associated, and mesenteric) based on the gross evaluation and analysis compatibility in animals with DCC 1–4. Additionally, pancreas (DCC 1–2), thymus (depending on the age), adrenal and thyroid glands, and urinary bladder (DCC 1–4) were collected for histological evaluation. Available abovementioned tissue samples were routinely collected in 3 aliquots for analyses: one was kept at 4 °C for bacteriology investigations, one at −80 °C for biomolecular exams, and one was preserved in neutral buffered formalin for microscopic and immunohistochemical (IHC) investigations. Additional sampling was considered depending on the gross findings. IHC for cetacean Morbillivirus (CeMV) and T. gondii was performed on selected tissues when the infection was suspected on gross, histopathological, and molecular evidence according to already-published methodologies [11,12].
For bacteriology, tissue samples (brain, lung, lymph nodes, liver, spleen, tonsils, kidney, and bladder), with samples of gross lesions consistent with localized or generalized bacterial infections (abscessation, hemorrhage), were processed for standard aerobic, anaerobic, and microaerobic (5% CO2) bacterial culture and identification by biochemical and/or molecular analyses, as well as MALDI-TOF. Additionally, samples from target tissues underwent specific bacteriological procedures to screen for Listeria spp., Salmonella spp., and Brucella spp. Molecular analyses were performed, targeting CeMV [13,14], Herpesvirus (HV) [15], Brucella spp. [16,17,18,19], Photobacterium damselae subsp. damselae [20,21], and T. gondii [22]. Regarding virology, molecular screening for Poxvirus was performed on skin lesions, consistent with this viral disease [23]. Frozen tissues from the brain and lung, as well as from other tissues with gross and/or microscopic features indicative of a fungal infection (i.e., with the presence of green-yellowish mucous–gelatinous material, pyogranulomatous inflammation, etc.), were inoculated onto Saboraud’s medium for attempted fungal isolation and speciation. The presence of macro-parasites was estimated by macroscopic and microscopic examinations of organs and tissues. Endoparasites were preserved in 70% alcohol for identification, according to microscopic morphological features [24,25,26]. The number of animals screened for these pathogens is summarized per year in Table 2.

Table 2.
Number of animals screened for pathogen prevalence per year.
Finally, frozen and formalin-fixed samples of specific tissues were sent for long-term preservation to the Mediterranean Marine Mammals Tissue Bank (MMMTB) of the University of Padova [27].
2.3. Data Analysis
The results of the analysis were summarized annually between 2015 and 2020. The present study integrates the results of the 6 annual reports, with further results published in the following years. The prevalence of different pathogens was calculated for the examined animals (Table 2).
Data processing and exploration were conducted in Python [28]. Categorical logistic regression analysis was used to assess the relationships among variables. As regression requires numerical inputs, categorical variables were recorded into a set of binary variables (dummy variables). Statistical significance was set at a p-value threshold of less than 0.05. Statistical analyses were conducted mainly on the species most represented in the dataset (Tt and Sc), analyzing different biological aspects.
3. Results
From January 2015 to December 2020, the ISN responded to 1236 single-stranding events. The logistics and functionality of regional stranding networks, together with the advanced decomposition of the animals, limited the post-mortem investigations to 585 individuals (Table S1), which are the focus of the present study (47.33%–585/1236).
As shown in Figure 1, very few animals were fresh (DCC 1–n: 14), including five animals stranded alive (DCC 2–n: 183; DDC 3–n: 186; DCC 4–n: 175; DCC 5–n: 27). Males were slightly more represented (48.03%–n: 281/585) than females (42.91%–n: 251/585), without any statistical difference, while in 53 individuals (9.06%), DCC impaired sex determination. Considering age categories, young animals (i.e., juveniles and newborns) were slightly more represented than adults (258 vs. 220). Only 151 individuals (25.81%) were deemed in good NCC.
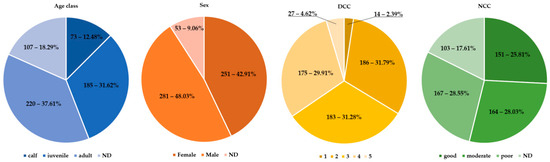
Figure 1.
Illustration of age classes, sexes, DCC, and NCC.
The following paragraphs describe the prevalence of different pathogens detected during post-mortem investigations (Table 3).

Table 3.
Pathogen prevalence per species. Sc and Tt are reported separately due to the higher number of stranded carcasses. The cetacean species names are abbreviated as reported in Table 1.
3.1. Viruses
Overall, 41.37% of the carcasses (242/585) were positive for viral infections, including 22 cases of CeMV-HV co-infection. The most common viral infection was CeMV, with 172 positive cases among 471 carcasses tested (36.52%), mostly represented by Sc (128/172) in poor NCC (53/128). With reference to the host range for CeMV, other species besides Tt and Sc were found positive, as shown in Table 3. It is interesting to note the positivity of 7 Pm among the 12 individuals tested (58.33% of CeMV positivity among the tested animals; 33.33% of CeMV positivity among the Pm stranded). HV tests were performed on 278 carcasses, with 45 positive cases (16.19%), mostly represented by Sc (34/44), but also Tt (10/44) and Gg (1/44). Poxvirus infection was diagnosed in two male Sc, one co-infected with CeMV.
3.2. Bacteria
Results obtained from standard microbiological investigations yielded the isolation of 28 different species of bacteria from 190 individuals (42.70% of the tested cases—n: 445). Among the 28 species, 15 bacteria with a potential zoonotic role were identified. P. damselae and Brucella spp. were the most represented bacterial species, respectively, with prevalences of 26.29% (117/445) and 4.92% (19/386). Other species were less represented, such as L. monocytogenes (8), Salmonella spp. (6), Leptospira spp. (4), Erysipelothrix rhusiopathiae (2), Mycoplasma spp. (6), Rhodococcus equi (1), Chlamydia abortus (1), Morganella morgani (2), S. aureus (11), Streptococcus spp. (1), E. coli (14), Pasteurella spp. (3), and Klebsiella spp. (1). All the details are included in the Supplementary Material (Table S1).
3.3. Parasitic and Fungal Infections
Parasitological investigations revealed the presence of common parasites in cetaceans. T. gondii was detected using molecular and/or IHC in 13.45% of all tested animals (55/409), often (45.45%, 25/55) with other pathogens, such as CeMV (18), HV (2), and CeMV-HV (5) (Table S1). Sc was the most represented species, with 30 positive individuals, followed by Tt (24). A peculiar case was one adult female Zc stranded along the Southern Adriatic Sea (Puglia) in 2019, already affected by other pathogens (CeMV, P. damselae). Interestingly, two Sc tested positive for Sarcocystis neurona and Giardia duodenalis.
