“One Health” Perspective on Prevalence of ESKAPE Pathogens in Africa: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Systematic Review Protocol, and Registration
2.2. Search Strategy
2.3. Selection of Studies and Data Extraction
2.4. Data Synthesis and Data Analysis
2.5. Quality Assessment of the Studies
3. Results
3.1. Search and Screening Results
3.2. Overall Number of Reported ESKAPE Bacterial Isolates
3.3. Meta-Analysis of ESKAPE Pathogens in Humans
3.4. Meta-Analysis of ESKAPE Pathogens in Animals
3.5. Meta-Analysis of ESKAPE Pathogens from the Environment
3.6. Meta-Analysis of ESKAPE Pathogens from Food
3.7. Meta-Analysis of ESKAPE Pathogens from Humans and Animals
3.8. Meta-Analysis of ESKAPE Pathogens in Humans and the Environment
3.9. Meta-Analysis of ESKAPE Pathogens in Humans, Animals, and the Environment
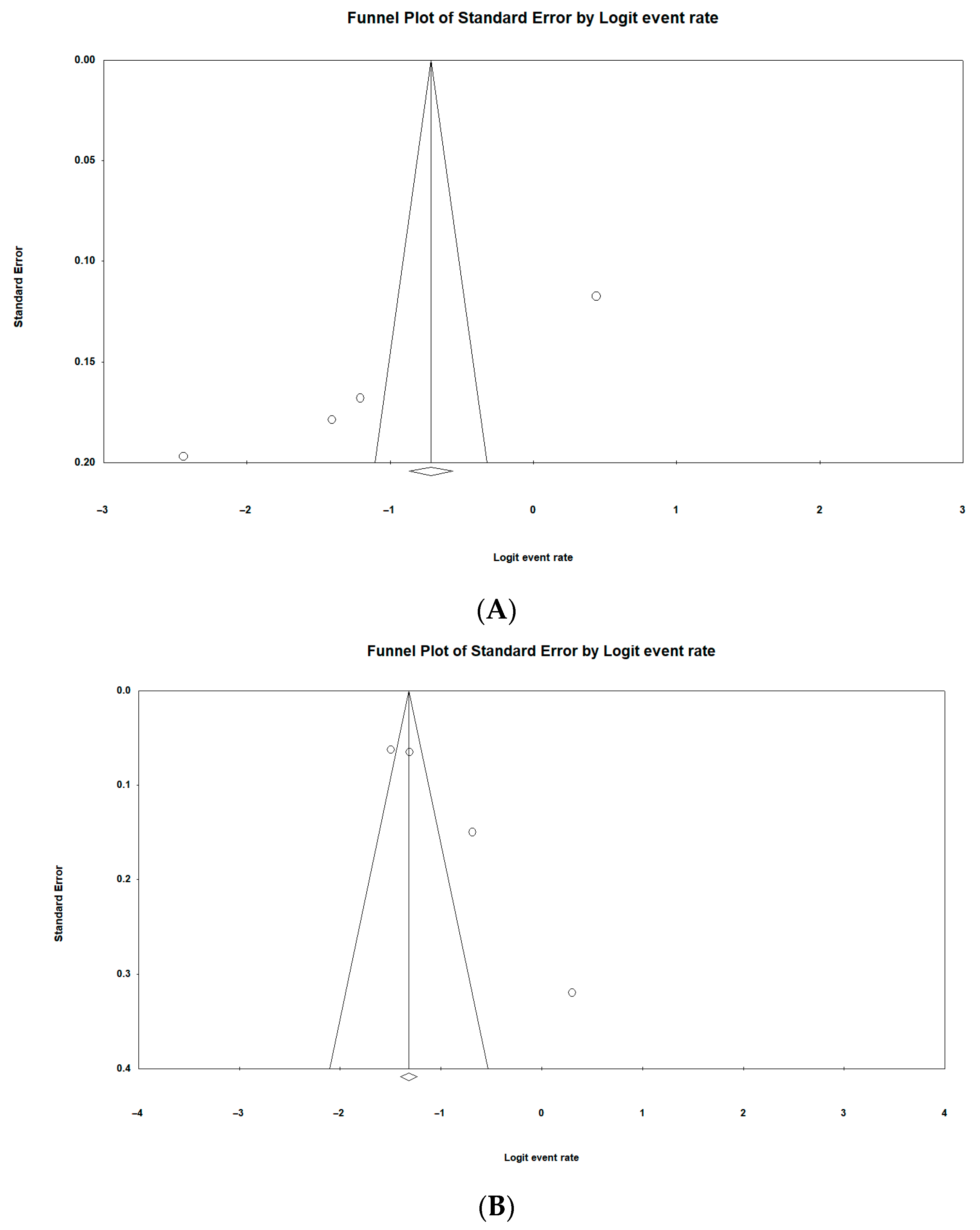
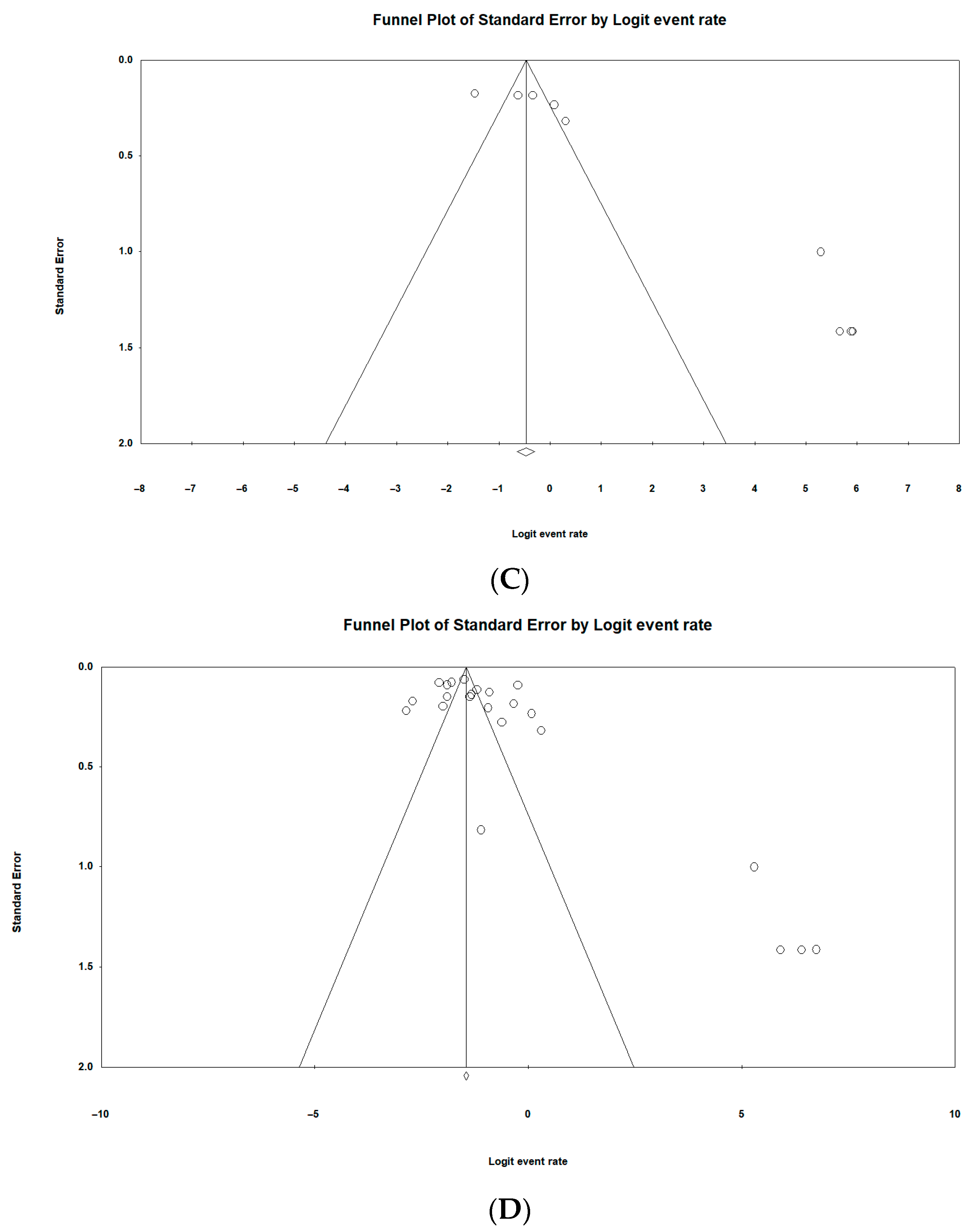
3.10. Risk of Publication Bias of Included Studies
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loyola-Cruz, M.Á.; Gonzalez-Avila, L.U.; Martínez-Trejo, A.; Saldaña-Padilla, A.; Hernández-Cortez, C.; Bello-López, J.M.; Castro-Escarpulli, G. ESKAPE and beyond: The burden of coinfections in the COVID-19 pandemic. Pathogens 