Pneumocystis Pneumonia Severity Is Associated with Taxonomic Shifts in the Respiratory Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. P. jirovecii Diagnosis
2.3. PCP Severity Scoring
2.4. P. jirovecii RT-PCR
2.5. 16S rDNA Sequencing
2.6. Metataxonomic and Functional Analysis
2.7. Statistical Analysis
3. Results
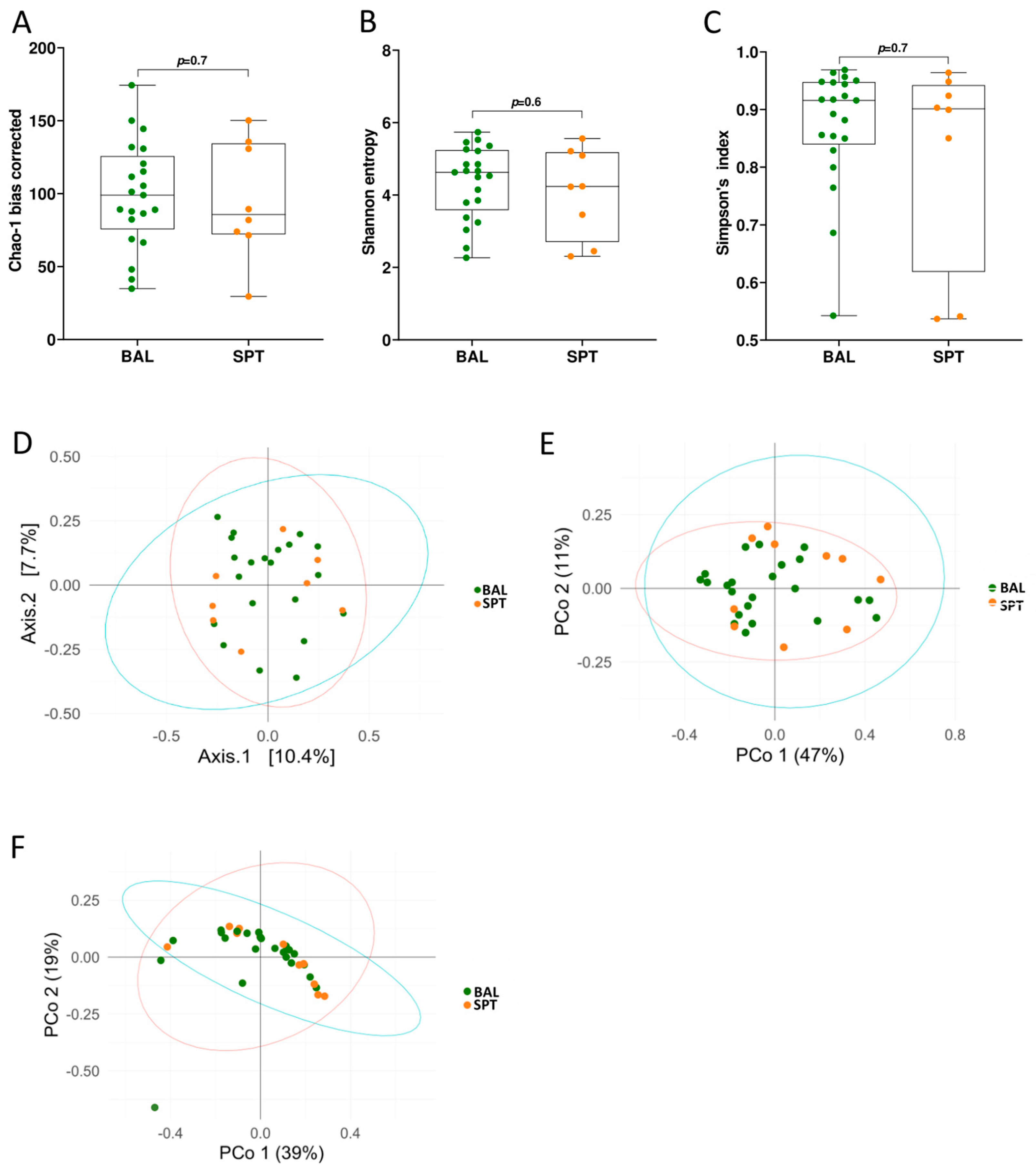
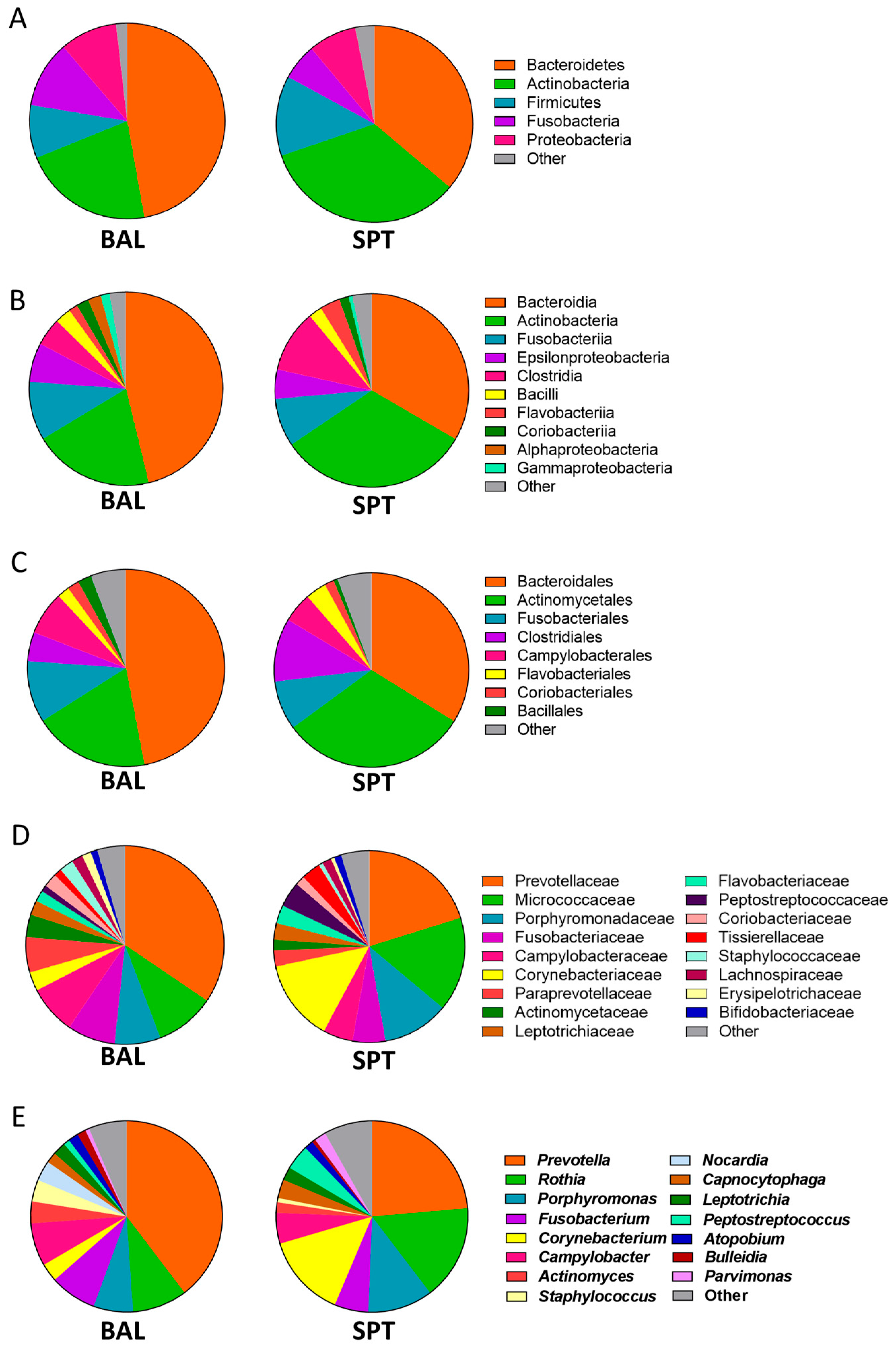
3.1. Upper and Lower Airways Have a Similar Community Structure in Patients with PCP
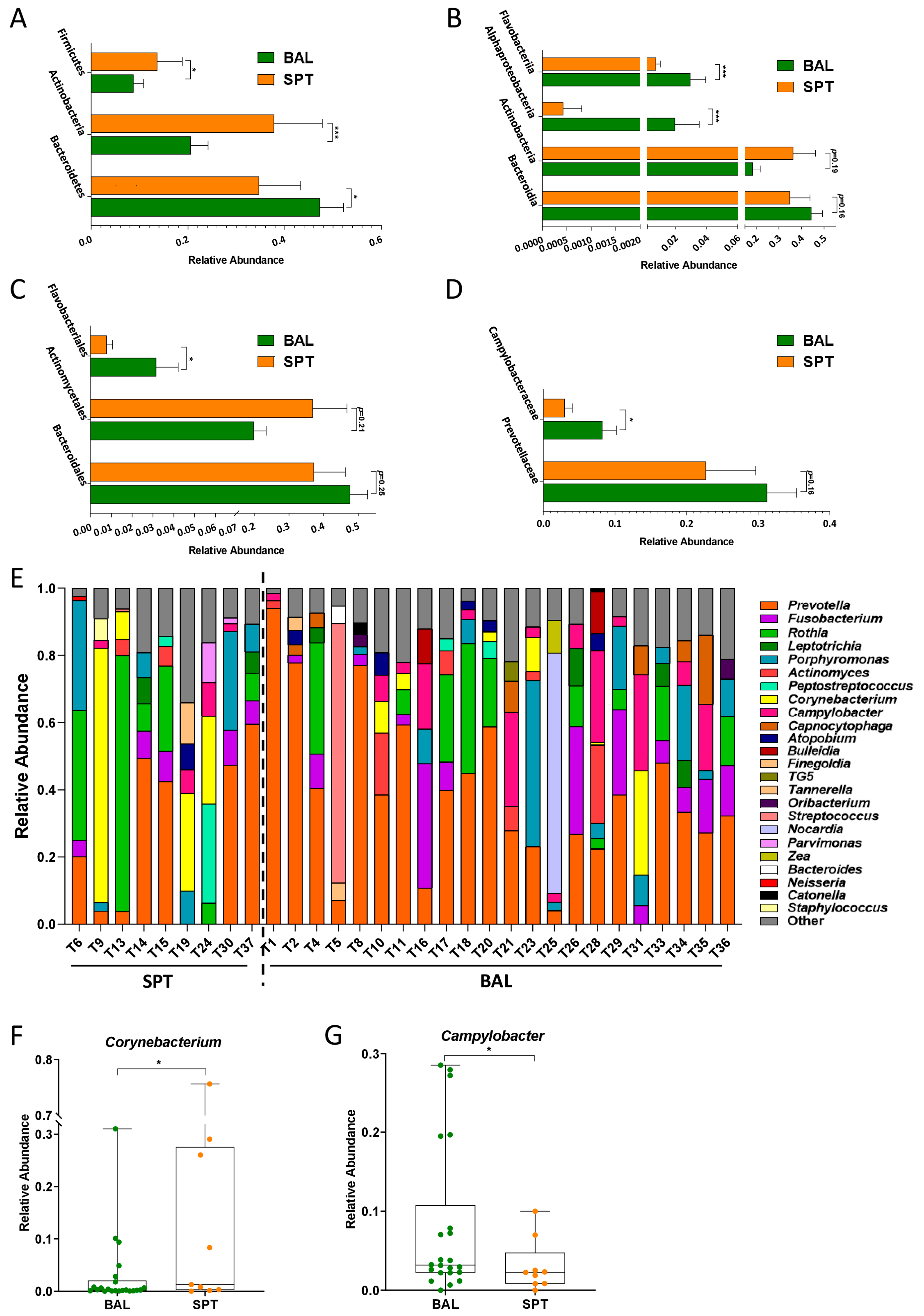
3.2. Subtle Taxonomic Shifts in Bacterial Communities Between Upper and Lower Airways
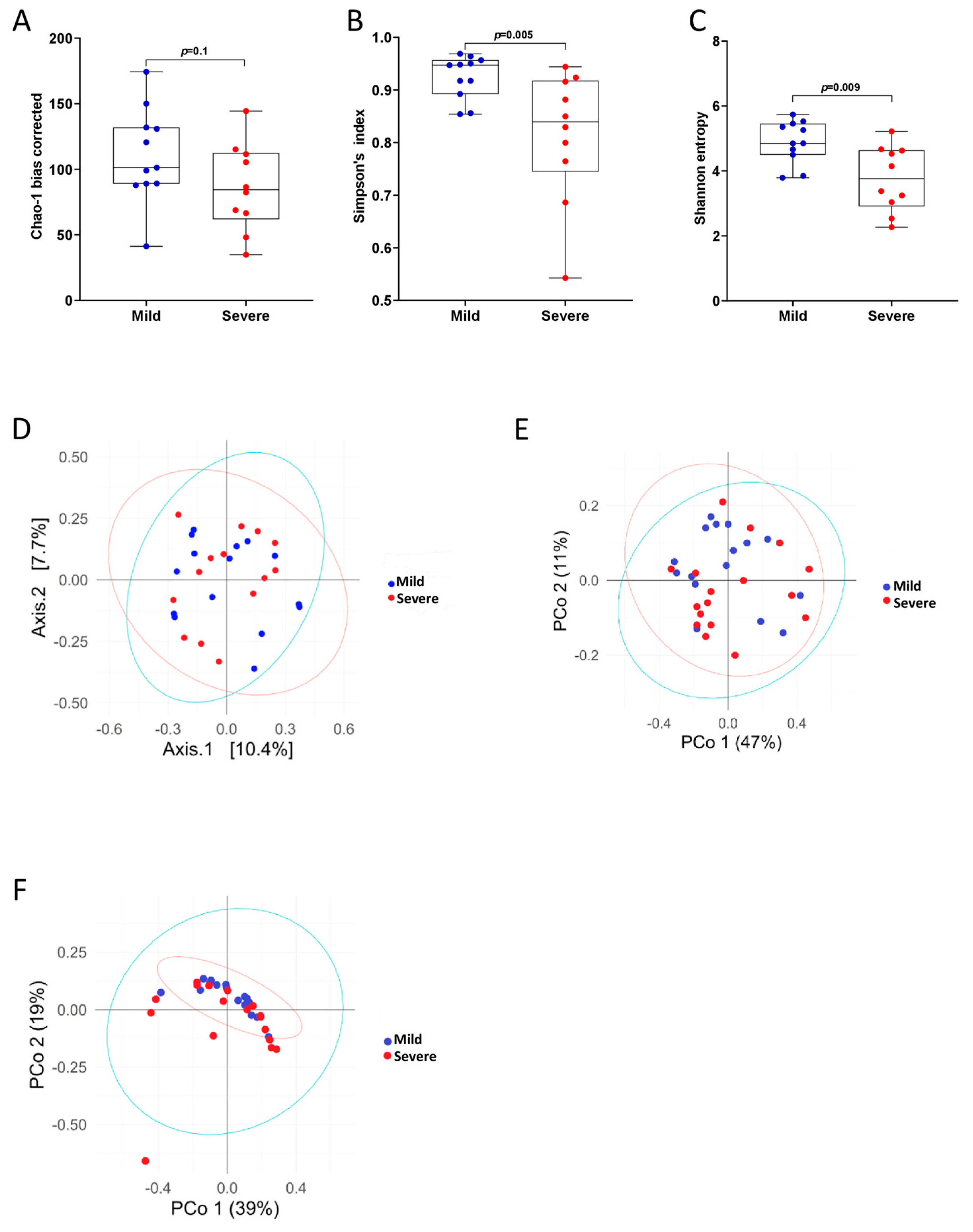
3.3. PCP Severity Is Associated with Reduced Bacterial Diversity
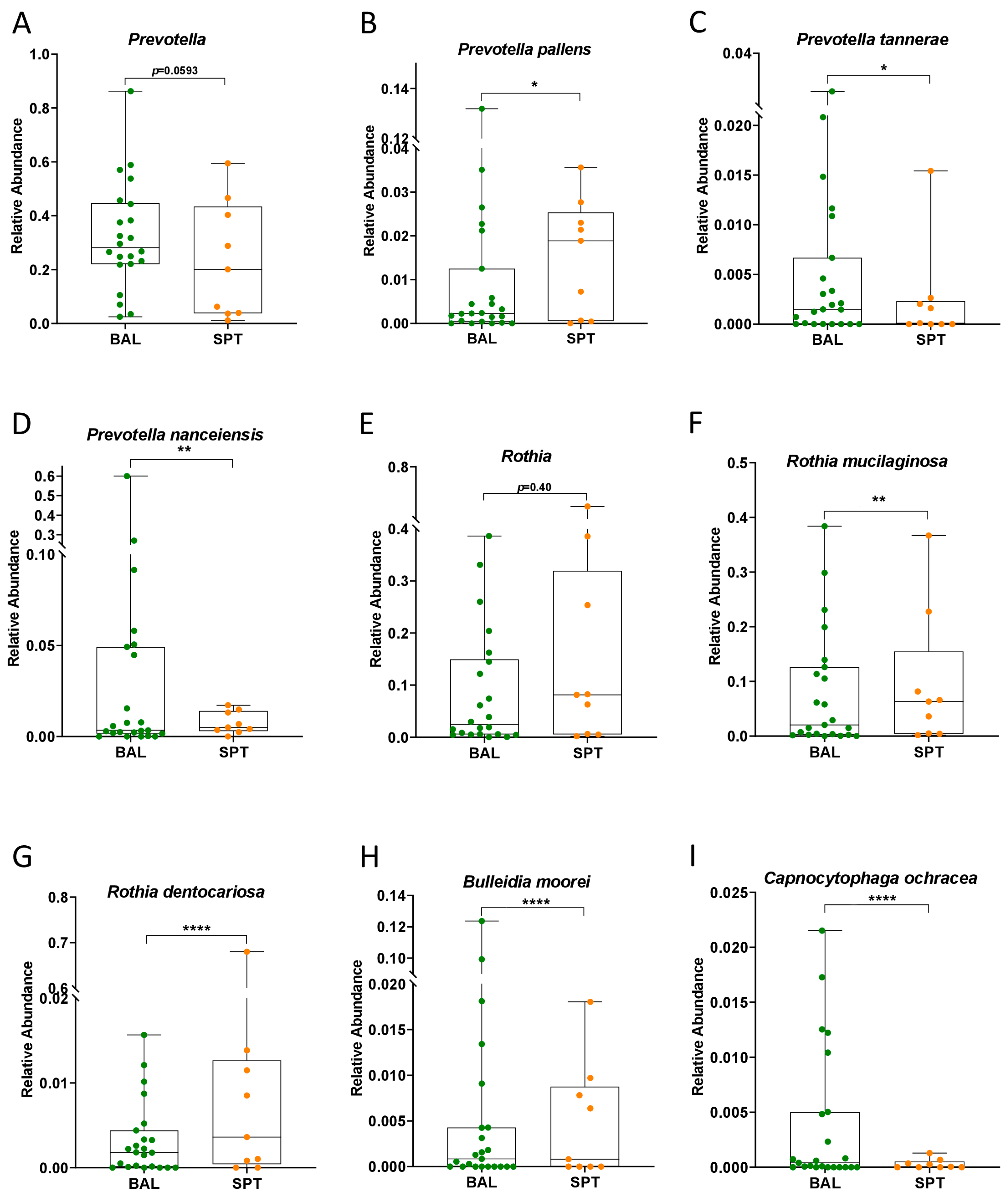
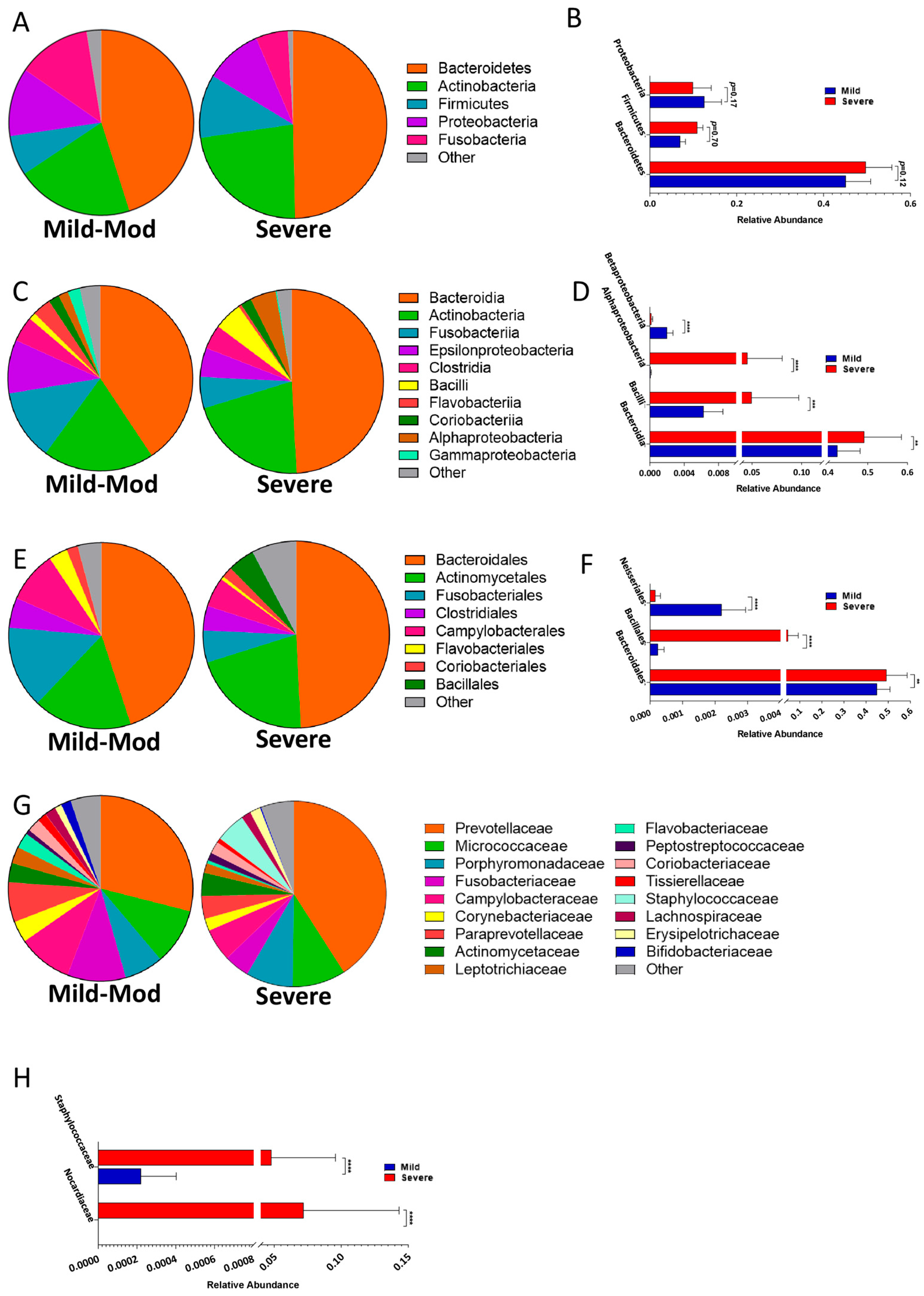
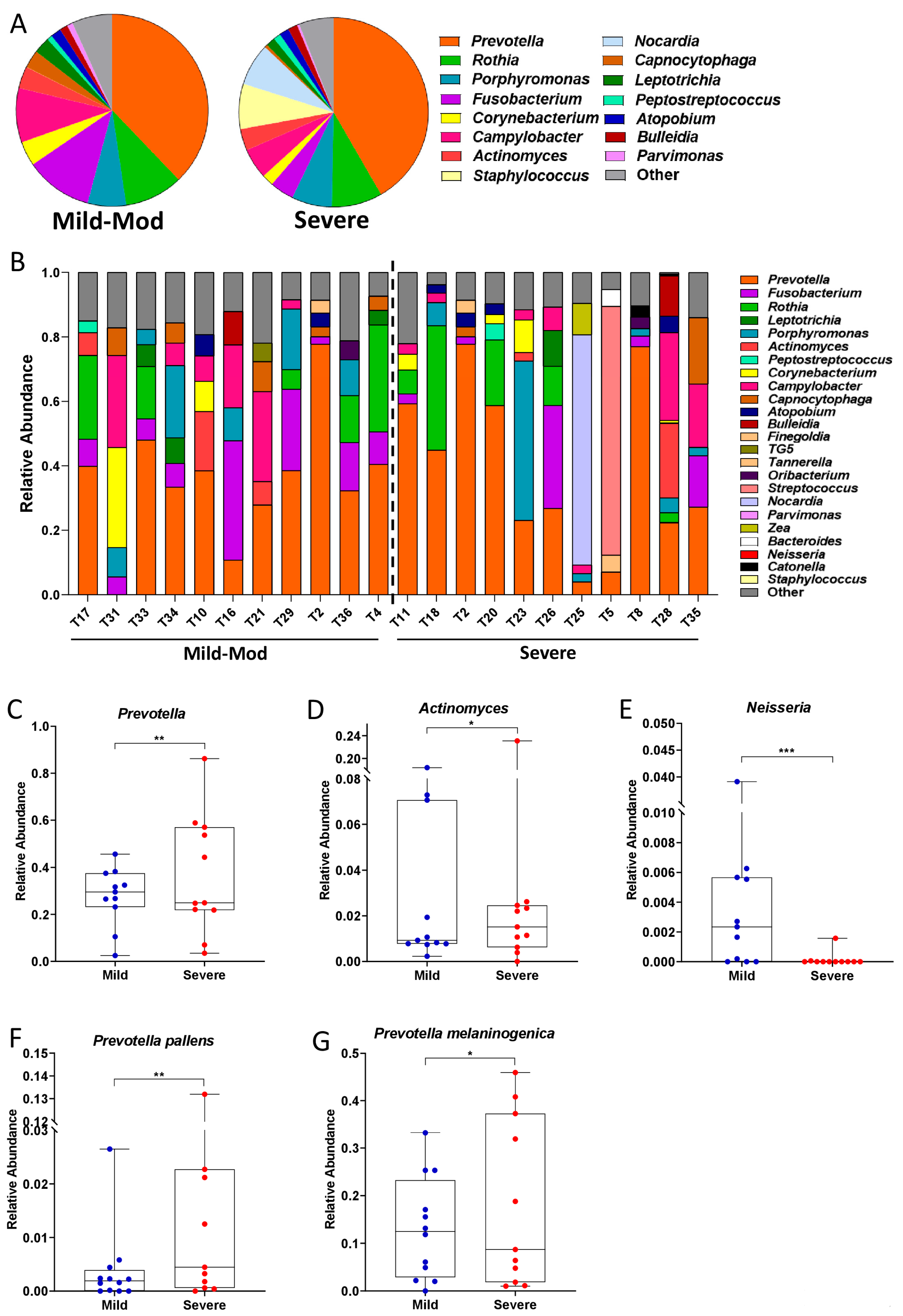
3.4. Taxonomic Shifts in the Microbiota of the Lower Airways Are Observed in Severe PCP
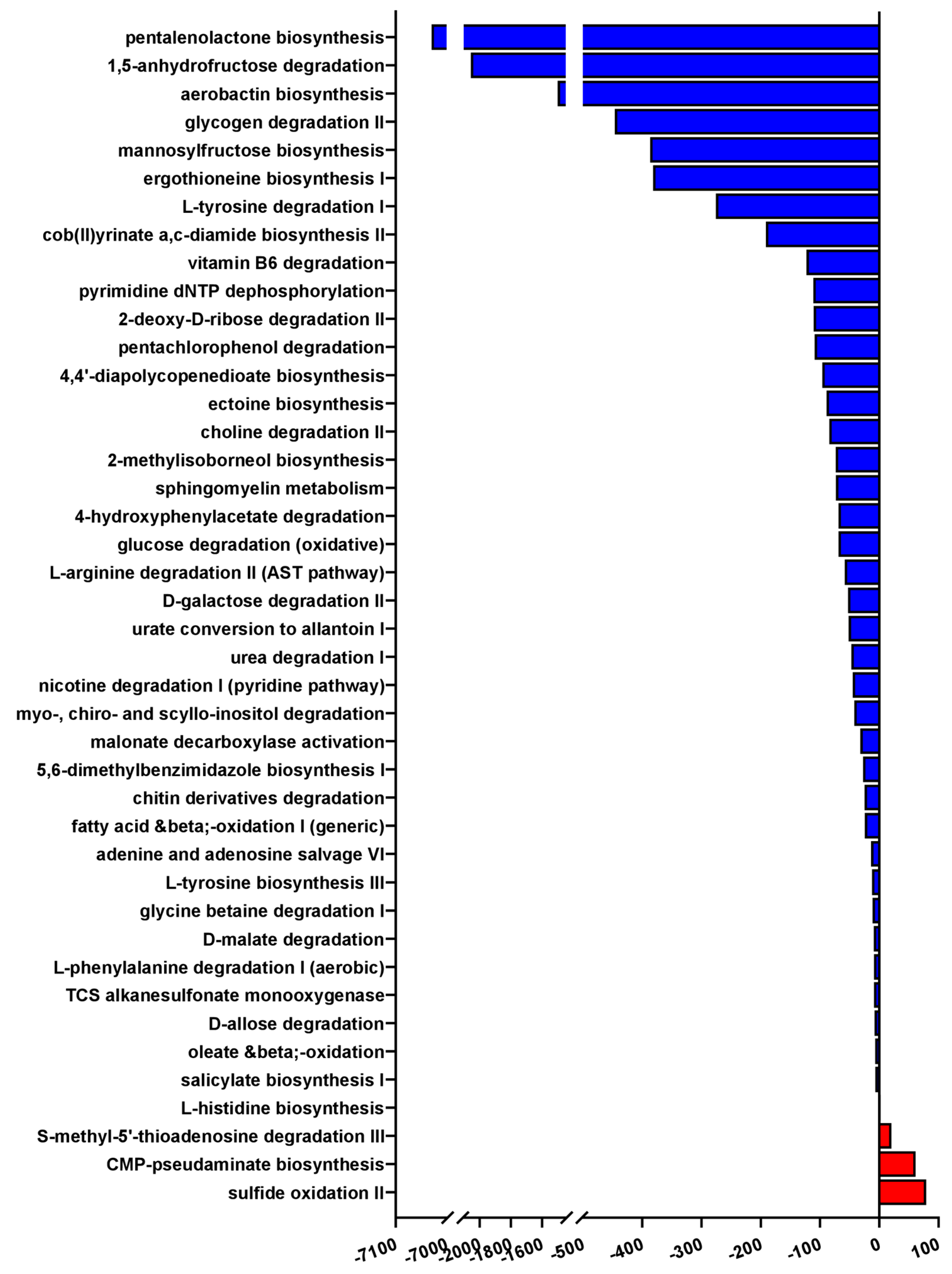
3.5. Severe PCP Is Associated with Altered Microbial Functional Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar lavage |
| GM | Gut microbiota |
| HIV | Human immunodeficiency virus |
| PCP | Pneumocystis pneumonia |
| SPT | Sputum |
References
- Catherinot, E.; Lanternier, F.; Bougnoux, M.-E.; Lecuit, M.; Couderc, L.-J.; Lortholary, O. Pneumocystis jirovecii Pneumonia. Infect. Dis. Clin. N. Am. 2010, 24, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Hatzl, S.; Posch, F.; Scholz, L.; Geiger, C.; Kriegl, L.; Kreuzer, P.; Eller, P.; Giacobbe, D.R.; Bassetti, M.; Hoenigl, M.; et al. Comparative Efficacy and Safety of Treatment Regimens for Pneumocystis Jirovecii Pneumonia in People Living with HIV—A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Cesaro, S.; Maschmeyer, G.; Einsele, H.; Donnelly, J.P.; Alanio, A.; Hauser, P.M.; Lagrou, K.; Melchers, W.J.G.; Helweg-Larsen, J.; et al. Pneumocystis jirovecii Pneumonia: Still a Concern in Patients with Haematological Malignancies and Stem Cell Transplant Recipients. J. Antimicrob. Chemother. 