Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development
Abstract
1. Introduction
2. Early Attempts and Setbacks in the Development of Pediatric RSV Vaccine
3. Current FDA-Approved RSV Passive Immunization for Children
3.1. Palivizumab
3.2. Nirsevimab
3.3. RSVpreF Vaccine (For Pregnant Women)
4. RSV Passive Immunization Under Development
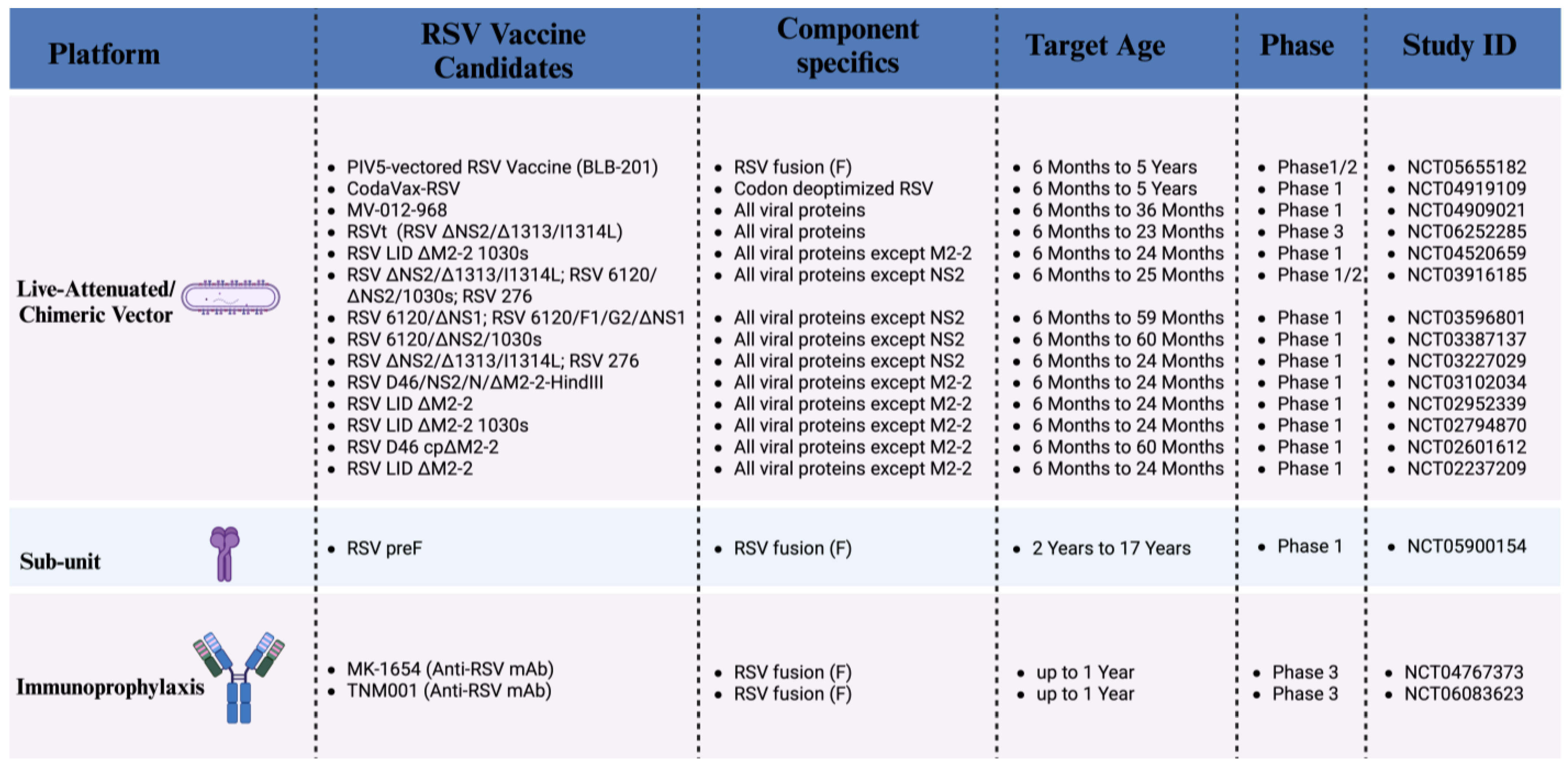
5. RSV Pediatric Vaccines Currently in Development (Phase 1, 2 Clinical Trials)
5.1. Live Attenuated Vaccines
5.2. Recombinant Vector-Based Vaccines
5.3. Protein-Based Vaccines
5.4. Nucleic Acid-Based Vaccines
6. Advanced-Stage RSV Pediatric Vaccine Candidate (Phase 3 Clinical Trial)
7. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Soto, J.A.; Stranger, V.; Rivera, T.; Vásquez, A.E.; Kalergis, A.M. Host Components That Modulate the Disease Caused by hMPV. Viruses 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. The Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, F.; Campanozzi, A.; Maffei, G.; Arena, F.; Carri, V.D.; Rollo, T.; Lopalco, P.L.; Martinelli, D. Respiratory syncytial virus-associated hospitalizations among children: An Italian retrospective observational study. Ital. J. Pediatr. 2024, 50, 45. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. The Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Boukhvalova, M.S.; Shirey, K.A.; Prince, G.A.; Vogel, S.N. New insights for development of a safe and protective RSV vaccine. Human. Vaccines 2010, 6, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Piedimonte, G. An overview on the RSV-mediated mechanisms in the onset of non-allergic asthma. Front. Pediatr. 2022, 10, 998296. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.K.; Kim, J.H.; Han, B.; Shin, J.; Lee, E.; Lee, K.J.; Shin, Y.H.; Han, M.Y. Viral respiratory infections requiring hospitalization in early childhood related to subsequent asthma onset and exacerbation risks. J. Med. Virol. 2024, 96, e29876. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Van-Tam, J.S.; O’Leary, M.; Martin, E.T.; Heijnen, E.; Callendret, B.; Fleischhackl, R.; Comeaux, C.; Tran, T.M.P.; Weber, K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022, 31, 220105. [Google Scholar] [CrossRef] [PubMed]
- Acosta, P.L.; Caballero, M.T.; Polack, F.P. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin. Vaccine Immunol. 2016, 23, 189–195. [Google Scholar] [CrossRef]
- Halasa, N.; Zambrano, L.D.; Amarin, J.Z.; Stewart, L.S.; Newhams, M.M.; Levy, E.R.; Shein, S.L.; Carroll, C.L.; Fitzgerald, J.C.; Michaels, M.G.; et al. Infants Admitted to US Intensive Care Units for RSV Infection During the 2022 Seasonal Peak. JAMA Netw. Open 2023, 6, e2328950. [Google Scholar] [CrossRef]
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, H.C.; Tripp, R.A. RSV Replication, Transmission, and Disease Are Influenced by the RSV G Protein. Viruses 2022, 14, 2396. [Google Scholar] [CrossRef] [PubMed]
