The Anthelmintic Activity of Nepeta racemosa Lam. Against Gastrointestinal Nematodes of Sheep: Rosmarinic Acid Quantification and In Silico Tubulin-Binding Studies
Abstract
1. Introduction
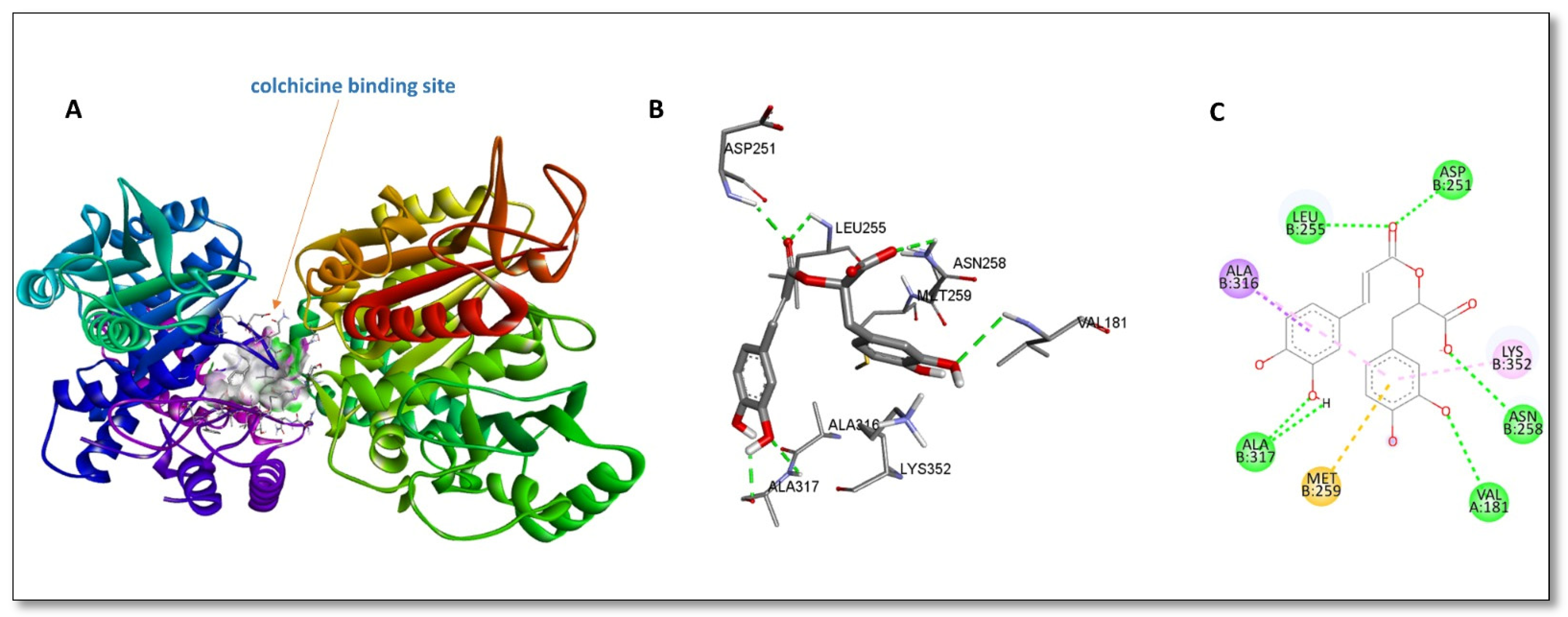
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Procedure
3.3. Anthelmintic Activity Studies
3.3.1. Egg Hatch Assay
3.3.2. Preparation and Maturation of Trichostrongylid Larvae
3.3.3. Larval Motility Assays
3.3.4. Statistical Analysis
3.4. Quantification of Rosmarinic Acid Using the RP-HPLC-DAD Method
Validation
3.5. In Silico Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Beleckė, A.; Kupčinskas, T.; Stadalienė, I.; Höglund, J.; Thamsborg, S.M.; Stuen, S.; Petkevičius, S. Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet. Scand. 2021, 63, 18. [Google Scholar] [CrossRef]
- Kozan, E.; Tatli, I.I.; Kahraman, C.; Kupeli Akkol, E.; Akdemir, Z. The in vivo Anthelmintic Efficacy of some Verbascum species growing in Turkey. Exp. Parasitol. 2011, 129, 211–214. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Claerebout, E. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Zeineldin, M.; Abdelmegeid, M.; Barakat, R.; Ghanem, M. A review: Herbal medicine as an effective therapeutic approach for treating digestive disorders in small ruminants. Alex. J. Vet. Sci. 2018, 56, 33–44. [Google Scholar] [CrossRef]
- Erez, M.S.; Kozan, E. Anthelmintic resistance in farm animals. Kocatepe Vet. J. 2018, 11, 322–330. [Google Scholar]
- Dag, S.; Erez, M.; Kozan, E.; Gençler Özkan, A.M.; Çankaya, İ. In vitro anthelmintic activity of five different Artemisia L. species growing in Türkiye. Pak. Vet. J. 2023, 43, 771–777. [Google Scholar]
- Sharma, A.; Cooper, R.; Bhardwaj, G.; Cannoo, D.S. The genus Nepeta: Traditional uses, phytochemicals and pharmacological properties. J. Ethnopharmacol. 2021, 268, 113679. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Custodio, L.; Polat, R.; Cakilcioglu, U.; Ayna, A.; et al. Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey. Plants 2021, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, A.; Di Simone, S.C.; Nilofar; Bouyahya, A.; Zengin, G.; Recinella, L.; Leone, S.; Brunetti, L.; Uba, A.I.; Guler, O.; et al. Screening for Chemical Characterization and Pharmacological Properties of Different Extracts from Nepeta italica. Plants 2023, 12, 2785. [Google Scholar] [CrossRef]
- Bandh, S.A.; Lone, B.; Chishti, M.Z.; Kamili, A.N.; Ganai, B.A.; Saleem, S. Evaluation of Anthelmintic and Antimicrobial Activity of the Methanolic Extracts of Nepeta cataria. N.Y. Sci. J. 2011, 4, 129–135. [Google Scholar]