2016, 71, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Canet, E.; Valade, S.; Gangneux-Robert, F.; Hamane, S.; Lafabrie, A.; Maubon, D.; Debourgogne, A.; Le Gal, S.; Dalle, F.; et al. Pneumocystis jirovecii Pneumonia in Patients with or without AIDS, France. Emerg. Infect. Dis. 2014, 20, 1490–1497. [Google Scholar] [CrossRef]
- Paterno, G.; Guarnera, L.; Palmieri, R.; Del Prete, V.; Bonanni, F.; Buzzatti, E.; Moretti, F.; Casciani, P.; Savi, A.; Di Cave, D.; et al. Pneumocystis jirovecii Pneumonia in Patients with Previously Untreated Acute Myeloid Leukaemia. Mycoses 2022, 65, 233–238. [Google Scholar] [CrossRef]
- Ghembaza, A.; Vautier, M.; Cacoub, P.; Pourcher, V.; Saadoun, D. Risk Factors and Prevention of Pneumocystis jirovecii Pneumonia in Patients with Autoimmune and Inflammatory Diseases. Chest 2020, 158, 2323–2332. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, S.; Friaza, V.; Girón-Moreno, R.M.; Gallego, E.Q.; Salcedo-Posadas, A.; Figuerola-Mulet, J.; Solé-Jover, A.; Campano, E.; Morilla, R.; Calderón, E.J.; et al. Fungal Microbiota Dynamics and Its Geographic, Age and Gender Variability in Patients with Cystic Fibrosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023, 29, 539.e1–539.e7. [Google Scholar] [CrossRef]
- Gantois, N.; Lesaffre, A.; Durand-Joly, I.; Bautin, N.; Le Rouzic, O.; Nseir, S.; Reboux, G.; Scherer, E.; Aliouat, E.M.; Fry, S.; et al. Factors Associated with Pneumocystis Colonization and Circulating Genotypes in Chronic Obstructive Pulmonary Disease Patients with Acute Exacerbation or at Stable State and Their Homes. Med. Mycol. 2021, 60, myab070. [Google Scholar] [CrossRef]