- Sutto-Ortiz, P.; Eléouët, J.-F.; Ferron, F.; Decroly, E. Biochemistry of the Respiratory Syncytial Virus L Protein Embedding RNA Polymerase and Capping Activities. Viruses 2023, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Rosenberg, H.F. Respiratory Syncytial Virus Infection: Immune Response, Immunopathogenesis, and Treatment. Clin. Microbiol. Rev. 1999, 12, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Karron, R.A. Respiratory Syncytial Virus and Metapneumovirus. In Fields Virolgy, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2013; Volume 1, pp. 1086–1118. [Google Scholar]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Kapoor, R.; Saharan, A.; Gautam, R.K. Targeting Molecular and Cellular Mechanisms in Respiratory Syncytial Virus (RSV) Infection; Springer: Singapore, 2021; pp. 501–516. [Google Scholar]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Churiso, G.; Husen, G.; Bulbula, D.; Abebe, L. Immunity Cell Responses to RSV and the Role of Antiviral Inhibitors: A Systematic Review. Infect. Drug Resist. 2022, 15, 7413–7430. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Woods, S.; Oliva, J.; Collins, P.L.; Buchholz, U.J. Evaluation of the Live-Attenuated Intranasal Respiratory Syncytial Virus (RSV) Vaccine RSV/6120/ΔNS2/1030s in RSV-Seronegative Young Children. J. Infect. Dis. 2024, 229, 346–354. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor. Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Petherbridge, L.; Davis, C.; Robinson, A.; Evans, T.; Sebastian, S. Pre-Clinical Development of an Adenovirus Vector Based RSV and Shingles Vaccine Candidate. Vaccines 2023, 11, 1679. [Google Scholar] [CrossRef]
- Shaw, C.A.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; El Asmar, L.; Schnyder-Ghamloush, S.; Schaefers, K.; August, A.; Stoszek, S.K.; et al. Safety, Tolerability and Immunogenicity of a mRNA-based RSV Vaccine in Healthy Young Adults in a Phase 1 Clinical Trial. J. Infect. Dis. 2024, 230, e637–e646. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine12. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Polack, F.P.; Alvarez-Paggi, D.; Libster, R.; Caballero, M.T.; Blair, R.V.; Hijano, D.R.; De La Iglesia Niveyro, P.X.; Menendez, D.R.; Gladwell, W.; Avendano, L.M.; et al. Fatal enhanced respiratory syncytial virus disease in toddlers. Sci. Transl. Med. 2021, 13, eabj7843. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ato, M. A critical role of Gas6/Axl signal in allergic airway responses during RSV vaccine-enhanced disease. Immunol. Cell Biol. 2017, 95, 906–915. [Google Scholar] [CrossRef]