Macro-parasites were found in 85.35% of all examined specimens (338/396). These reported parasites included pulmonary nematodes (143/396–36.11%), Pholeter gastrophilus (130/396–32.83%), Clistobothrium grimaldii (129–32.58%), Pennella balaenopterae (28/396–7.07%), Anisakis spp. (26/396–6.57%), and Crassicauda spp. (20/396–5.05%) as cofactors influencing health impairment.
In total, fungi were detected in 7.81% of the examined carcasses (10/128 tested animals), mostly as opportunistic infections secondary to other relevant pathogens (Table S1). Three Sc were positive for Aspergillus spp., one Sc and one Tt for Penicillium spp., two Sc for Candida spp., one Sc positive for Cladosporium spp., and one Tt for Mucorales spp. Interestingly, one Pm stranded along the Tyrrhenian Sea tested positive for Aspergillus spp., Trichophyton spp. (pharynx), and CeMV; one female Ks stranded along the Tyrrhenian Sea tested positive for Penicillum spp. (lung) and Geotrichum spp. (lung), as well as for CeMV and Brucella spp.
3.4. Statistical Analysis of Natural Diseases
All the data regarding pathological natural conditions were analyzed using statistics by comparing demographic factors (age, sex, and species limited to Sc and Tt) with findings of specific pathogens. Data processing and exploration were conducted in Python. As shown in Table 4, Sc are statistically more susceptible to infections by different pathogens, such as CeMV, HV, Brucella spp., and T. gondii. On the other hand, the statistics also revealed a lower probability of macro-parasites in this species. Regarding Tt, the species is less susceptible to CeMV and Brucella spp. and, in contrast, is more susceptible to T. gondii. In both the species, adult individuals are more susceptible to infections, and females showed the highest prevalence of infections compared to males for all the most relevant tested pathogens.

Table 4.
Summary of the statistical results per species (Sc and Tt) and demographic factors. The cetacean species names are abbreviated as reported in Table 1.
4. Discussion
The present study summarizes the results of the pathogen prevalence in cetaceans stranded along Italian coastlines between 2015 and 2020. The ISN adopted a multidisciplinary approach to offer, under a One Health perspective, an overview of the health status of the marine mammal population [29,30]. Almost 50% of the stranded carcasses (47.33%) were analyzed, a lower percentage compared to similar studies conducted in smaller regions, such as the Canary Islands [3,31] and the Pelagos Sanctuary [10], but higher when comparing the efforts of many other stranding networks in Europe [32,33,34], America [35,36,37,38,39,40,41], and Oceania [42,43]. This effort can influence the prevalence of pathogens found during this survey.
Like other studies, the logistics in collecting fresh carcasses at the stranding sites, in particular for large whales, as well as the different expertise of the regional responders and laboratories for standard analysis and harmonized interpretation, limited the analysis and influenced the diagnoses and the data obtained from stranded animals. These limitations are also evident in the numbers of animals tested for different pathogens, requiring constant coordination by C.Re.Di.Ma of the 10 Institutes involved in the stranding monitoring of the 15 Italian coastal regions. These difficulties are likely related to the regional organization and complexity of the logistics, but also to discrepancies in training and expertise in different areas. Despite these challenges, the present study represents the first systematic review of pathogen prevalence from post-mortem investigations on stranded cetaceans in Italian waters.
Among the most relevant viral agents, CeMV was detected in 36.52% of screened animals. This finding is consistent with prior publications and is among the highest ever reported worldwide, also confirmed by data reported for the same area in a partially overlapping period (2018–2021) by Vargas-Castro et al. [44]. This latter work reported a prevalence of 31.9%, a slightly lower percentage compared to the present study; this could be related to different outbreaks reported in different areas in Italy between 2015 and 2020 [45,46], and different viral strains circulating [44]. A lower percentage of positive animals has also been reported in other similar studies: 2.9% in the Canary Islands [3]; 5.7% along the Atlantic coastline of the Iberic peninsula [47]; 14.6% along Catalonian coastlines [34]; and in Parana State in Brazil, a prevalence varying from 4.2% between 2007 and 2012 to 27.5% between 2016 and 2018 [3,48]. CeMV is well known for causing outbreaks worldwide, including in the Mediterranean Sea [49]. The continuous findings in Italian waters since the early 1990s, excluding the Adriatic Sea, suggests an endemic viral circulation in all the regular cetacean species living in the Mediterranean Sea. In this respect, during the study period, an expanding viral host range was documented, with CeMV infection being reported in Sc, Tt, Dd, Gg, Gm, Bp [29], and Zc [50], and in the single occasional stranding of Ks in 2017. Moreover, a viral spillover was reported for phylogenetically distant species, such as the Eurasian otter (Lutra lutra) [51] and the Mediterranean Monk Seal (Monachus monachus) [52]. It is interesting to stress the relevant impact of this virus on some particular species, such as Pm. CeMV had already been reported in the Mediterranean Pm subpopulation in the investigation of the mass stranding that occurred in 2014 [53]; between 2015 and 2020, an additional seven animals were found to be positive for a morbilliviral infection, with four animals of the eleven animals stranded in the first 7 months of 2019. Unfortunately, the DCC limited the post-mortem investigations and it was not possible to hypothesize the COD, but, considering molecular and epidemiological data, a relevant role for CeMV in this unusual mortality event (UME) was hypothesized. For these reasons, this viral disease, along with human interaction, should be considered as one of the most severe menaces to the conservation of this species listed as “Endangered” according to the last International Union for Conservation of Nature (IUCN) assessment [54].
CeMV has often been reported in co-infection with other pathogens (other viruses, bacteria, fungi, and parasites), as expected for this virus [49]. In these cases, it was difficult to discriminate the exact role of all the involved pathogens due to their common effects on natural hosts (i.e., non-purulent meningoencephalitis, pneumonia, etc.) and the overlapping of different pathological changes. In this respect, considering that a peculiar form of immune memory loss has recently been described in Measles virus (MeV)-infected humans, as well as in MeV experimentally challenged macaques [55], similar biological behavior could also characterize CeMV infection in susceptible cetacean species, given the close genomic and antigenic similarities existing between MeV and CeMV [49,56,57,58].
The prevalence of HV was 16.19% (45/278), similar to what was observed in Portugal [59], lower compared to other areas in Europe, such as Spain [60,61], and slightly higher compared to the North Sea [62]. The role of HV as an opportunistic pathogen was highlighted by the relation between positive results and empty stomachs, considered a possible indicator of poor health conditions, similar to what was observed for opportunistic bacteria reported below.