- Kaska, A.; Deniz, N.; Çiçek, M.; Mammadov, R. Evaluation of Antioxidant Properties, Phenolic Compounds, Anthelmintic, and Cytotoxic Activities of Various Extracts Isolated from Nepeta cadmea: An Endemic Plant for Turkey. J. Food Sci. 2018, 83, 1552–1559. [Google Scholar] [CrossRef]
- Fennell, B.; Naughton, J.; Barlow, J.; Brennan, G.; Fairweather, I.; Hoey, E.; McFerran, N.; Trudgett, A.; Bell, A. Microtubules as antiparasitic drug targets. Expert. Opin. Drug Discov. 2008, 3, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Kumar, S.P.; Kari, V.; Jha, P.C. Exploration of interaction zones of β-tubulin colchicine binding domain of helminths and binding mechanism of anthelmintics. Comput. Biol. Chem. 2017, 68, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.Á.; Tosat-Bitrián, C.; Barrado-Gil, L.; Bonato, F.; Galindo, I.; Garaigorta, U.; Álvarez-Bernad, B.; París-Ogáyar, R.; Lucena-Agell, D.; Giménez-Abián, J.F.; et al. Effect of Clinically Used Microtubule Targeting Drugs on Viral Infection and Transport Function. Int. J. Mol. Sci. 2022, 23, 3448. [Google Scholar] [CrossRef]
- Canela, M.D.; Noppen, S.; Bueno, O.; Prota, A.E.; Bargsten, K.; Sáez-Calvo, G.; Jimeno, M.L.; Benkheil, M.; Ribatti, D.; Velázquez, S.; et al. Antivascular and antitumor properties of the tubulin-binding chalcone TUB091. Oncotarget 2017, 8, 14325–14342. [Google Scholar] [CrossRef]
- de la Roche, N.M.; Mühlethaler, T.; Di Martino, R.M.C.; Ortega, J.A.; Gioia, D.; Roy, B.; Prota, A.E.; Steinmetz, M.O.; Cavalli, A. Novel fragment-derived colchicine-site binders as microtubule-destabilizing agents. Eur. J. Med. Chem. 2022, 241, 114614. [Google Scholar] [CrossRef] [PubMed]
- Jost, M.; Chen, Y.; Gilbert, L.A.; Horlbeck, M.A.; Krenning, L.; Menchon, G.; Rai, A.; Cho, M.Y.; Stern, J.J.; Prota, A.E.; et al. Combined CRISPRi/a-Based Chemical Genetic Screens Reveal that Rigosertib Is a Microtubule-Destabilizing Agent. Mol. Cell 2017, 68, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Memoona, A.; Amir, M.K.; Sultan, A.; Razia, P.; Sumera, P.; Lal, M.; Nisar, A. Evaluation of Nepeta laevigata, Nepeta kurramensis and Rhynchosia reniformis on antimalarial and antileishmanial activities. Int. J. Bioassays 2012, 1, 122–127. [Google Scholar]
- Mahnaz, K.; Alireza, F.; Hassan, V.; Mahdi, S.; Reza, A.M.; Abbas, H. Larvicidal activity of essential oil and methanol extract of Nepeta menthoides against malaria vector Anopheles stephensi. Asian Pac. J. Trop. Med. 2012, 5, 962–965. [Google Scholar] [CrossRef]