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Pellegrino, M.; Giuzio, F.; Marra, M.; Rosano, C.; Saturnino, C.; Sinicropi, M.S.; Aquaro, S. Antibiotic-resistant ESKAPE pathogens and COVID-19: The pandemic beyond the pandemic. Viruses 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for which New Antibiotics are Urgently Needed. 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-listof-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 April 2024).
- Benkő, R.; Gajdács, M.; Matuz, M.; Bodó, G.; Lázár, A.; Hajdú, E.; Papfalvi, E.; Hannauer, P.; Erdélyi, P.; Pető, Z. Prevalence and antibiotic resistance of ESKAPE pathogens isolated in the emergency department of a tertiary care teaching hospital in hungary: A 5-year retrospective survey. Antibiotics 2020, 9, 624. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Stull, J.W.; Weese, J.S. Hospital-associated infections in small animal practice. Vet. Clin. Small Anim. Pract. 2015, 45, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Tedin, K.; Lübke-Becker, A. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet. Microbiol. 2017, 200, 71–78. [Google Scholar] [CrossRef]
- Hrenovic, J.; Durn, G.; Music, M.S.; Dekic, S.; Troskot-Corbic, T.; Skoric, D. Extensively and multi drug-resistant Acinetobacter baumannii recovered from technosol at a dump site in Croatia. Sci. Total Environ. 2017, 607, 1049–1055. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Akanbi, O.E.; Njom, H.A.; Fri, J.; Otigbu, A.C.; Clarke, A.M. Antimicrobial susceptibility of Staphylococcus aureus isolated from recreational waters and beach sand in Eastern Cape Province of South Africa. Int. J. Environ. Res. Public Health 2017, 14, 1001. [Google Scholar] [CrossRef]
- Gwenzi, W. Occurrence, behaviour, and human exposure pathways and health risks of toxic geogenic contaminants in serpentinitic ultramafic geological environments (SUGEs): A medical geology perspective. Sci. Total Environ. 2020, 700, 134622. [Google Scholar] [CrossRef]
- Ebomah, K.E.; Okoh, A.I. An African perspective on the prevalence, fate and effects of carbapenem resistance genes in hospital effluents and wastewater treatment plant (WWTP) final effluents: A critical review. Heliyon 2020, 6, e03899. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Munn, Z.; Peters, M.D.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019 (2019 AR Threats Report). 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 1 April 2024).