A similar prevalence was reported also for T. gondii (13.45%–n: 55/409). This prevalence was lower than the worldwide percentage of positive animals described in the meta-analysis by Li et al. [63], who collected reports of confirmed infections by serology, molecular screening and immunohistochemistry; all the selected studies show a higher prevalence, except those conducted on Delphinidae [63]. Additionally, it is also lower compared to data obtained from all the aquatic species investigated in Italy (21.09%) and other European countries, but very similar to the European average (15.02%) for all the marine mammals. These discrepancies could be partially explained by the absence of seroepidemiological data in the present study. The prevalence of T. gondii is positively related to Tt and adult age, likely due to their longer and coastal life with a greater risk of exposure and infection [63].
With reference to bacteria, 42.70% of the examined animals tested positive for different bacterial species, including B. ceti, considered as the likely COD in six animals mainly stranded in the Adriatic Sea, as already reported by Grattarola et al. [64]. This latter study partially overlaps the period considered in the present retrospective study, confirming the pathogenic role of this bacterial species responsible for severe meningoencephalitis, distinguishable from those caused by other agents for their peculiar features in case of co-infections, due to the peculiar neurotropism of B. ceti in Sc. Furthermore, ST26 and, to a lesser extent, ST49 were identified as the most common sequence types circulating in the Italian coastline [64], confirming, along with other reports, the presence and circulation of this bacterial species in the Mediterranean Sea, even if with a lower prevalence compared to other studies [34,65]. Brucellosis was statistically related to sex, showing a clear prevalence for females, confirming already-published data in the same area [64], even if in contrast with other meta-analyses on marine mammals [65]. This difference could be related to social and behavioral factors like the sexual segregation between weaning and sexual maturity, which reduces the number of males in large groups [66].
Considering the other bacterial pathogens’ genera and species, they have often been considered opportunistic pathogens due to the immune impairment of different factors, including CeMV [67]. It is interesting to note that most of these bacteria have zoonotic potential and are generally reported in terrestrial animal husbandry. In particular, bacteria like L. monocytogenes, Salmonella spp., Leptospira spp., E. rhusiopathiae, Mycoplasma spp., and C. abortus, but also parasites such as T. gondii, are often found in poultry, cattle, and pig farms and are associated with foodborne infection in humans [68,69]. Although these are often single case reports, these findings could highlight the risk of biological marine pollution from different mainland activities as a relevant threat to marine animal conservation. A similar concern was also raised from investigations on live marine vertebrates hosting zoonotic bacteria and parasites [70,71], stressing the need for further studies to understand their real origin. These pathogens are not considered native to seawater but can likely reach the sea via run-off freshwater feeding rivers during unusual rainy periods or storms [72]. These weather events, possibly linked to climate change, can lead to an overloading of sewage plants, resulting in fecal bacterial accumulation in sediment and the filtering of animals with untreated wastewater [73]. Also, increasing evidence confirms that plastic waste can help in the environmental resistance of bacteria released through human and animal discharges [74]. Similar considerations have also been addressed in sea turtles in the same areas for some of the above-mentioned pathogens [75,76], expressing concern for these food-borne zoonotic diseases and confirming the value of surveillance actions on wildlife overlapping with human habitats and the food supply chain. The increasing climate change-driven fecal contamination of marine waters could also be of relevance for the transmission of the SARS-CoV-2 betacoronavirus to susceptible cetacean species, with special emphasis on inshore species like Tt [73,77].
In many animals (83.84%), macro-parasites were found mainly in the respiratory (nematodes and Crassicauda spp.) and gastrointestinal (P. gastrophilus and Anisakis spp.) tracts, as well as the tegument, the peritoneal cavity (Pennella balaenopterae and Clistobothrium grimaldii) and the urinary system (Crassicauda spp.). Similar surveys reported a lower prevalence of multi-parasitic infections [3,31]. In some of the previous surveys conducted in the Pelagos Sanctuary, parasitic infections were associated with poor nutritional conditions [10,78]. The role of parasites in strandings is still under debate, being alternatively considered as a cause of debilitation and death or a consequence of poor health conditions [78]. Results in the present study did not allow any statistical association between metazoan parasites and species, sex, age, NCC, or diseases, except for a moderate negative correlation between the presence of parasitic elements in Sc, which is also the species more frequently affected by different pathogens. Additional and more focused studies should be carried out to investigate the potential association between ecological or pathological aspects and parasitic infection.
5. Conclusions
In conclusion, the present data represent the first systematic analysis of the prevalence of different pathogens in cetaceans stranded along the Italian coastline over a 6-year period. This study underlines that these data can drive an impacting action for the conservation of marine mammals to focus on threats and spatiotemporal hotspots underlined by constant passive monitoring. Even if spontaneous infection were often identified, it should be stressed that epidemiology could be affected by global warming [79], frequently contributing to the occurring mass mortalities in all animal taxa and influencing the temporal occurrence of these outbreaks in marine mammals [80]. In this context and under the One Health approach, the above-reported evidence should lead to the consideration of other threats, such as diseases and microbial pollution, among the possible threats menacing marine animal conservation, as it is influenced by global warming effects, pollution, and other anthropic factors. Since baseline data are limited for many marine mammal species, their surveillance, along with infectious disease investigations, should be improved and implemented for long-term population health monitoring. As global climate evolution represents one of the major threats causing worry to marine mammal conservation [79,80], and an aspect that should be better addressed in the IUCN status assessment of the different species, mitigation measures cannot be realistically implemented at a local scale but worldwide action should be taken to slowly change the impact on marine species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13090762/s1, Table S1: Master table of the analyzed carcasses and pathogen prevalence. References [44,45,46,50,64,67,70,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), G.P. and C.G.; methodology, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), C.C. (Cinzia Centelleghe), D.B., E.B., C.C. (Cristiano Cocumelli ), C.C. (Cristina Canonico), S.C., D.D., A.D.D., G.D.F., G.D.G., F.D.N., L.D.R., S.G., F.G., G.L., L.M. (Letizia Marsili), F.M., L.M. (Leonardo Marino), S.M. (Sergio Migliore), I.P., A.P. (Antonio Petrella), A.P. (Antonio Pintore), R.P., S.R., G.T., A.T., G.P. and C.G.; formal analysis, C.G., G.P. and S.M. (Sergio Migliore); investigation, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), C.C. (Cinzia Centelleghe), D.B., E.B., C.C. (Cristiano Cocumelli), C.C. (Cristina Canonico), S.C., D.D., A.D.D., G.D.F., G.D.G., F.D.N., L.D.R., S.G., F.G., G.L., L.M. (Letizia Marsili), F.M., L.M. (Leonardo Marino), S.M. (Sergio Migliore), I.P., A.P. (Antonio Petrella), A.P. (Antonio Pintore), R.P., S.R., G.T., A.T., G.P. and C.G.; data curation, C.G. and G.P.; writing—original draft preparation, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), G.P., G.D.G. and C.G.; writing—review and editing, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), C.C. (Cinzia Centelleghe), D.B., E.B., C.C. (Cristiano Cocumelli), C.C. (Cristina Canonico), S.C., D.D., A.D.D., G.D.F., G.D.G., F.D.N., L.D.R., S.G., F.G., G.L., L.M. (Letizia Marsili), F.M., L.M. (Leonardo Marino), S.M. (Sergio Migliore), I.P., A.P. (Antonio Petrella), A.P. (Antonio Pintore), R.P., S.R., G.T., A.T., G.P. and C.G.; visualization, G.P. and C.G.; supervision, S.M. (Sandro Mazzariol), C.C. (Cristina Casalone), and C.G.; funding acquisition, S.M. (Sandro Mazzariol) and C.C. (Cristina Casalone). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by 1. the Italian Ministry of Health (Ricerca Corrente 2015 IZSPLV 12/15; Ricerca Corrente 2016 IZSPLV 05/16; Ricerca Corrente 2018 IZSPLV 09/18; Ricerca Corrente IZSPLV 05/19); 2. EU funding: a. NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT); b. LIFE DELFI project (LIFE18 NAT/IT/000942).