- Moghaddam, A.; Olszewska, W.; Wang, B.; Tregoning, J.S.; Helson, R.; Sattentau, Q.J.; Openshaw, P.J.M. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006, 12, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.-Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S. The Journey to RSV Vaccines—Heralding an Era of Structure-Based Design. N. Engl. J. Med. 2023, 388, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, K.M.; Kosanovich, J.L.; Lipp, M.; Empey, K.M.; Petrovsky, N. Strategies for active and passive pediatric RSV immunization. Ther. Adv. Vaccines Immunother. 2021, 9, 251513552098151. [Google Scholar] [CrossRef] [PubMed]
- Sande, C.J.; Cane, P.A.; Nokes, D.J. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 2014, 32, 4726–4729. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Chirkova, T.; Anderson, L.J. Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development. Front. Immunol. 2019, 10, 1675. [Google Scholar] [CrossRef]
- Bolt, G.; Pedersen, L.O.; Birkeslund, H.H. Cleavage of the respiratory syncytial virus fusion protein is required for its surface expression: Role of furin. Virus Res. 2000, 68, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Day, N.D.; Branigan, P.J.; Liu, C.; Gutshall, L.L.; Luo, J.; Melero, J.A.; Sarisky, R.T.; Del Vecchio, A.M. Contribution of cysteine residues in the extracellular domain of the F protein of human respiratory syncytial virus to its function. Virol. J. 2006, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Schaerlaekens, S.; Jacobs, L.; Stobbelaar, K.; Cos, P.; Delputte, P. All Eyes on the Prefusion-Stabilized F Construct, but Are We Missing the Potential of Alternative Targets for Respiratory Syncytial Virus Vaccine Design? Vaccines 2024, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Sevendal, A.T.K.; Hurley, S.; Bartlett, A.W.; Rawlinson, W.; Walker, G.J. Systematic Review of the Efficacy and Safety of RSV-Specific Monoclonal Antibodies and Antivirals in Development. Rev. Med. Virol. 2024, 34, e2576. [Google Scholar] [CrossRef]
- Joyce, M.G.; Zhang, B.; Ou, L.; Chen, M.; Chuang, G.Y.; Druz, A.; Kong, W.P.; Lai, Y.T.; Rundlet, E.J.; Tsybovsky, Y.; et al. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat. Struct. Mol. Biol. 2016, 23, 811–820. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Yang, Y.; Graham, B.S.; Kwong, P.D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 2011, 85, 7788–7796. [Google Scholar] [CrossRef] [PubMed]
- López, J.A.; Andreu, D.; Carreño, C.; Whyte, P.; Taylor, G.; Melero, J.A. Conformational constraints of conserved neutralizing epitopes from a major antigenic area of human respiratory syncytial virus fusion glycoprotein. J. Gen. Virol. 1993, 74 Pt 12, 2567–2577. [Google Scholar] [CrossRef]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef]