- Lanternier, F.; Cypowyj, S.; Picard, C.; Bustamante, J.; Lortholary, O.; Casanova, J.-L.; Puel, A. Primary Immunodeficiencies Underlying Fungal Infections. Curr. Opin. Pediatr. 2013, 25, 736–747. [Google Scholar] [CrossRef]
- Thomas, C.F.J.; Limper, A.H. Pneumocystis Pneumonia. N. Engl. J. Med. 2004, 350, 2487–2498. [Google Scholar] [CrossRef]
- Bellamy, R.J. HIV: Treating Pneumocystis Pneumonia (PCP). BMJ Clin. Evid. 2008, 2008, 2501. [Google Scholar] [PubMed]
- Schäfer, G.; Hoffmann, C.; Arasteh, K.; Schürmann, D.; Stephan, C.; Jensen, B.; Stoll, M.; Bogner, J.R.; Faetkenheuer, G.; Rockstroh, J.; et al. Immediate versus Deferred Antiretroviral Therapy in HIV-Infected Patients Presenting with Acute AIDS-Defining Events (Toxoplasmosis, Pneumocystis jirovecii-Pneumonia): A Prospective, Randomized, Open-Label Multicenter Study (IDEAL-Study). AIDS Res. Ther. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Pates, K.; Periselneris, J.; Russell, M.D.; Mehra, V.; Schelenz, S.; Galloway, J.B. Rising Incidence of Pneumocystis Pneumonia: A Population-Level Descriptive Ecological Study in England. J. Infect. 2023, 86, 385–390. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Saimi, M.; Huang, X.; Sun, T.; Fan, G.; Zhan, Q. Risk Factors of Mortality from Pneumocystis Pneumonia in Non-HIV Patients: A Meta-Analysis. Front. Public Health 2021, 9, 680108. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-W.; Lin, F.-C.; Tsai, H.-C.; Chang, S.-C. The Impact of Concomitant Pulmonary Infection on Immune Dysregulation in Pneumocystis jirovecii Pneumonia. BMC Pulm. Med. 2014, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Blaser, M.J. A Brave New World: The Lung Microbiota in an Era of Change. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. S1), S21–S27. [Google Scholar] [CrossRef]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef]