- Pavaraj, M.; Bakavathiappan, G.A.; Baskaran, S. Evaluation of some plant extracts for their nematicidal properties against root-knot nematode, Meloidogyne incognita. J. Biopest. 2012, 5, 106. [Google Scholar]
- Musso, L.; Scaglia, B.; Haj, G.A.; Arnold, N.A.; Adani, F.; Scarì, G.; Dallavalle, S.; Iriti, M. Chemical characterization and nematicidal activity of the essential oil of Nepeta nuda L. ssp. pubescens and Nepeta curviflora Boiss. from Lebanon. J. Essent. Oil Bear. Plants 2017, 20, 1424–1433. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef]
- Brunet, S.; Hoste, H. Monomers of condensed tannins affect the larval exsheathment of parasitic nematodes of ruminants. J. Agric. Food Chem. 2006, 54, 7481–7487. [Google Scholar] [CrossRef]
- Quijada, J.; Fryganas, C.; Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I.; Hoste, H. Anthelmintic activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J. Agric. Food Chem. 2015, 63, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, L.S.; Gamble, H.R.; Fetterer, R.H. Characterization of the eggshell of Haemonchus contortus—I. Structural components. Comp. Biochem. Physiol. B 1992, 103, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.; Costa Júnior, L.M.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.S.; Mesquista, J.W.C.; Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W. Anthelmintic activity of plant extracts from Brazilian savana. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef]
- Zabré, G.; Kaboré, A.; Bayala, B.; Katiki, L.M.; Costa-Júnior, L.M.; Tamboura, H.H.; Belem, A.; Abdalla, A.L.; Niderkorn, V.; Hoste, H.; et al. Comparison of the in vitro anthelmintic effects of Acacia nilotica and Acacia raddiana. Parasite 2017, 24, 44. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, X.; Han, Y.; Guo, D.; Guo, Q.; Li, R. Rosmarinic acid from eelgrass shows nematicidal and antibacterial activities against pine wood nematode and its carrying bacteria. Mar. Drugs 2012, 10, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.B.; Castro, L.M.; Azambuja, R.H.M.; Capella, G.A.; Moura, M.Q.; Terto, W.D.; Freitag, R.A.; Jeske, S.T.; Villela, M.M.; Cleff, M.B.; et al. Ovicidal and larvicidal potential of Rosmarinus officinalis to control gastrointestinal nematodes of sheep. Brazilian J. Vet. Parasitol. 2019, 28, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Eylek, B. Investigating the Anthelmintic Effects of Rosmarinus officinalis L. and Rosmarinic Acid in Mice Naturally İnfected with Aspiculuris tetraptera. Master’s Thesis, Bursa Uludağ University, Bursa, Turkey, 2021. [Google Scholar]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Wang, L.J.; Cao, Y.; Shi, H.N. Helminth infections and intestinal inflammation. World J. Gastroenterol. 2008, 14, 5125–5132. [Google Scholar] [CrossRef]