- Scaglione, E.; Mantova, G.; Caturano, V.; Fanasca, L.; Carraturo, F.; Farina, F.; Pagliarulo, C.; Vitiello, M.; Pagliuca, C.; Salvatore, P.; et al. Molecular epidemiology of genital infections in Campania Region: A retrospective study. Diagnostics 2022, 12, 1798. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, R.; Karoma, S.; Al Atrouni, A. Multidrug-Resistant Gram-Negative ESKAPE Pathogens from a Tertiary-Care Hospital: Prevalence and Risk Factors. Egypt. J. Med. Microbiol. 2022, 31, 135–142. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low-and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]
- Schmidt, T.; Kock, M.M.; Ehlers, M.M. Molecular characterization of Staphylococcus aureus isolated from bovine mastitis and close human contacts in South African dairy herds: Genetic diversity and inter-species host transmission. Front. Microbiol. 2017, 8, 511. [Google Scholar] [CrossRef]
- Iyamba, J.M.; Wambale, J.M.; Lukukula, C.M. High prevalence of methicillin resistant staphylococci strains isolated from surgical site infections in Kinshasa. Pan Afr. Med. J. 2014, 18, 322. [Google Scholar] [CrossRef]
- Ojulong, J.; Mwambu, T.P.; Joloba, M.; Bwanga, F.; Kaddu-Mulindwa, D.H. Relative prevalence of methicilline resistant Staphylococcus aureus and its susceptibility pattern in Mulago Hospital, Kampala, Uganda. Tanzan. J. Health Res. 2009, 11, 149–153. [Google Scholar] [CrossRef]
- Dilnessa, T.; Bitew, A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect. Dis. 2016, 16, 398. [Google Scholar] [CrossRef]
- Kahsay, A.G.; Hagos, D.G.; Abay, G.K.; Mezgebo, T.A. Prevalence and antimicrobial susceptibility patterns of methicillin-resistant Staphylococcus aureus among janitors of Mekelle University, North Ethiopia. BMC Res. Notes 2018, 11, 294. [Google Scholar] [CrossRef]
- Nirmal, K.; Gupta, P.; Ahmad, N.; Nath, S.; Singh, N.P.; Das, S. Bacteriological profile and their antimicrobial susceptibility pattern among clinical suspected adult septicemia admitted patients: A study from tertiary care and teaching hospital. East. J. Med. Sci. 2024, 9, 4–9. [Google Scholar] [CrossRef]
- Tenssaie, Z.W. Multiple antimicrobial resistance in gram negative bacilli isolated from clinical specimens, Jimma Hospital, southwest Ethiopia. Ethiop. Med. J. 2001, 39, 305–312. [Google Scholar]
- Penes, N.O.; Muntean, A.A.; Moisoiu, A.; Muntean, M.M.; Chirca, A.; Bogdan, M.A.; Popa, M.I. An overview of resistance profiles ESKAPE pathogens from 2010–2015 in a tertiary respiratory center in Romania. Rom. J. Morphol. Embryol. 2017, 58, 909–922. [Google Scholar]