- Resch, B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum. Vaccin. Immunother. 2017, 13, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.C.J. Nirsevimab: Review of pharmacology, antiviral activity and emerging clinical experience for respiratory syncytial virus infection in infants. J. Antimicrob. Chemother. 2023, 78, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Nirsevimab: First Approval. Drugs 2023, 83, 181–187. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Bollani, L.; Baraldi, E.; Chirico, G.; Dotta, A.; Lanari, M.; Del Vecchio, A.; Manzoni, P.; Boldrini, A.; Paolillo, P.; Di Fabio, S.; et al. Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV). Ital. J. Pediatr. 2015, 41, 97. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.A.; Hoet, B.; Hochrein, H.; De Moerlooze, L. The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines 2023, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Garegnani, L.; Styrmisdóttir, L.; Roson Rodriguez, P.; Escobar Liquitay, C.M.; Esteban, I.; Franco, J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst. Rev. 2021, 11, Cd013757. [Google Scholar]
- Blanken, M.O.; Rovers, M.M.; Molenaar, J.M.; Winkler-Seinstra, P.L.; Meijer, A.; Kimpen, J.L.L.; Bont, L. Respiratory Syncytial Virus and Recurrent Wheeze in Healthy Preterm Infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Pedraz, C.; Carbonell-Estrany, X.; Figueras-Aloy, J.; Quero, J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr. Infect. Dis. J. 2003, 22, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Gouyon, B.; Sagot, P.; Quantin, C.; Huet, F.; Gouyon, J.B. Palivizumab efficacy in preterm infants with gestational age ≤30 weeks without bronchopulmonary dysplasia. Pediatr. Pulmonol. 2007, 42, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.Q.; Oduoye, M.O. Revitalizing hope for older adults: The use of the novel Arexvy for immunization against respiratory syncytial virus. Health Sci. Rep. 2023, 6, e1648. [Google Scholar] [CrossRef] [PubMed]
- Rogovik, A.L.; Carleton, B.; Solimano, A.; Goldman, R.D. Palivizumab for the prevention of respiratory syncytial virus infection. Can. Fam. Physician 2010, 56, 769–772. [Google Scholar] [PubMed]
- Mazur, N.I.; Löwensteyn, Y.N.; Terstappen, J.; Leusen, J.; Schobben, F.; Cianci, D.; van de Ven, P.M.; Nierkens, S.; Bont, L.J. Daily intranasal palivizumab to prevent respiratory syncytial virus infection in healthy preterm infants: A phase 1/2b randomized placebo-controlled trial. EClinicalMedicine 2023, 66, 102324. [Google Scholar] [CrossRef]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Esposito, S.; Abu-Raya, B.; Bonanni, P.; Cahn-Sellem, F.; Flanagan, K.L.; Martinon Torres, F.; Mejias, A.; Nadel, S.; Safadi, M.A.P.; Simon, A. Coadministration of Anti-Viral Monoclonal Antibodies with Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper. Front. Immunol. 2021, 12, 708939. [Google Scholar] [CrossRef]