Institutional Review Board Statement
The study was conducted on dead animals under the authorization of the Italian Ministry of Health and the Ministry of Environment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available on the C.Re.Di.Ma. website: https://www.izsplv.it/it/istituto/213-centri-eccellenza/centri-referenza-nazionali/428-credima.html#:~:text=Il%20Centro%20di%20Referenza%20Nazionale,post%20mortem%20sui%20cetacei%20spiaggiati (accessed on 31 December 2022).
Acknowledgments
This research could not have been carried out without the collaboration of all the members of the ISN who contributed to the stranding management and post-mortem investigations during the study period. We also acknowledge the contribution of Bruno Cozzi (Dep. BCA, University of Padova), Michela Podestà (Natural History Museum of Milano, Italy), and Cecilia Mancusi (Agenzia Regionale per la Protezione Ambientale Toscana-ARPAT, Italy) for their kind support and assistance in conducting stranding monitoring and post-mortem investigations, and Maria Ines Crescio (Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d’Aosta, Italy) and Luca Ceolotto (Dep. BCA, University of Padova) for their support with statistical analysis. This work is dedicated to the loving memory of Gianni Pavan (University of Pavia and CIBRA, Italy), a passionate and committed conservation scientist who inspired and supported the work of all the ISN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Profeta, F.; Di Francesco, C.E.; Marsilio, F.; Mignone, W.; Di Nocera, F.; De Carlo, E.; Lucifora, G.; Pietroluongo, G.; Baffoni, M.; Cocumelli, C.; et al. Retrospective seroepidemiological investigations against Morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coast-line (1998–2014). Res. Vet. Sci. 2015, 10, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar]
- Bogomolni, A.L.; Gast, R.J.; Ellis, J.C.; Dennett, M.; Pugliares, K.R.; Lentell, B.J.; Moore, M.J. Victims or vectors: A survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Dis. Aquat. Org. 2008, 81, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G.; Castagnaro, M.; Marrucchella, G.; Mazzariol, S.; Mignone, W.; Olivieri, V.; Ponzio, P.; Cozzi, B. Human-induced mortality in cetaceans found stranded on the Italian coastline (1995–2005). In Document SC/58/BC1, Presented at the 58th Meeting of the Scientific Committee of the International Whaling Commission, St. Kitts & Nevis; Universita Degli Studi di Torino: Turin, Italy, 2006. [Google Scholar]
- Notarbartolo di Sciara, G.; Tonay, A.M. Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas: An ACCOBAMS Status Report; ACCOBAMS: Monaco, 2021. [Google Scholar]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings; National Aquarium: Baltimore, MD, USA, 2005. [Google Scholar]
- Mazzariol, S.; Cozzi, B.; Centelleghe, C. Handbook for Cetacean’ Strandings; NETCET: Malmo, Sweden, 2015. [Google Scholar]
- Accobams-mop6/2016/Resolution 6.22. Cetacean Live Stranding. 2016. Available online: https://www.accobams.org/wp-content/uploads/2016/06/ACCOBAMS_MOP6_Res6.22.pdf (accessed on 7 August 2024).
- Giorda, F.; Ballardini, M.; Di Guardo, G.; Pintore, M.D.; Grattarola, C.; Iulini, B.; Mignone, W.; Goria, M.; Serracca, L.; Varello, K.; et al. Post-mortem findings in cetaceans found stranded in the Pelagos Sanctuary, Italy, 2007–2014. J. Wildl. Dis. 2017, 53, 795. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G.; Proietto, U.; Di Francesco, C.E.; Marsilio, F.; Zaccaroni, A.; Scaravelli, D.; Mignone, W.; Garibaldi, F.; Kennedy, S.; Forster, F.; et al. Cerebral Toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet. Pathol. 2010, 47, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Casalone, C.; Mazzariol, S.; Pautasso, A.; Di Guardo, G.; Di Nocera, F.; Lucifora, G.; Ligios, C.; Franco, A.; Fichi, G.; Cocumelli, C.; et al. Cetacean strandings in Italy: An unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis. Aquat. Org. 2014, 109, 81–86. [Google Scholar] [CrossRef]
- Centelleghe, C.; Beffagna, G.; Zanetti, R.; Zappulli, V.; Di Guardo, G.; Mazzariol, S. Molecular analysis of dolphin Morbillivirus: A new sensitive detection method based on nested RT-PCR. J. Virol. Methods 2016, 235, 85–91. [Google Scholar] [CrossRef]