- Bastidas-Caldes, C.; Cisneros-Vásquez, E.; Zambrano, A.; Mosquera-Maza, A.; Calero-Cáceres, W.; Rey, J.; Yamamoto, Y.; Yamamoto, M.; Calvopiña, M.; de Waard, J.H. Co-harboring of beta-lactamases and mcr-1 genes in Escherichia coli and Klebsiella pneumoniae from healthy carriers and backyard animals in rural communities in Ecuador. Antibiotics 2023, 12, 856. [Google Scholar] [CrossRef]
- Kerr, K.G.; Snelling, A.M. Pseudomonas aeruginosa: A formidable and ever-present adversary. J. Hosp. Infect. 2009, 73, 338–344. [Google Scholar] [CrossRef]
- Ndubuisi, J.C.; Olonitola, O.S.; Olayinka, A.T.; Jatau, E.D.; Iregbu, K.C. Prevalence and antibiotics susceptibility profile of Enterococcus spp. Isolated from some hospitals in Abuja, Nigeria. Afr. J. Clin. Exp. Microbiol. 2017, 18, 154–158. [Google Scholar] [CrossRef]
- Olawale, K.O.; Fadiora, S.O.; Taiwo, S.S. Prevalence of hospital-acquired enterococci infections in two primary-care hospitals in osogbo, southwestern Nigeria. Afr. J. Infect. Dis. 2011, 5, 40–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falco, A.; Guerrero, D.; García, I.; Correa, A.; Rivera, S.; Olaya, M.B.; Aranaga, C. Molecular characterization of KPC-2-Producing Enterobacter cloacae complex isolates from cali, Colombia. Antibiotics 2021, 10, 694. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Miladi, R.; Janbakhsh, A.; Mansouri, F.; Sayad, B.; Vaziri, S.; Afsharian, M.; Zamanian, M.H.; Shirvani, M.; Yavari, S.; et al. The Prevalence and Pattern of Enterobacter Antibiotic Resistance in the Patients Admitted to Imam Reza Hospital in Kermanshah, Iran (2016–2018). J. Kermanshah Univ. Med. Sci. 2021, 25, e112518. [Google Scholar]
- Wang, Y.; Xiong, Y.; Wang, Z.; Zheng, J.; Xu, G.; Deng, Q.; Wen, Z.; Yu, Z. Comparison of solithromycin with erythromycin in Enterococcus faecalis and Enterococcus faecium from China: Antibacterial activity, clonality, resistance mechanism, and inhibition of biofilm formation. J. Antibiot. 2021, 74, 143–151. [Google Scholar] [CrossRef]
- Thu, W.P.; Sinwat, N.; Bitrus, A.A.; Angkittitrakul, S.; Prathan, R.; Chuanchuen, R. Prevalence, antimicrobial resistance, virulence gene, and class 1 integrons of Enterococcus faecium and Enterococcus faecalis from pigs, pork and humans in Thai-Laos border provinces. J. Glob. Antimicrob. Resist. 2019, 18, 130–138. [Google Scholar] [CrossRef]