- Fly, J.H.; Eiland, L.S.; Stultz, J.S. Nirsevimab: Expansion of Respiratory Syncytial Virus Prevention Options in Neonates, Infants, and At-Risk Young Children. Ann. Pharmacother. 2025, 59, 81–91. [Google Scholar] [CrossRef]
- Brady, T.; Cayatte, C.; Roe, T.L.; Speer, S.D.; Ji, H.; Machiesky, L.; Zhang, T.; Wilkins, D.; Tuffy, K.M.; Kelly, E.J. Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection. Front. Immunol. 2023, 14, 1283120. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Wählby Hamren, U.; Basavaraju, B.; Koen, A.; Leach, A.; Mankad, V.S.; Mori, M.; Ndibmun, C.; Soler-Palacin, P.; Pannaraj, P.S.; et al. Safety, Tolerability, and Pharmacokinetics of Nirsevimab for the Prevention of RSV Disease in Immunocompromised Children Aged ≤24 Months: Music, an Open Label, Phase 2 Trial. Blood 2023, 142, 1173. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, D.; Brust-Sisti, L.A.; Bridgeman, M.; Bridgeman, M.B. Vaccines for Respiratory Syncytial Virus Prevention in Older Adults. Ann. Pharmacother. 2024, 58, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Schmoele-Thoma, B.; Zareba, A.M.; Jiang, Q.; Maddur, M.S.; Danaf, R.; Mann, A.; Eze, K.; Fok-Seang, J.; Kabir, G.; Catchpole, A.; et al. Vaccine Efficacy in Adults in a Respiratory Syncytial Virus Challenge Study. N. Engl. J. Med. 2022, 386, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants. Pediatric Drugs 2023, 25, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Wolford, D.; Caro, L.; Maas, B.M.; Ma, H.; Montgomery, D.L.; Sterling, L.M.; Hunt, A.; Cox, K.S.; Vora, K.A.; et al. A Phase 1 Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety, Tolerability, and Pharmacokinetics of a Respiratory Syncytial Virus Neutralizing Monoclonal Antibody MK-1654 in Healthy Adults. Clin. Pharmacol. Drug Dev. 2021, 10, 556–566. [Google Scholar] [CrossRef]
- Phuah, J.Y.; Maas, B.M.; Tang, A.; Zhang, Y.; Caro, L.; Railkar, R.A.; Swanson, M.D.; Cao, Y.; Li, H.; Roadcap, B.; et al. Quantification of clesrovimab, an investigational, half-life extended, anti-respiratory syncytial virus protein F human monoclonal antibody in the nasal epithelial lining fluid of healthy adults. Biomed. Pharmacother. 2023, 169, 115851. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, P.; van der Plas, J.L.; van Brummelen, E.M.J.; Jeeninga, R.E.; de Haan, C.A.M.; Roestenberg, M.; Burggraaf, J.; Kamerling, I.M.C. First-in-human administration of a live-attenuated RSV vaccine lacking the G-protein assessing safety, tolerability, shedding and immunogenicity: A randomized controlled trial. Vaccine 2020, 38, 6088–6095. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E.J.; Karron, R.A.; Muresan, P.; Cunningham, C.K.