- Kehrmann, J.; Veckollari, B.; Schmidt, D.; Schildgen, O.; Schildgen, V.; Wagner, N.; Zeschnigk, M.; Klein-Hitpass, L.; Witzke, O.; Buer, J.; et al. The Lung Microbiome in Patients with Pneumocystosis. BMC Pulm. Med. 2017, 17, 170. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung Microbiome: New Insights into the Pathogenesis of Respiratory Diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Sommariva, M.; Le Noci, V.; Bianchi, F.; Camelliti, S.; Balsari, A.; Tagliabue, E.; Sfondrini, L. The Lung Microbiota: Role in Maintaining Pulmonary Immune Homeostasis and Its Implications in Cancer Development and Therapy. Cell. Mol. Life Sci. 2020, 77, 2739–2749. [Google Scholar] [CrossRef]
- Natalini, J.G.; Singh, S.; Segal, L.N. The Dynamic Lung Microbiome in Health and Disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Marimón, J.M.; Sorarrain, A.; Ercibengoa, M.; Azcue, N.; Alonso, M.; Vidaur, L. Lung Microbiome on Admission in Critically Ill Patients with Acute Bacterial and Viral Pneumonia. Sci. Rep. 2023, 13, 17724. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Dickson, R.P. The Lung Microbiome in HIV. Getting to the HAART of the Host-Microbe Interface. Am. J. Respir. Crit. Care Med. 2016, 194, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, R.F.J.; de Brabander, J.; Boers, L.S.; Biemond, J.J.; Nossent, E.J.; Heunks, L.M.A.; Vlaar, A.P.J.; Bonta, P.I.; van der Poll, T.; Duitman, J.; et al. Lung Microbiota of Critically Ill Patients with COVID-19 Are Associated with Nonresolving Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2022, 206, 846–856. [Google Scholar] [CrossRef]