- Lozano, C.; Gharsa, H.; Ben Slama, K.; Zarazaga, M.; Torres, C. Staphylococcus aureus in animals and food: Methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms 2016, 4, 12. [Google Scholar] [CrossRef]
- Cuny, C.; Layer-Nicolaou, F.; Werner, G.; Witte, W. A look at staphylococci from the one health perspective. Int. J. Med. Microbiol. 2024, 314, 151604. [Google Scholar] [CrossRef]
- Mekhloufi, O.A.; Chieffi, D.; Hammoudi, A.; Bensefia, S.A.; Fanelli, F.; Fusco, V. Prevalence, enterotoxigenic potential and antimicrobial resistance of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) isolated from Algerian ready to eat foods. Toxins 2021, 13, 835. [Google Scholar] [CrossRef]
- Chaalal, W.; Chaalal, N.; Bourafa, N.; Kihal, M.; Diene, S.M.; Rolain, J.M. Characterization of Staphylococcus aureus isolated from food products in Western Algeria. Foodborne Pathog. Dis. 2018, 15, 353–360. [Google Scholar] [CrossRef]
- Titouche, Y.; Houali, K.; Ruiz-Ripa, L.; Vingadassalon, N.; Nia, Y.; Fatihi, A.; Cauquil, A.; Bouchez, P.; Bouhier, L.; Torres, C.; et al. Enterotoxin genes and antimicrobial resistance in Staphylococcus aureus isolated from food products in Algeria. J. Appl. Microbiol. 2020, 129, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Madoroba, E.; Magwedere, K.; Chaora, N.S.; Matle, I.; Muchadeyi, F.; Mathole, M.A.; Pierneef, R. Microbial communities of meat and meat products: An exploratory analysis of the product quality and safety at selected enterprises in South Africa. Microorganisms 2021, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.E.; Mousa, W.S.; Abdelsalam, S.Y.; Heikal, H.S.; Shawish, R.R.; Nooruzzaman, M.; Soliman, M.M.; Batiha, G.E.; Hamad, A.; Abdeen, A. Prevalence and characterization of coagulase positive Staphylococci from food products and human specimens in Egypt. Antibiotics 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.M.; Hassanin, F.S.; Shaltout, F.A.; Nassif, M.Z.; Seif, M.Z. Prevalence of methicillin-resistant Staphylococcus aureus in some ready-to-eat meat products. Am. J. Biomed. Sci. Res. 2019, 4, 460–464. [Google Scholar]
- Ire, F.; Imuh, V. Bacteriological quality evaluation and safety of randomly selected ready-to-eat foods sold in Port Harcourt City, Nigeria. J. Appl. Life Sci. Int. 2016, 7, 1–10. [Google Scholar] [CrossRef]


| Risk Factors | Number of Studies | Pooled Estimates | Measure of Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample Size | ESKAPE Positive | I2 (95% CI) | Q Value | I2 | ||
| Overall | ||||||
| S. aureus | 28 | 8804 | 1885 | 22.5% (17.1–28.9) | 804.4 | 96.6 |