- Verna, F.; Giorda, F.; Miceli, I.; Rizzo, G.; Pautasso, A.; Romano, A.; Iulini, B.; Pintore, M.D.; Mignone, W.; Grattarola, C.; et al. Detection of morbillivirus infection by RT-PCR RFLP analysis in cetaceans and carnivores. J. Virol. Methods 2017, 247, 22–27. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Bounaadja, L.; Albert, D.; Chénais, B.; Hénault, S.; Zygmunt, M.S.; Poliak, S.; Garin-Bastuji, B. Real-time PCR for identification of Brucella spp.: A comparative study of IS711, bcsp31 and per target genes. Vet. Microbiol. 2009, 137, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.R.; Karczmarski, L.; Au, W.W.L.; Rickards, S.H.; Vanderlip, C.A.; Bowen, B.W.; Grau, G.; Toonen, R.J. Rolling stones, and stable homes: Social structure, habitat diversity and population genetics of the Hawaiian spinner dolphin (Stenella longirostris). Mol. Ecol. 2010, 19, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Her, M.; Kim, J.W.; Kim, J.Y.; Ko, K.Y.; Ha, Y.M.; Jung, S.C. Advanced multiplex PCR assay for differentiation of Brucella species. Appl. Environ. Microbiol. 2011, 77, 6726–6728. [Google Scholar] [CrossRef]
- Baily, G.G.; Krahn, J.B.; Drasar, B.S.; Stoker, N.G. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J. Trop. Med. Hyg. 1992, 95, 271–275. [Google Scholar]
- Osorio, C.R.; Collins, M.D.; Romalde, J.L.; Toranzo, A.E. Characterization of the 23S and 5S rRNA genes and 23S-5S intergenic spacer region (ITS-2) of Photobacterium damselae. Dis. Aquat. Org. 2004, 61, 33–39. [Google Scholar] [CrossRef]
- Amagliani, G.; Omiccioli, E.; Andreoni, F.; Boiani, R.; Bianconi, I.; Zaccone, R.; Mancuso, M.; Magnani, M. Development of a multiplex PCR assay for Photobacterium damselae subsp. piscicida identification in fish samples. J. Fish. Dis. 2009, 32, 645–653. [Google Scholar] [PubMed]
- Vitale, M. A high sensitive nested PCR for Toxoplasma gondii detection in animal and food samples. Microb. Biochem. Technol. 2013, 5, 39–41. [Google Scholar] [CrossRef]
- Barnett, J.; Dastjerdi, A.; Davison, N.; Deaville, R.; Everest, D.; Peake, J.; Finnegan, C.; Jepson, P.; Steinbach, F. Identification of novel cetacean poxviruses in cetaceans stranded in South West England. PLoS ONE 2015, 10, e0124315. [Google Scholar] [CrossRef]
- Anderson, R.C. Keys to genera of the superfamily. Metastrongyloidea 1978, 5, 40. [Google Scholar]
- Khalil, L.F.; Jones, A.; Bray, R.A. Keys to the Cestode Parasites of Vertebrates; CAB International: Wallingford, UK, 1994; p. 752. [Google Scholar]
- Gibson, I.D.; Jones, A.; Bray, R.A. Keys to the Trematoda, Vol 1. CABI; The Natural History Museum: London, UK, 2002. [Google Scholar]
- Available online: https://marinemammals.bca.unipd.it (accessed on 1 July 2024).
- Rossum, G.V.; Drake, F.L. The Python Language Reference Manual; Network Theory Ltd.: Godalming, UK, 2011; p. 578. [Google Scholar]
- Mazzariol, S.; Marsili, L.; Di Guardo, G. Cetacean mass strandings and multidisciplinary work. Chemosphere 2016, 148, 32–33. [Google Scholar] [CrossRef]
- Williams, R.S.; Brownlow, A.; Baillie, A.; Barber, J.L.; Barnett, J.; Davison, N.J.; Deaville, R.; ten Doeschate, M.; Penrose, R.; Perkins, M.; et al. Evaluation of a marine mammal status and trends contaminants indicator for European waters. Sci. Total Environ. 2023, 866, 161301. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, M.; Espinosa de los Monteros, A.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernández, A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Org. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.K.; Bennett, P.M.; Jepson, P.D.; Kuiken, T.; Simpson, V.R.; Baker, J.R. Entanglement in fishing gear and other causes of death in cetaceans stranded on the coasts of England and Wales. Vet. Rec. 1997, 141, 94–98. [Google Scholar] [CrossRef]
- Wright, A.J.; Maar, M.; Mohn, C.; Nabe-Nielsen, J.; Siebert, U.; Jensen, L.F.; Baagøe, H.J.; Teilmann, J. Possible causes of a harbour porpoise mass stranding in Danish waters in 2005. PLoS ONE 2013, 8, e55553. [Google Scholar] [CrossRef] [PubMed]
- Cuvertoret-Sanz, M.; López-Figueroa, C.; O’Byrne, A.; Canturri, A.; Martí-Garcia, B.; Pintado, E.; Pérez, L.; Ganges, L.; Cobos, A.; Abarca, M.L. Causes of cetacean stranding and death on the Catalonian coast (Western Mediterranean Sea), 2012–2019. Dis. Aquat. Org. 2020, 142, 239–253. [Google Scholar] [CrossRef]
- Meirelles, A.C.O.; Monteiro-Neto, C.; Martins, A.M.A.; Costa, A.F.; Barros, H.M.D.R.; Alves, M.D.O. Cetacean strandings on the coast of Ceará, North-eastern Brazil (1992–2005). J. Mar. Biol. Assoc. UK 2009, 89, 1083–1090. [Google Scholar] [CrossRef]
- McFee, W.E.; Lipscomb, T.P. Major pathologic findings and probable causes of mortality in bottlenose dolphins stranded in South Carolina from 1993 to 2006. J. Wildl. Dis. 2009, 45, 575–593. [Google Scholar]
- Bogomolni, A.; Pugliares, K.; Sharp, S.; Patchett, K.; Harry, C.; LaRocque, J.; Touhey, K.M.; Moore, M. Mortality trends of stranded marine mammals on Cape Cod and southeastern Massachusetts, USA, 2000 to 2006. Dis. Aquat. Org. 2010, 88, 143–155. [Google Scholar] [CrossRef]
- Nemiroff, L.; Wimmer, T.; Daoust, P.Y.; McAlpine, D.F. Cetacean strandings in the Canadian Maritime provinces, 1990–2008. Can. Field-Nat. 2010, 124, 32–44. [Google Scholar] [CrossRef]