- Marsland, B.J.; Gollwitzer, E.S. Host-Microorganism Interactions in Lung Diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B.; et al. Analysis of the Lung Microbiome in the “Healthy” Smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef]
- Naidoo, C.C.; Nyawo, G.R.; Wu, B.G.; Walzl, G.; Warren, R.M.; Segal, L.N.; Theron, G. The Microbiome and Tuberculosis: State of the Art, Potential Applications, and Defining the Clinical Research Agenda. Lancet Respir. Med. 2019, 7, 892–906. [Google Scholar] [CrossRef]
- Alvarado-Peña, N.; Galeana-Cadena, D.; Gómez-García, I.A.; Mainero, X.S.; Silva-Herzog, E. The Microbiome and the Gut-Lung Axis in Tuberculosis: Interplay in the Course of Disease and Treatment. Front. Microbiol. 2023, 14, 1237998. [Google Scholar] [CrossRef]
- Guillaud-Saumur, T.; Nevez, G.; Bazire, A.; Virmaux, M.; Papon, N.; Le Gal, S. Comparison of a Commercial Real-Time PCR Assay, RealCycler® PJIR Kit, Progenie Molecular, to an in-House Real-Time PCR Assay for the Diagnosis of Pneumocystis jirovecii Infections. Diagn. Microbiol. Infect. Dis. 2017, 87, 335–337. [Google Scholar] [CrossRef]
- Totet, A.; Meliani, L.; Lacube, P.; Pautard, J.C.; Raccurt, C.; Roux, P.; Nevez, G. Immunocompetent Infants as a Human Reservoir for Pneumocystis Jirovecii: Rapid Screening by Non-Invasive Sampling and Real-Time PCR at the Mitochondrial Large Subunit RRNA Gene. J. Eukaryot. Microbiol. 2003, 50, 668–669. [Google Scholar] [CrossRef]
- Wakefield, A.E. DNA Sequences Identical to Pneumocystis Carinii f. Sp. Carinii and Pneumocystis Carinii f. Sp. Hominis in Samples of Air Spora. J. Clin. Microbiol. 1996, 34, 1754–1759. [Google Scholar] [CrossRef]