- Seyfried, M.; Soldera-Silva, A.; Campestrini, L.H.; Siebert, D.A.; Vitali, L.; Micke, G.A.; Zawadzki-Baggio, S.F.; Molento, M.B.; Maurer, J.B.B. In vitro anthelmintic activity of Polygonum acre (Linnaeus, 1754) extracts against the ruminant nematode Haemonchus contortus (Rudolphi, 1803). Arch. Vet. Sci. 2022, 27, e83890. [Google Scholar] [CrossRef]
- Váradyová, Z.; Mravčáková, D.; Babják, M.; Bryszak, M.; Grešáková, L.; Čobanová, K.; Kišidayová, S.; Plachá, I.; Königová, A.; Cieslak, A.; et al. Effects of herbal nutraceuticals and/or zinc against Haemonchus contortus in lambs experimentally infected. BMC Vet. Res. 2018, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.A.; Platzer, E.G. Interaction of anthelmintic benzimidazoles and benzimidazole derivatives with bovine brain tubulin. Biochim. Biophys. Acta BBA 1978, 544, 605–614. [Google Scholar] [CrossRef]
- Kohler, P.; Bachmann, R. Intestinal tubulin as possible target for the chemotherapeutic action of mebendazole in parasitic nematodes. Mol. Biochem. Parasitol. 1981, 4, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Lacey, E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 1988, 18, 885–936. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.H.; Paul, A.; Mojumdar, M.; Hasanat, A.; Islam, M.; Nabila, A.; Islam, J.; Hossain, R.; Chakrabarty, N.; Islam, N.M. In vitro anthelmintic activity of Macaranga denticulata and in silico molecular docking analysis of its isolated compounds with tubulin. Int. J. Pharm. 2016, 6, 131–136. [Google Scholar]
- Horton, J. Albendazole: A review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121 (Suppl. S1), 113–132. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Molan, A.L.; Meagher, L.P.; Spencer, P.A.; Sivakumaran, S. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Int. J. Parasitol. 2003, 33, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.C.; Clifford, S.; O’grady, J.; Behnke, J.M.; McCarthy, J.S. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am. J. Trop. Med. Hyg. 2004, 71, 608–616. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest Graph™ LC50 Calculator. AAT Bioquest. Available online: https://www.aatbio.com/tools/lc50-calculator (accessed on 22 December 2024).