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; Schappell, E.; et al. Live-Attenuated Respiratory Syncytial Virus Vaccine With M2-2 Deletion and With Small Hydrophobic Noncoding Region Is Highly Immunogenic in Children. J. Infect. Dis. 2020, 221, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Billard, M.N.; Bont, L.J. Live-attenuated Respiratory Syncytial Virus Vaccines: Time for the Next Step. Am. J. Respir. Crit. Care Med. 2021, 203, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Luongo, C.; Mateo, J.S.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Safety and Immunogenicity of the Respiratory Syncytial Virus Vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-Seronegative Children. J. Infect. Dis. 2020, 222, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Alamares-Sapuay, J.; Kishko, M.; Lai, C.; Parrington, M.; Delagrave, S.; Herbert, R.; Castens, A.; Swerczek, J.; Luongo, C.; Yang, L.; et al. Mutations in the F protein of the live-attenuated respiratory syncytial virus vaccine candidate ΔNS2/Δ1313/I1314L increase the stability of infectivity and content of prefusion F protein. PLoS ONE 2024, 19, e0301773. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E.J.; Karron, R.A.; Muresan, P.; Cunningham, C.K.; Valentine, M.E.; Perlowski, C.; Thumar, B.; Gnanashanmugam, D.; Siberry, G.K.; Schappell, E.; et al. Live-Attenuated Respiratory Syncytial Virus Vaccine Candidate With Deletion of RNA Synthesis Regulatory Protein M2-2 is Highly Immunogenic in Children. J. Infect. Dis. 2018, 217, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, A.; Collins, P.L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 1999, 96, 11259–11264. [Google Scholar] [CrossRef] [PubMed]
- Ghildyal, R.; Ho, A.; Jans, D.A. Central role of the respiratory syncytial virus matrix protein in infection. FEMS Microbiol. Rev. 2006, 30, 692–705. [Google Scholar] [CrossRef]
- McFarland, E.J.; Karron, R.A.; Muresan, P.; Cunningham, C.K.; Libous, J.; Perlowski, C.; Thumar, B.; Gnanashanmugam, D.; Moye, J.; Schappell, E.; et al. Live Respiratory Syncytial Virus Attenuated by M2-2 Deletion and Stabilized Temperature Sensitivity Mutation 1030s Is a Promising Vaccine Candidate in Children. J. Infect. Dis. 2020, 221, 534–543. [Google Scholar] [CrossRef]
- Stobart, C.C.; Rostad, C.A.; Ke, Z.; Dillard, R.S.; Hampton, C.M.; Strauss, J.D.; Yi, H.; Hotard, A.L.; Meng, J.; Pickles, R.J.; et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat. Commun. 2016, 7, 13916. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Cizmeci, D.; Yuan, D.; Mehta, N.; Tolboom, J.; De Paepe, E.; van Heesbeen, R.; Sadoff, J.; Comeaux, C.A.; Heijnen, E.; et al. Vaccine-induced antibody Fc-effector functions in humans immunized with a combination Ad26.RSV.preF/RSV preF protein vaccine. J. Virol. 2023, 97, e0077123. [Google Scholar] [CrossRef] [PubMed]