- Segal, L.N.; Alekseyenko, A.V.; Clemente, J.C.; Kulkarni, R.; Wu, B.; Gao, Z.; Chen, H.; Berger, K.I.; Goldring, R.M.; Rom, W.N.; et al. Enrichment of Lung Microbiome with Supraglottic Taxa Is Associated with Increased Pulmonary Inflammation. Microbiome 2013, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Xiu, R.; Jia, J.; Zhang, Q.; Liu, F.; Jia, Y.; Zhang, Y.; Song, B.; Liu, X.; Chen, J.; Huang, D.; et al. Three Sesquiterpene Lactones Suppress Lung Adenocarcinoma by Blocking TMEM16A-Mediated Ca2+-Activated Cl− Channels. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2023, 27, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Conroy, L.R.; Clarke, H.A.; Allison, D.B.; Valenca, S.S.; Sun, Q.; Hawkinson, T.R.; Young, L.E.A.; Ferreira, J.E.; Hammonds, A.V.; Dunne, J.B.; et al. Spatial Metabolomics Reveals Glycogen as an Actionable Target for Pulmonary Fibrosis. Nat. Commun. 2023, 14, 2759. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Kawahara, K.; Matsushita, K.; Nawa, Y.; Shrestha, B.; Kikuchi, K.; Sameshima, H.; Hashiguchi, T.; Maruyama, I. Attenuation of LPS-Induced INOS Expression by 1,5-Anhydro-d-Fructose. Biochem. Biophys. Res. Commun. 2009, 387, 42–46. [Google Scholar] [CrossRef]
- Yu, S.; Bojsen, K.; Svensson, B.; Marcussen, J. Alpha-1,4-Glucan Lyases Producing 1,5-Anhydro-D-Fructose from Starch and Glycogen Have Sequence Similarity to Alpha-Glucosidases. Biochim. Biophys. Acta 1999, 1433, 1–15. [Google Scholar] [CrossRef]
- Lian, Q.; Song, X.; Yang, J.; Wang, L.; Xu, P.; Wang, X.; Xu, X.; Yang, B.; He, J.; Ju, C. Alterations of Lung Microbiota in Lung Transplant Recipients with Pneumocystis jirovecii Pneumonia. Respir. Res. 2024, 25, 125. [Google Scholar] [CrossRef]
- Boutin, S.; Graeber, S.Y.; Stahl, M.; Dittrich, A.S.; Mall, M.A.; Dalpke, A.H. Chronic but Not Intermittent Infection with Pseudomonas Aeruginosa Is Associated with Global Changes of the Lung Microbiome in Cystic Fibrosis. Eur. Respir. J. 2017, 50, 701086. [Google Scholar] [CrossRef]