- Domiciano, I.G.; Domit, C.; Broadhurst, M.K.; Koch, M.S.; Bracarense, A.P.F.R.L. Assessing disease and mortality among small cetaceans stranded at a world heritage site in Southern Brazil. PLoS ONE 2016, 11, e0149295. [Google Scholar] [CrossRef]
- Fenton, H.; Daoust, P.; Forzán, M.; Vanderstichel, R.; Ford, J.; Spaven, L.; Lair, S.; Raverty, S. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis. Aquat. Org. 2017, 122, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Bloodgood, J.; Deming, A.; Colegrove, K.; Russell, M.; Díaz Clark, C.; Carmichael, R. Causes of death and pathogen prevalence in bottlenose dolphins Tursiops truncatus stranded in Alabama, USA, between 2015 and 2020, following the Deepwater Horizon oil spill. Dis. Aquat. Org. 2023, 155, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Stockin, K.A.; Duignan, P.J.; Roe, W.D.; Meynier, L.; Alley, M.; Fettermann, T. Causes of mortality in stranded common dolphin (Delphinus sp.) from New Zealand waters between 1998 and 2008. Pac. Conserv. Biol. 2009, 15, 217. [Google Scholar] [CrossRef]
- Tomo, I.; Kemper, C.M. Strandings in St Vincent Gulf Bioregion, South Australia: 12-year study monitors biology and pathology of cetaceans. Oceans 2022, 3, 439–463. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Peletto, S.; Mattioda, V.; Goria, M.; Serracca, L.; Varello, K.; Sánchez-Vizcaíno, J.M.; Puleio, R.; Di Nocera, F.; Lucifora, G.; et al. Epidemiological and genetic analysis of cetacean Morbillivirus circulating on the Italian coast between 2018 and 2021. Front. Vet. Sci. 2023, 10, 1216838. [Google Scholar] [CrossRef] [PubMed]
- Mira, F.; Rubio-Guerri, C.; Purpari, G.; Puleio, R.; Caracappa, G.; Gucciardi, F.; Russotto, L.; Loria, G.R.; Guercio, A. Circulation of a novel strain of dolphin Morbillivirus (DMV) in stranded cetaceans in the Mediterranean Sea. Sci. Rep. 2019, 9, 9792. [Google Scholar] [CrossRef]
- Pautasso, A.; Iulini, B.; Grattarola, C.; Giorda, F.; Goria, M.; Peletto, S.; Masoero, L.; Mignone, W.; Varello, K.; Petrella, A.; et al. Novel dolphin Morbillivirus (DMV) outbreak among Mediterranean striped dolphins Stenella coeruleoalba in Italian waters. Dis. Aquat. Org. 2019, 132, 215–220. [Google Scholar] [CrossRef]
- Bento, M.C.R.d.M.; Eira, C.I.C.S.; Vingada, J.V.; Marçalo, A.L.; Ferreira, M.C.T.; Fernandez, A.L.; Tavares, L.M.M.; Duarte, A.I.S.P. New insight into dolphin Morbillivirus phylogeny and epidemiology in the northeast Atlantic: Opportunistic study in cetaceans stranded along the Portuguese and Galician coasts. BMC Vet. Res. 2016, 12, 176. [Google Scholar] [CrossRef]
- Marutani, V.H.B.; Miyabe, F.; Alfieri, A.F.; Domit, C.; de Matos, A.M.R.N.; Filho, M.R.C.M.; Bracarense, A.P.F.R.L. Systematic beach monitoring as a health assessment tool: Cetacean Morbillivirus under non-epizootic circumstances in stranded dolphins. Transbound. Emerg. Dis. 2021, 69, e96–e103. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; Duignan, P.; Banyard, A.; Barbieri, M.; Colegrove, K.; De Guise, S.; Di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean Morbillivirus: Current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef]
- Centelleghe, C.; Beffagna, G.; Palmisano, G.; Franzo, G.; Casalone, C.; Pautasso, A.; Giorda, F.; Di Nocera, F.; Iaccarino, D.; Santoro, M.; et al. Dolphin Morbillivirus in a Cuvier’s beaked whale (Ziphius cavirostris), Italy. Front. Microbiol. 2017, 8, 233261. [Google Scholar] [CrossRef] [PubMed]
- Padalino, I.; Di Guardo, G.; Carbone, A.; Troiano, P.; Parisi, A.; Galante, D.; Cafiero, M.A.; Caruso, M.; Palazzo, L.; Guarinoet, L.; et al. Dolphin Morbillivirus in Eurasian otters, Italy. Emerg. Infect. Dis. 2019, 25, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Petrella, A.; Mazzariol, S.; Padalino, I.; Di Francesco, G.; Casalone, C.; Grattarola, C.; Di Guardo, G.; Smoglica, C.; Centelleghe, C.; Gili, C. Cetacean Morbillivirus and Toxoplasma gondii co-infection in Mediterranean monk seal pup, Italy. Emerg. Infect. Dis. 2021, 27, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, S.; Centelleghe, C.; Di Provvido, A.; Di Renzo, L.; Cardeti, G.; Cersini, A.; Fichi, G.; Petrella, A.; Di Francesco, C.E.; Mignone, W.; et al. Dolphin Morbillivirus associated with a mass stranding of sperm whales, Italy. Emerg. Infect. Dis. 2017, 23, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, E.; Carpinelli, E.; Frantzis, A.; Gauffier, P.; Lanfredi, C.; Pace, D.S.; Rendell, L.E. Physeter macrocephalus (Mediterranean subpopulation). The IUCN Red List of Threatened Species. 2021. e.T16370739A50285671. Available online: http://www.pelagosinstitute.gr/gr/pelagos/pdfs/Pirotta%20et%20al.%202021.pdf (accessed on 7 August 2024).