- Awar, M.; Mylonakis, E. In older adults, an Ad26.RSV.preF-RSV preF protein vaccine reduced RSV-related lower respiratory tract disease. Ann. Intern. Med. 2023, 176, Jc63. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, C.A.; Bart, S.; Bastian, A.R.; Klyashtornyy, V.; De Paepe, E.; Omoruyi, E.; Van Der Fits, L.; Van Heesbeen, R.; Heijnen, E.; Callendret, B.; et al. Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.preF–Based Vaccine Combinations: A Randomized, Double-blind, Placebo-Controlled, Phase 1/2a Study. J. Infect. Dis. 2024, 229, 19–29. [Google Scholar] [CrossRef]
- Widagdo, W.; Bastian, A.R.; Jastorff, A.M.; Scheys, I.; De Paepe, E.; Comeaux, C.A.; Ligtenberg, N.; Callendret, B.; Heijnen, E. Concomitant Administration of Ad26.RSV.preF/RSV preF Protein Vaccine and High-Dose Influenza Vaccine in Adults 65 Years and Older: A Noninferiority Trial. J. Infect. Dis. 2024, 230, e374–e383. [Google Scholar] [CrossRef]
- Hosman, T.; van Heesbeen, R.; Bastian, A.R.; Hu, W.; Comeaux, C.; Ligtenberg, N.; van Montfort, B.; Callendret, B.; Heijnen, E. Immunogenicity and safety of Ad26.RSV.preF/RSV preF protein vaccine at predicted intermediate- and end-of-shelf-life as an evaluation of potency throughout shelf life. Hum. Vaccin. Immunother. 2024, 20, 2344970. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hosman, T.; Bastian, A.R.; Vandenberghe, S.; Chan, E.K.H.; Douoguih, M.; Heijnen, E.; Comeaux, C.A.; Callendret, B. Long-term efficacy and immunogenicity of Ad26.RSV.preF-RSV preF protein vaccine (CYPRESS): A randomised, double-blind, placebo-controlled, phase 2b study. Lancet Infect. Dis. 2024, 24, 1015–1024. [Google Scholar] [CrossRef]
- Eto, T.; Okubo, Y.; Momose, A.; Tamura, H.; Zheng, R.; Callendret, B.; Bastian, A.R.; Comeaux, C.A. A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of Single Vaccination of Ad26.RSV.preF-Based Regimen in Japanese Adults Aged 60 Years and Older. Influenza Other Respir. Viruses 2024, 18, e13336. [Google Scholar] [CrossRef]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal parainfluenza virus type 5 (PIV5)-vectored RSV vaccine is safe and immunogenic in healthy adults in a phase 1 clinical study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2024, 78, 1732–1744. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Srikantiah, P. Respiratory syncytial virus: Promising progress against a leading cause of pneumonia. Lancet Glob. Health 2021, 9, e1644–e1645. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Nolan, T.M.; Rämet, M.; Richmond, P.C.; Rosário Filho, N.; Haazen, W.; van den Berg, S.P.H.; Williams, K.; Bastian, A.R.; Omoruyi, E.; et al. A Phase 1/2a Study Evaluating Safety and Immunogenicity of Ad26.RSV.preF in RSV-seronegative Toddlers Aged 12-24 Months. Open Forum Infect. Dis. 2024, 11, ofae453. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Tran, K.C.; Teng, M.N. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 2009, 83, 3734–3742. [Google Scholar] [CrossRef] [PubMed]