- ICH. ICH Guideline Q2(R2), Validation of Analytical Procedures; ICH: Geneva, Switzerland, 2022. [Google Scholar]

| Treatment Group | Concentration (mg/mL) | Mortality (%) ± S.E.M | LC50 for L3 Stages of Gastrointestinal Nematodes of Sheep | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 6 h | 8 h | 24 h | |||
| NR-MeOH | 50 | 48.0 ± 0.8 b | 55.0 ± 0.0 b | 68.0 ± 1.4 b | 72.0 ± 1.2 b | 74.0 ± 0.0 b | 74.0 ± 0.0 b | 74.0 ± 0.0 b | 5.432 |
| 25 | 41.7 ± 0.6 b | 46.3 ± 0.6 b | 52.3 ± 0.6 b | 65.7 ± 1.2 b | 69.3 ± 1.0 b | 69.3 ± 1.0 b | 69.3 ± 1.0 b | ||
| 12.50 | 26.0 ± 0.7 b | 41.7 ± 0.6 b | 48.7 ± 0.6 b | 60.7 ± 1.2 b | 65.7 ± 0.5 b | 65.7 ± 0.5 b | 65.7 ± 0.5 b | ||
| 6.25 | 22.7 ± 0.6 b | 39.7 ± 0.2 b | 45.7 ± 0.5 b | 49.7 ± 1.5 b | 57.7 ± 0.5 b | 57.7 ± 0.5 b | 57.7 ± 0.5 b | ||
| 3.125 | 14.3 ± 0.5 b | 20.7 ± 0.5 b | 26.0 ± 0.7 b | 27.7 ± 0.3 b | 39.7 ± 0.6 b | 39.7 ± 0.6 b | 39.7 ± 0.6 b | ||
| 1.5625 | 9.7 ± 0.2 b | 13.3 ± 0.0 b | 18.7 ± 0.3 b | 34.4 ± 1.2 b | 34.4 ± 1.2 b | 34.4 ± 1.2 b | 34.4 ± 1.2 b | ||
| NR-n-Hexane | 50 | 40.3 ± 0.6 b | 45.3 ± 0.6 b | 53.0 ± 1.1 b | 64.7 ± 0.9 b | 67.0 ± 0.0 b | 67.0 ± 0.0 b | 67.0 ± 0.0 b | 7.3035 |
| 25 | 35.0 ± 0.4 b | 38.3 ± 0.6 b | 45.7 ± 0.9 b | 60.7 ± 0.7 b | 64.0 ± 0.4 b | 64.5 ± 0.5 b | 64.5 ± 0.5 b | ||
| 12.50 | 22.0 ± 0.0 b | 34.7 ± 0.5 b | 40.7 ± 0.5 b | 54.7 ± 0.3 b | 58.3 ± 0.6 b | 58.0 ± 0.0 b | 58.0 ± 0.0 b | ||
| 6.25 | 17.7 ± 0.6 b | 26.0 ± 0.7 b | 31.0 ± 0.7 b | 36.7 ± 0.7 b | 42.0 ± 0.8 b | 46.0 ± 0.0 b | 46.0 ± 0.0 b | ||
| 3.125 | 10.7 ± 0.5 b | 14.7 ± 0.5 b | 20.0 ± 0.0 b | 23.0 ± 0.6 b | 30.7 ± 0.5 b | 36.0 ± 0.0 b | 36.0 ± 0.0 b | ||
| 1.5625 | 7.3 ± 0.2 a | 10.7 ± 0.5 b | 14.0 ± 0.4 b | 15.7 ± 0.3 b | 26.7 ± 0.6 b | 30.0 ± 0.8 b | 30.0 ± 0.8 b | ||
| NR-DCM | 50 | 32.7 ± 0.5 b | 40.0 ± 0.8 b | 45.7 ± 0.6 b | 53.7 ± 0.9 b | 58.7 ± 0.6 b | 61.0 ± 1.1 b | 61.0 ± 1.1 b | 9.8452 |
| 25 | 23.7 ± 0.6 b | 33.7 ± 0.6 b | 41.7 ± 0.6 b | 46.3 ± 0.9 b | 51.0 ± 0.7 b | 58.0 ± 1.1 b | 58.0 ± 1.1 b | ||
| 12.50 | 16.7 ± 0.9 b | 26.0 ± 0.8 b | 36.7 ± 0.5 b | 45.7 ± 1.2 b | 50.7 ± 0.8 b | 50.7 ± 0.8 b | 50.7 ± 0.8 b | ||