| K. pneumoniae | 24 | 12,292 | 1531 | 16.1% (10.9–23.3) | 1248 | 98.1 |
| Enterobacter spp. | 12 | 7789 | 149 | 2.5% (1.3–4.8) | 166.1 | 93.3 |
| P. aeruginosa | 7 | 1392 | 234 | 9.0% (3.4–17.7) | 84.9 | 92.9 |
| E. faecium | 6 | 2004 | 143 | 5.1% (1.3–17.5) | 228.4 | 97.8 |
| A. baumannii | 6 | 2774 | 92 | 4.6% (1.6–12.4) | 115.0 | 95.6 |
| Study year | ||||||
| 2010–2020 | 23 | 10,681 | 2812 | 28.9% (22.4–36.4) | 675.2 | 96.7 |
| 2021–2024 | 31 | 13,006 | 3932 | 41.2% (31.9–51.2) | 1725.1 | 99.2 |
| Samples | ||||||
| Mixed samples | 28 | 12,650 | 3536 | 34.5% (26.5–43.4) | 1370.9 | 98.0 |
| Nasal Swabs | 7 | 1930 | 518 | 26.9% (17.2–39.6) | 130.5 | 95.4 |
| Stool | 4 | 2834 | 749 | 44.0% (6.6–89.8) | 288.6 | 99.3 |
| Blood | 4 | 3786 | 711 | 22.9% (17.1–29.9) | 46.3 | 93.5 |
| Urine | 3 | 709 | 353 | 37.3% (15.0–66.6) | 67.8 | 97.0 |
| Methods | ||||||
| PCR | 5 | 1321 | 462 | 33.2% (19.3–50.8) | 141.8 | 97.1 |
| Culturing, Biochemical | 31 | 11,425 | 3147 | 29.1% (22.8–36.2) | 981.5 | 96.9 |
| VITEK-MS | 6 | 3765 | 1160 | 55.2% (32.2–76.1) | 512.8 | 99.0 |
| WGS | 3 | 2295 | 693 | 37.1% (11.4–71.0) | 88.6 | 97.7 |
| MALDI-TOF MS | 4 | 3369 | 704 | 28.4% (20.8–37.5) | 51.7 | 94.2 |
| Regions | ||||||
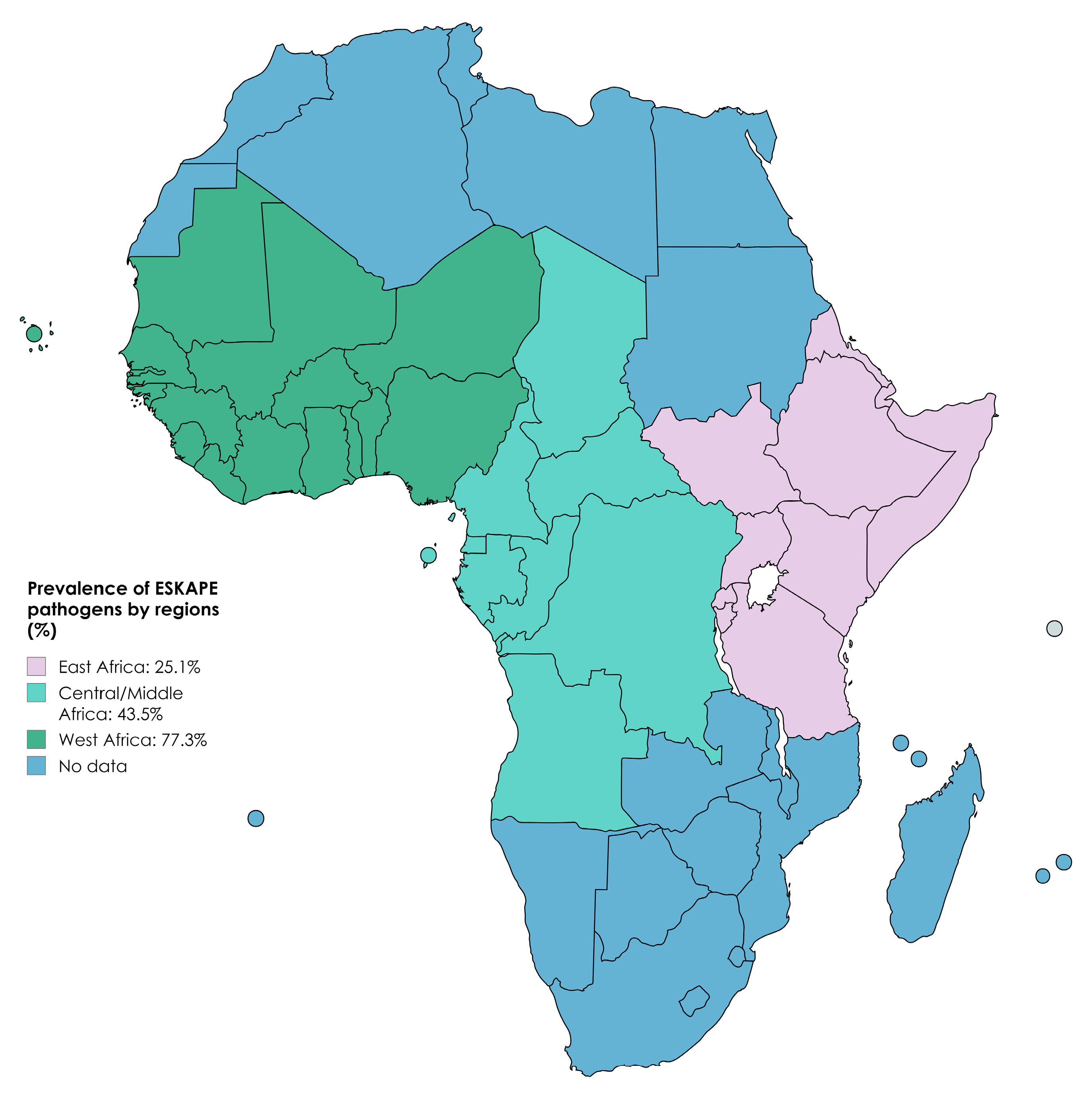
| East Africa | 30 | 14,210 | 3858 | 25.1% (20.1–30.9) | 1011.2 | 97.1 |
| West Africa | 9 | 1285 | 904 | 77.3% (58.3–89.2) | 137.4 | 94.1 |
| Central/Middle Africa | 8 | 3239 | 1135 | 43.5% (23.9–65.4) | 591.3 | 98.8 |
| Countries | ||||||
| Ethiopia | ||||||
| S. aureus | 9 | 3505 | 693 | 21.5% (16.2–28.0) | 115.3 | 93.0 |
| K. pneumoniae | 6 | 4364 | 445 | 12.5% (7.3–20.8) | 176.9 | 97.1 |
| Enterobacter spp. | 5 | 2436 | 84 | 4.1% (2.2–7.5) | 25.0 | 88.0 |
| Nigeria | ||||||
| S. aureus | 5 | 792 | 344 | 45.7% (21.7–71.9) | 105.4 | 96.2 |
| Tanzania | ||||||
| E. faecium | 7 | 3014 | 837 | 25.3% (14.8–39.8) | 209.1 | 97.1 |
| K. pneumoniae | 5 | 2616 | 261 | 7.5% (3.9–13.8) | 83.3 | 95.2 |
| A. baumannii | 4 | 1052 | 298 | 24.1% (8.1–53.3) | 197.2 | 98.4 |
| Cameroon | ||||||
| S. aureus | 3 | 632 | 275 | 42.2% (21.4–66.1) | 65.6 | 96.9 |
| K. pneumoniae | 3 | 386 | 45 | 10.6% (4.3–23.9) | 14.8 | 86.5 |
| Risk Factors | Number of Studies | Pooled Estimates | Measure of Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample Size | ESKAPE Positive | I2 (95%CI) | Q Value | I2 | ||
| Overall | ||||||
| S. aureus | 7 | 2188 | 617 | 26.8% (15.3–42.7) | 285.2 | 97.8 |
| Enterobacter spp. | 3 | 1908 | 181 | 10.5% (5.4–19.6) | 22.7 | 91.2 |
| Methods | ||||||