- Mina, M.J.; Kula, T.; Leng, Y.; Li, M.; de Vries, R.D.; Knip, M.; Siljander, H.; Rewers, M.; Choy, D.F.; Wilson, M.S.; et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019, 366, 599–606. [Google Scholar] [CrossRef]
- Di Guardo, G.; Mazzariol, S. Cetacean Morbillivirus: A land-to-sea journey and back? Virol. Sin. 2019, 34, 240–242. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Groch, K.R.; Ressio, R.; Riskallah, I.P.J.; Sierra, E.; Sacchini, S.; Quesada-Canales, Ó.; Arbelo, M.; Fernández, A.; Santos-Neto, E.; et al. Comparative Immunopathology of Cetacean morbillivirus Infection in Free-Ranging Dolphins from Western Mediterranean, Northeast-Central, and Southwestern Atlantic. Front. Immunol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Sierra, E.; Fernández, A.; Felipe-Jiménez, I.; Zucca, D.; Díaz-Delgado, J.; Puig-Lozano, R.; Câmara, N.; Consoli, F.; Díaz-Santana, P.; Suárez-Santana, C.; et al. Histopathological differential diagnosis of meningoencephalitis in cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front. Vet. Sci. 2020, 7, 650. [Google Scholar] [CrossRef]
- Bento, M.C.; Canha, R.; Eira, C.; Vingada, J.; Nicolau, L.; Ferreira, M.; Domingo, M.; Tavares, L.; Duarte, A. Herpesvirus infection in marine mammals: A retrospective molecular survey of stranded cetaceans in the Portuguese coastline. Infect. Genet. Evol. 2019, 67, 222–233. [Google Scholar] [CrossRef]
- Bellière, E.N.; Esperón, F.; Arbelo, M.; Muñoz, M.J.; Fernández, A.; Sánchez-Vizcaíno, J.M. Presence of herpesvirus in striped dolphins stranded during the cetacean Morbillivirus epizootic along the Mediterranean Spanish coast in 2007. Arch. Virol. 2010, 155, 1307–1311. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Melero, M.; Crespo-Picazo, J.L.; Jiménez, M.d.l.Á.; Sierra, E.; Rubio-Guerri, C.; Arbelo, M.; Fernández, A.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Systematic Determination of Herpesvirus in Free-Ranging Cetaceans Stranded in the Western Mediterranean: Tissue Tropism and Associated Lesions. Viruses 2021, 13, 2180. [Google Scholar] [CrossRef]
- van Elk, C.; van de Bildt, M.; van Run, P.; de Jong, A.; Getu, S.; Verjans, G.; Osterhaus, A.; Kuiken, T. Central nervous system disease and genital disease in harbor porpoises (Phocoena phocoena) are associated with different herpesviruses. Vet. Res. 2016, 47, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Kang, Y.H.; Sun, W.C.; Hao, Z.P.; Elsheikha, H.M.; Cong, W. Terrestrial runoff influences the transport and contamination levels of Toxoplasma gondii in marine organisms. Sci. Total Environ. 2022, 851, 158168. [Google Scholar] [CrossRef] [PubMed]
- Grattarola, C.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Nocera, F.; Pintore, A.; Cocumelli, C.; Terracciano, G.; Battisti, A.; Di Renzo, L.; et al. Brucella ceti infection in striped dolphins from Italian Seas: Associated lesions and epidemiological data. Pathogens 2023, 12, 1034. [Google Scholar] [CrossRef]
- Dadar, M.; Shahali, Y.; Fakhri, Y.; Godfroid, J. A comprehensive meta-analysis of Brucella infections in aquatic mammals. Vet. Ital. 2022, 58, 30. [Google Scholar]
- Roca-Monge, K.; González-Barrientos, R.; Suárez-Esquivel, M.; Palacios-Alfaro, J.D.; Castro-Ramírez, L.; Jiménez-Soto, M.; Cordero-Chavarría, M.; García-Párraga, D.; Barratclough, A.; Moreno, E.; et al. Age and Sexual Maturity Estimation of Stranded Striped Dolphins, Stenella coeruleoalba, Infected with Brucella ceti. Oceans 2022, 3, 494–508. [Google Scholar] [CrossRef]
- Di Renzo, L.; Di Francesco, G.; Profico, C.; Di Francesco, C.E.; Ferri, N.; Averaimo, D.; Di Guardo, G. Vibrio parahaemolyticus—And V. alginolyticus -associated meningo-encephalitis in a bottlenose dolphin (Tursiops truncatus) from the Adriatic coast of Italy. Res. Vet. Sci. 2017, 115, 363–365. [Google Scholar] [CrossRef]
- Fayer, R.; Esposito, D.H.; Dubey, J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015, 28, 295–311. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Grattarola, C.; Gallina, S.; Giorda, F.; Pautasso, A.; Ballardini, M.; Iulini, B.; Varello, K.; Goria, M.; Peletto, S.; Masoero, L.; et al. First report of Salmonella 1,4,[5],12:i:- in free-ranging striped dolphins (Stenella coeruleoalba), Italy. Sci. Rep. 2019, 9, 6061. [Google Scholar] [CrossRef]
- Marangi, M.; Carlucci, R.; Carlino, P.; Fanizza, C.; Cirelli, G.; Maglietta, R.; Beneduce, L. Dolphins and sea turtles may host zoonotic parasites and pathogenic bacteria as indicators of anthropic pressure in the Gulf of Taranto (Northern Ionian Sea, Central-Eastern Mediterranean Sea). Vet. Res. Commun. 2022, 46, 1157–1166. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Saco, M.; de Novoa, J.; Perez-Piñeiro, P.; Peiteado, J.; Lozano-Leon, A.; Garcia-Martin, O. Influence of environmental factors and human activity on the presence of Salmonella serovars in a marine environment. Appl. Environ. Microbiol. 2004, 70, 2089–2097. [Google Scholar] [CrossRef]
- Audino, T.; Grattarola, C.; Centelleghe, C.; Peletto, S.; Giorda, F.; Florio, C.L.; Caramelli, M.; Bozzetta, E.; Mazzariol, S.; Di Guardo, G.; et al. SARS-CoV-2, a Threat to Marine Mammals? A Study from Italian Seawaters. Animals 2021, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, M.J.; Woodford, L.; Quilliam, R.S. Can plastic pollution drive the emergence and dissemination of novel zoonotic diseases? Environ. Res. 2024, 246, 118172. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; De Angelis, M.E.; Torresi, M.; Di Lollo, V.; Di Teodoro, G.; Averaimo, D.; Defourny, S.V.P.; Di Giacinto, F.; Profico, C.; Olivieri, V.; et al. First Report of Septicaemic Listeriosis in a Loggerhead Sea Turtle (Caretta caretta) Stranded along the Adriatic Coast: Strain Detection and Sequencing. Animals 2022, 12, 2364. [Google Scholar] [CrossRef] [PubMed]
- Rubini, S.; Baruffaldi, M.; Taddei, R.; D’Annunzio, G.; Scaltriti, E.; Tambassi, M.; Menozzi, I.; Bondesan, G.; Mazzariol, S.; Centelleghe, C.; et al. Loggerhead Sea Turtle as Possible Source of Transmission for Zoonotic Listeriosis in the Marine Environment. Vet. Sci. 2023, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Audino, T.; Berrone, E.; Grattarola, C.; Giorda, F.; Mattioda, V.; Martelli, W.; Pintore, A.; Terracciano, G.; Cocumelli, C.; Lucifora, G.; et al. Potential SARS-CoV-2 Susceptibility of Cetaceans Stranded along the Italian Coastline. Pathogens 2022, 11, 1096. [Google Scholar] [CrossRef]