| 6.25 | 12.3 ± 0.8 b | 16.0 ± 0.7 b | 22.7 ± 1.0 b | 27.0 ± 0.6 b | 40.7 ± 1.2 b | 40.7 ± 1.2 b | 40.7 ± 1.2 b | ||
| 3.125 | 14.4 ± 0.9 b | 16.0 ± 0.4 b | 17.0 ± 0.4 b | 21.3 ± 1.2 b | 32.0 ± 0.8 b | 32.0 ± 0.8 b | 32.0 ± 0.8 b | ||
| 1.5625 | 11.0 ± 0.7 b | 15.0 ± 0.0 b | 17.0 ± 0.7 b | 19.7 ± 0.9 b | 23.3 ± 0.6 b | 28.0 ± 0.8 b | 28.0 ± 0.8 b | ||
| NR-EtOAc | 50 | 55.3 ± 1.2 b | 65.0 ± 0.7 b | 72.3 ± 1.0 b | 75.0 ± 1.2 b | 79.7 ± 1.4 b | 79.7 ± 1.4 b | 79.7 ± 1.4 b | 7.5025 |
| 25 | 50.3 ± 0.8 b | 57.0 ± 0.8 b | 63.3 ± 0.6 b | 69.7 ± 0.9 b | 72.3 ± 0.8 b | 72.3 ± 0.8 b | 72.3 ± 0.8 b | ||
| 12.50 | 48.3 ± 0.8 b | 54.3 ± 0.8 b | 56.0 ± 1.1 b | 62.0 ± 2.1 b | 62.7 ± 1.2 b | 66.3 ± 0.8 b | 66.3 ± 0.8 b | ||
| 6.25 | 44.3 ± 0.2 b | 46.7 ± 0.6 b | 50.7 ± 0.8 b | 56.0 ± 1.0 b | 61.0 ± 1.1 b | 61.0 ± 1.1 b | 61.0 ± 1.1 b | ||
| 3.125 | 28.0 ± 0.4 b | 32.3 ± 0.8 b | 38.0 ± 1.1 b | 45.7 ± 0.7 b | 50.7 ± 0.8 b | 50.7 ± 0.8 b | 50.7 ± 0.8 b | ||
| 1.5625 | 24.7 ± 0.2 b | 26.7 ± 0.8 b | 33.3 ± 0.8 b | 39.7 ± 1.2 b | 45.3 ± 0.6 b | 45.3 ± 0.6 b | 45.3 ± 0.6 b | ||
| NR-n-BuOH | 50 | 20.0 ± 0.0 b | 23.3 ± 0.5 b | 31.7 ± 0.8 b | 46.7 ± 0.9 b | 51.7 ± 0.6 b | 51.7 ± 0.6 b | 51.7 ± 0.6 b | 7.8801 |
| 25 | 18.0 ± 0.4 b | 20.7 ± 0.6 b | 27.0 ± 0.4 b | 41.3 ± 0.9 b | 46.0 ± 0.7 b | 46.0 ± 0.7 b | 46.0 ± 0.7 b | ||
| 12.50 | 17.3 ± 0.5 b | 19.3 ± 0.2 b | 23.7 ± 0.8 b | 39.0 ± 1.0 b | 39.7 ± 0.8 b | 39.7 ± 0.8 b | 39.7 ± 0.8 b | ||
| 6.25 | 15.0 ± 0.4 b | 18.0 ± 0.4 b | 19.7 ± 0.2 b | 29.7 ± 0.9 b | 33.0 ± 0.4 b | 33.0 ± 0.4 b | 33.0 ± 0.4 b | ||
| 3.125 | 12.7 ± 0.2 b | 12.0 ± 0.0 b | 16.0 ± 0.7 b | 23.0 ± 1.2 b | 26.3 ± 0.6 b | 26.3 ± 0.6 b | 26.3 ± 0.6 b | ||
| 1.5625 | 8.0 ± 0.0 b | 9.7 ± 0.5 b | 10.3 ± 0.2 b | 16.3 ± 0.3 b | 21.0 ± 0.7 b | 21.0 ± 0.7 b | 21.0 ± 0.7 b | ||
| NR-H2O | 50 | 26.0 ± 0.7 b | 34.3 ± 0.8 b | 47.0 ± 0.8 b | 54.7 ± 0.9 b | 64.0 ± 0.7 b | 64.5 ± 0.3 b | 64.0 ± 0.7 b | 6.8703 |
| 25 | 22.0 ± 0.4 b | 30.7 ± 0.8 b | 41.7 ± 0.8 b | 51.3 ± 0.9 b | 59.0 ± 0.4 b | 59.0 ± 0.4 b | 59.0 ± 0.4 b | ||
| 12.50 | 17.3 ± 0.6 b | 26.3 ± 0.6 b | 34.0 ± 1.1 b | 45.0 ± 0.6 b | 52.7 ± 1.5 b | 52.7 ± 1.5 b | 52.7 ± 1.5 b | ||
| 6.25 | 14.3 ± 0.2 b | 20.3 ± 0.2 b | 26.7 ± 0.5 b | 32.0 ± 1.2 b | 40.3 ± 1.0 b | 40.3 ± 1.0 b | 40.3 ± 1.0 b | ||