- Li, L.; Mac Aogáin, M.; Xu, T.; Jaggi, T.K.; Chan, L.L.Y.; Qu, J.; Wei, L.; Liao, S.; Cheng, H.S.; Keir, H.R.; et al. Neisseria Species as Pathobionts in Bronchiectasis. Cell Host Microbe 2022, 30, 1311–1327.e8. [Google Scholar] [CrossRef]
- Iaffaldano, L.; Granata, I.; Pagliuca, C.; Esposito, M.V.; Casaburi, G.; Salerno, G.; Colicchio, R.; Piccirillo, M.; Ciacci, C.; Del Vecchio Blanco, G.; et al. Oropharyngeal Microbiome Evaluation Highlights Neisseria Abundance in Active Celiac Patients. Sci. Rep. 2018, 8, 11047. [Google Scholar] [CrossRef]
- Mathilde, A.; Catherine, R.-M.; Jean-Philippe, B.; Benoit, G.; Bruno, S.; Alain, S.; Sophia, S.-H.; Renaud, L.; Xavier, N.; Mathieu, C. Airway Mucus Restricts Neisseria Meningitidis Away from Nasopharyngeal Epithelial Cells and Protects the Mucosa from Inflammation. mSphere 2019, 4, 10-1128. [Google Scholar] [CrossRef]
- Kidd, S.E.; Chen, S.C.-A.; Meyer, W.; Halliday, C.L. A New Age in Molecular Diagnostics for Invasive Fungal Disease: Are We Ready? Front. Microbiol. 2020, 10, 2903. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review Article: The Future of Microbiome-Based Therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef]
| Clinical Variables | BAL | SPT |
|---|---|---|
| Number of Subjects | 24 | 9 |
| Male | 18 | 5 |
| Female | 6 | 4 |
| Median Age | 60.5 | 69 |
| Underlying Pathology | ||
| COPD | 2 | 1 |
| Cancer | 9 | 4 |
| HIV | 5 | 2 |
| Transplant Recipient | 1 | 1 |
| Other/no known pathology | 7 | 1 |
| Immunosuppressive Therapy | 16 | 6 |
| Immunodeficiency Status | ||
| Mild Neutropenia | 1 | 1 |
| Moderate Neutropenia | 1 | 0 |
| Severe Neutropenia | 2 | 0 |
| Lymphopenia | 9 | 6 |
| Pulmonary Co-infections | ||
| Bacterial | 13 | 7 |
| Fungal | 9 | 1 |
| SARS-CoV 2 | 2 | 1 |
| Cytomegalovirus | 1 | 0 |
| None | 6 | 1 |
| Clinical Variables | Mild-Moderate | Severe |
|---|---|---|