- Terracciano, G.; Fichi, G.; Comentale, A.; Ricci, E.; Mancusi, C.; Perrucci, S. Dolphins Stranded along the Tuscan Coastline (Central Italy) of the “Pelagos Sanctuary”: A Parasitological Investigation. Pathogens 2020, 9, 612. [Google Scholar] [CrossRef]
- Barratclough, A.; Ferguson, S.H.; Lydersen, C.; Thomas, P.O.; Kovacs, K.M. A Review of Circumpolar Arctic Marine Mammal Health—A Call to Action in a Time of Rapid Environmental Change. Pathogens 2023, 12, 937. [Google Scholar] [CrossRef]
- Sanderson, C.E.; Alexander, K.A. Unchartered waters: Climate change likely to intensify infectious disease outbreaks causing mass mortality events in marine mammals. Glob. Chang. Biol. 2020, 26, 4284–4301. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Giorda, F.; Mattioda, V.; Audino, T.; Di Nocera, F.; Lucifora, G.; Varello, K.; Grattarola, C.; Ortega-Mora, L.; Casalone, C.; et al. Toxoplasma gondii genetic diversity in Mediterranean dolphins. Pathogens 2022, 11, 909. [Google Scholar] [CrossRef]
- Grattarola, C.; Giorda, F.; Iulini, B.; Pintore, M.; Pautasso, A.; Zoppi, S.; Goria, M.; Romano, A.; Peletto, S.; Varello, K.; et al. Meningoencephalitis and Listeria monocytogenes, Toxoplasma gondii and Brucella spp. coinfection in a dolphin in Italy. Dis. Aquat. Organ. 2016, 118, 169–174. [Google Scholar] [CrossRef]
- Giorda, F.; Crociara, P.; Iulini, B.; Gazzuola, P.; Favole, A.; Goria, M.; Serracca, L.; Dondo, A.; Crescio, M.; Audino, T.; et al. Neuropathological characterization of dolphin Morbillivirus infection in cetaceans stranded in Italy. Animals 2022, 12, 452. [Google Scholar] [CrossRef]
- Cocumelli, C.; Fichi, G.; Marsili, L.; Senese, M.; Cardeti, G.; Cersini, A.; Ricci, E.; Garibaldi, F.; Scholl, F.; Di Guardo, G.; et al. Cetacean Poxvirus in two striped dolphins (Stenella coeruleoalba) stranded on the Tyrrhenian coast of Italy: Histopathological, ultrastructural, biomolecular, and ecotoxicological findings. Front. Vet. Sci. 2018, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Grattarola, C.; Giorda, F.; Iulini, B.; Pautasso, A.; Ballardini, M.; Zoppi, S.; Marsili, L.; Peletto, S.; Masoero, L.; Varello, K.; et al. Occlusive mycotic tracheobronchitis and systemic Alphaherpesvirus coinfection in a free-living striped dolphin Stenella coeruleoalba in Italy. Dis. Aquat. Organ. 2018, 127, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Piredda, I.; Palmas, B.; Noworol, M.; Tola, S.; Longheu, C.; Bertasio, C.; Scaltriti, E.; Denurra, D.; Cherchi, M.; Picardeau, M.; et al. Isolation of Leptospira interrogans from a bottlenose dolphin (Tursiops truncatus) in the Mediterranean Sea. J. Wildl. Dis. 2020, 56, 727. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D.; Sciortino, S.; Migliore, S.; Galuppo, L.; Puleio, R.; Dara, S.; Vicari, D.; Seminara, S.; Gargano, V. Preliminary results on the prevalence of Salmonella spp. in marine animals stranded in sicilian coasts: Antibiotic susceptibility profile and ARGs detection in the isolated strains. Pathogens 2021, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Garofolo, G.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Guardo, G.; Pautasso, A.; Iulini, B.; Varello, K.; Giorda, F.; Goria, M.; et al. Occurrence of Brucella ceti in striped dolphins from Italian Seas. Plos ONE 2020, 15, e0240178. [Google Scholar] [CrossRef]
- Giorda, F.; Romani-Cremaschi, U.; Marsh, A.E.; Grattarola, C.; Iulini, B.; Pautasso, A.; Varello, K.; Berio, E.; Gazzuola, P.; Marsili, L.; et al. Evidence for unknown Sarcocystis-like infection in stranded striped dolphins (Stenella coeruleoalba) from the Ligurian Sea, Italy. Animals 2021, 11, 1201. [Google Scholar] [CrossRef]
- Sévellec, Y.; Torresi, M.; Félix, B.; Palma, F.; Centorotola, G.; Bilei, S.; Senese, M.; Terracciano, G.; Leblanc, J.C.; Pomilio, F.; et al. First Report on the finding of Listeria mnocytogenes ST121 strain in a dolphin brain. Pathogens 2020, 9, 802. [Google Scholar] [CrossRef]
- Scarpa, F.; Grattarola, C.; Arillo, A.; Mattioda, V.; Testori, C.; Terracciano, G.; Senese, M.; Giorda, F.; Zoppi, S.; Sanna, D.; et al. Draft genome of three isolates of Listeria monocytogenes isolated from Stenella coeruleoalba in Italy. Microbiol. Resour. Announc. 2024, e01221-23. [Google Scholar] [CrossRef] [PubMed]
- Corazzola, G.; Baini, M.; Grattarola, C.; Panti, C.; Marcer, F.; Garibaldi, F.; Berio, E.; Mancusi, C.; Galli, M.; Mazzariol, S.; et al. analysis of the gastro-intestinal tract of marine mammals: A multidisciplinary approach with a new multi-sieves tool. Animals 2021, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Iaccarino, D.; Di Nocera, F.; Degli Uberti, B.; Lucibelli, M.; Borriello, G.; De Luca, G.; D’Amore, M.; Cerrone, A.; Galiero, G. Molecular detection of Chlamydia abortus in a stranded Mediterranean striped dolphin Stenella coeruleoalba. Dis. Aquat. Organ. Inter.-Res. Sci. Center 2019, 132, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, R.; Cipriano, G.; Santacesaria, F.C.; Ricci, P.; Maglietta, R.; Petrella, A.; Mazzariol, S.; De Padova, D.; Mossa, M.; Bellomo, S.; et al. Exploring data from an individual stranding of a Cuvier’s beaked whale in the Gulf of Taranto (Northern Ionian Sea, Central-eastern Mediterranean Sea). J. Exp. Mar. Biol. Ecol. 2020, 533, 151473. [Google Scholar] [CrossRef]
- Romani-Cremaschi, U.; Zoppi, S.; Mattioda, V.; Audino, T.; Marsili, L.; Varello, K.; Iulini, B.; Marra, C.; Zoccola, R.; Battistini, R.; et al. Morganella morganii septicemia and concurrent renal crassicaudiasis in a Cuvier’s beaked whale (Ziphius cavirostris) stranded in Italy. Front. Mar. Sci. 2023, 9, 1058724. [Google Scholar] [CrossRef]
- Ascheri, D.; Fontanesi, E.; Marsili, L.; Berio, E.; Garibaldi, F.; Goria, M.; Serracca, L.; Dondo, A.; di Francesco, C.; Varello, K.; et al. Post-mortem examination on a striped dolphin (Stenella coeruleoalba) reveals a potential fatal interaction with bottlenose dolphins (Tursiops truncatus) in Italian waters. J. Mar. Biol. Assoc. UK 2024, 104, e4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).