| 3.125 | 12.0 ± 0.0 b | 15.0 ± 0.0 b | 18.0 ± 0.4 b | 22.0 ± 1.5 b | 30.0 ± 0.0 b | 30.0 ± 0.0 b | 30.0 ± 0.0 b | ||
| 1.5625 | 9.0 ± 0.4 b | 10.7 ± 0.5 b | 13.0 ± 0.0 b | 16.0 ± 0.0 b | 18.7 ± 0.6 b | 18.7 ± 0.6 b | 18.7 ± 0.6 b | ||
| Albendazole | 0.25 | 23.0 ± 0.0 b | 30.0 ± 0.0 b | 42.0 ± 0.0 b | 52.0 ± 0.0 b | 59.0 ± 1.0 b | 59.0 ± 1.0 b | 59.0 ± 1.0 b | |
| PBS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| DSMO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Compound | Retention Time (min) | Standard Curve | R2 | Test Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|
| Rosmarinic acid | 22.441 | y = 69.095x + 2194.4 | 0.9939 | 10–1000 | 0.6198 | 1.8782 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ağören, B.K.; Erez, M.S.; Kozan, E.; Dağyaran, A.; Akdağ, M.; Sobarzo-Sánchez, E.; Küpeli Akkol, E. The Anthelmintic Activity of Nepeta racemosa Lam. Against Gastrointestinal Nematodes of Sheep: Rosmarinic Acid Quantification and In Silico Tubulin-Binding Studies. Pathogens 2025, 14, 77. https://doi.org/10.3390/pathogens14010077
Ağören BK, Erez MS, Kozan E, Dağyaran A, Akdağ M, Sobarzo-Sánchez E, Küpeli Akkol E. The Anthelmintic Activity of Nepeta racemosa Lam. Against Gastrointestinal Nematodes of Sheep: Rosmarinic Acid Quantification and In Silico Tubulin-Binding Studies. Pathogens. 2025; 14(1):77. https://doi.org/10.3390/pathogens14010077
Chicago/Turabian StyleAğören, Büşra Karpuz, Mahmut Sinan Erez, Esma Kozan, Aydın Dağyaran, Mevlüt Akdağ, Eduardo Sobarzo-Sánchez, and Esra Küpeli Akkol. 2025. "The Anthelmintic Activity of Nepeta racemosa Lam. Against Gastrointestinal Nematodes of Sheep: Rosmarinic Acid Quantification and In Silico Tubulin-Binding Studies" Pathogens 14, no. 1: 77. https://doi.org/10.3390/pathogens14010077
APA StyleAğören, B. K., Erez, M. S., Kozan, E., Dağyaran, A., Akdağ, M., Sobarzo-Sánchez, E., & Küpeli Akkol, E. (2025). The Anthelmintic Activity of Nepeta racemosa Lam. Against Gastrointestinal Nematodes of Sheep: Rosmarinic Acid Quantification and In Silico Tubulin-Binding Studies. Pathogens, 14(1), 77. https://doi.org/10.3390/pathogens14010077