- Sedeyn, K.; Schepens, B.; Saelens, X. Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses. PLoS Pathog. 2019, 15, e1007984. [Google Scholar] [CrossRef] [PubMed]
- Liesman, R.M.; Buchholz, U.J.; Luongo, C.L.; Yang, L.; Proia, A.D.; DeVincenzo, J.P.; Collins, P.L.; Pickles, R.J. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J. Clin. Investig. 2014, 124, 2219–2233. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Winter, C.C.; Collins, P.L.; Buchholz, U.J. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J. Virol. 2013, 87, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Atwell, J.E.; McFarland, E.J.; Cunningham, C.K.; Muresan, P.; Perlowski, C.; Libous, J.; Spector, S.A.; Yogev, R.; Aziz, M.; et al. Live-attenuated Vaccines Prevent Respiratory Syncytial Virus-associated Illness in Young Children. Am. J. Respir. Crit. Care Med. 2021, 203, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Papayanni, P.G.; Koukoulias, K.; Kuvalekar, M.; Watanabe, A.; Velazquez, Y.; Ramos, C.A.; Leen, A.M.; Vasileiou, S. T cell immune profiling of respiratory syncytial virus for the development of a targeted immunotherapy. Br. J. Haematol. 2023, 202, 874–878. [Google Scholar] [CrossRef] [PubMed]
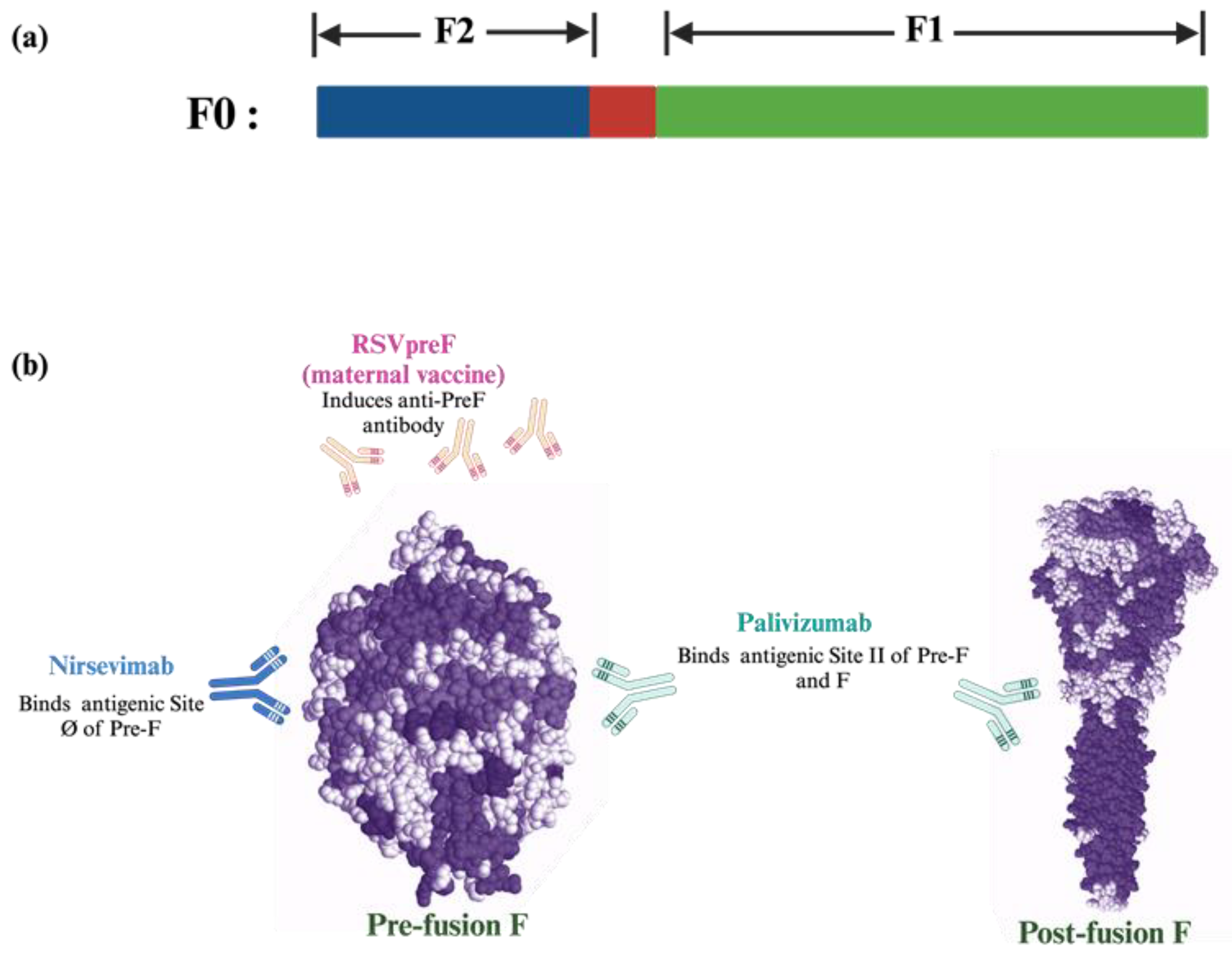
| Immunization | Target Population | Mean Half Life | Duration of Protection | Target Protein | Ref. |
|---|---|---|---|---|---|
| Palivizumab | High risk and Preterm infants (born <36 weeks of gestation) | ~20 days | ~30 days | Antigenic site II of the F protein | [42,43,44] |
| Nirsevimab | 8-month-old infants (born during RSV season or entering their first RSV season). | 68.7 ± 10.9 days | ~150 days | Site Ø on the RSV pre-F protein and neonatal Fc receptor (FcRn) | [45,46,47] |
| RSV PreF vaccine | Pregnant women between 32 and 36 weeks of pregnancy | Up to 6 months after birth | RSV prefusion F protein | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babawale, P.I.; Martínez-Espinoza, I.; Mitchell, A.M.; Guerrero-Plata, A. Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development. Pathogens 2025, 14, 104. https://doi.org/10.3390/pathogens14020104
Babawale PI, Martínez-Espinoza I, Mitchell AM, Guerrero-Plata A. Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development. Pathogens. 2025; 14(2):104. https://doi.org/10.3390/pathogens14020104
Chicago/Turabian StyleBabawale, Pius I., Iván Martínez-Espinoza, Alaine’ M. Mitchell, and Antonieta Guerrero-Plata. 2025. "Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development" Pathogens 14, no. 2: 104. https://doi.org/10.3390/pathogens14020104
APA StyleBabawale, P. I., Martínez-Espinoza, I., Mitchell, A. M., & Guerrero-Plata, A. (2025). Preventing RSV Infection in Children: Current Passive Immunizations and Vaccine Development. Pathogens, 14(2), 104. https://doi.org/10.3390/pathogens14020104