| PCR | 5 | 1586 | 514 | 30.6% (16.1–50.4) | 203.8 | 99.0 |
| Biochemical | 3 | 2092 | 289 | 17.4% (7.6–34.9) | 28.3 | 92.9 |
| Samples | ||||||
| Milk | 3 | 2158 | 239 | 14.4% (6.7–28.2) | 58.0 | 96.6 |
| Meat (Beef, chicken and sheep) | 4 | 1066 | 528 | 41.7% (19.3–68.1) | 97.3 | 96.9 |
| Years | ||||||
| 2010–2020 | 5 | 1313 | 658 | 70.4% (39.0–89.8) | 201.8 | 98.01 |
| 2021–2024 | 7 | 3078 | 453 | 16.6% (10.3–25.7) | 151.9 | 96.0 |
| Countries | ||||||
| South Africa | 4 | 1015 | 428 | 39.2% (24.5–56.2) | 78.3 | 96.1 |
| Risk Factors | Number of Studies | Pooled Estimates | Measure of Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample Size | ESKAPE Positive | I2 (95%CI) | Q Value | I2 | ||
| Overall | ||||||
| S. aureus | 4 | 1180 | 110 | 9.5% (2.6–29.4) | 116.6 | 95.5 |
| K. pneumoniae | 3 | 910 | 37 | 8.1% (1.3–37.4) | 45.0 | 95.5 |
| Enterobacter spp. | 3 | 903 | 29 | 7% (1.0–5.9) | 35.8 | 94.4 |
| A. baumannii | 4 | 1698 | 324 | 23.0% (12.9–37.8) | 36.8 | 91.8 |
| Methods | ||||||
| PCR | 5 | 1044 | 221 | 38.3% (13.8–70.7) | 99.3 | 95.9 |
| Culturing, Biochemical | 4 | 592 | 197 | 44.5% (12.7–81.6) | 89.1 | 96.6 |
| Countries | ||||||
| South Africa | 6 | 1851 | 394 | 33.8% (19.6–51.8) | 64.7 | 92.2 |
| Risk Factors | Number of Studies | Pooled Estimates | Measure of Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample Size | ESKAPE Positive | I2 (95%CI) | Q Value | I2 | ||
| Overall | ||||||
| S. aureus | 6 | 877 | 219 | 21.1% (13.4–31.4) | 44.6 | 88.7 |
| Methods | ||||||
| PCR | 5 | 867 | 569 | 38.5% (19.9–61.1) | 78.4 | 94.8 |
| Countries | ||||||
| South Africa | 3 | 515 | 462 | 57.6% (12.2–93.0) | 50.1 | 96.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khasapane, N.G.; Nkhebenyane, S.J.; Lekota, K.; Thekisoe, O.; Ramatla, T. “One Health” Perspective on Prevalence of ESKAPE Pathogens in Africa: A Systematic Review and Meta-Analysis. Pathogens 2024, 13, 787. https://doi.org/10.3390/pathogens13090787
Khasapane NG, Nkhebenyane SJ, Lekota K, Thekisoe O, Ramatla T. “One Health” Perspective on Prevalence of ESKAPE Pathogens in Africa: A Systematic Review and Meta-Analysis. Pathogens. 2024; 13(9):787. https://doi.org/10.3390/pathogens13090787
Chicago/Turabian StyleKhasapane, Ntelekwane George, Sebolelo Jane Nkhebenyane, Kgaugelo Lekota, Oriel Thekisoe, and Tsepo Ramatla. 2024. "“One Health” Perspective on Prevalence of ESKAPE Pathogens in Africa: A Systematic Review and Meta-Analysis" Pathogens 13, no. 9: 787. https://doi.org/10.3390/pathogens13090787