| Number of Subjects | 12 | 12 |
| Male | 9 | 9 |
| Female | 3 | 3 |
| Median Age | 58 | 63 |
| Underlying Pathology | ||
| COPD | 1 | 1 |
| Cancer | 1 | 8 |
| HIV | 4 | 1 |
| Transplant Recipient | 1 | 0 |
| Other/no known pathology | 6 | 1 |
| Immunosuppressive Therapy | 8 | 8 |
| Immunodeficiency Status | ||
| Mild Neutropenia | 1 | 0 |
| Moderate Neutropenia | 0 | 1 |
| Severe Neutropenia | 0 | 2 |
| Lymphopenia | 5 | 4 |
| Pulmonary Co-infections | ||
| Bacterial | 6 | 7 |
| Fungal | 3 | 6 |
| SARS-CoV 2 | 1 | 1 |
| Cytomegalovirus | 0 | 1 |
| None | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prete, V.; Piazzesi, A.; Scanu, M.; Toto, F.; Pane, S.; Berrilli, F.; Paterno, G.; Putignani, L.; di Cave, D. Pneumocystis Pneumonia Severity Is Associated with Taxonomic Shifts in the Respiratory Microbiota. Pathogens 2025, 14, 82. https://doi.org/10.3390/pathogens14010082
Del Prete V, Piazzesi A, Scanu M, Toto F, Pane S, Berrilli F, Paterno G, Putignani L, di Cave D. Pneumocystis Pneumonia Severity Is Associated with Taxonomic Shifts in the Respiratory Microbiota. Pathogens. 2025; 14(1):82. https://doi.org/10.3390/pathogens14010082
Chicago/Turabian StyleDel Prete, Valentina, Antonia Piazzesi, Matteo Scanu, Francesca Toto, Stefania Pane, Federica Berrilli, Giovangiacinto Paterno, Lorenza Putignani, and David di Cave. 2025. "Pneumocystis Pneumonia Severity Is Associated with Taxonomic Shifts in the Respiratory Microbiota" Pathogens 14, no. 1: 82. https://doi.org/10.3390/pathogens14010082
APA StyleDel Prete, V., Piazzesi, A., Scanu, M., Toto, F., Pane, S., Berrilli, F., Paterno, G., Putignani, L., & di Cave, D. (2025). Pneumocystis Pneumonia Severity Is Associated with Taxonomic Shifts in the Respiratory Microbiota. Pathogens, 14(1), 82. https://doi.org/10.3390/pathogens14010082







