Utility of 28S Ribosomal RNA Gene Domains for Molecular Classification and Phylogeny of Rhinonyssid Mites
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Species Identification
2.3. Molecular Study
2.4. Sequence Alignments and Phylogenetic Analysis
3. Results
3.1. Species Identification
3.2. Molecular Analysis
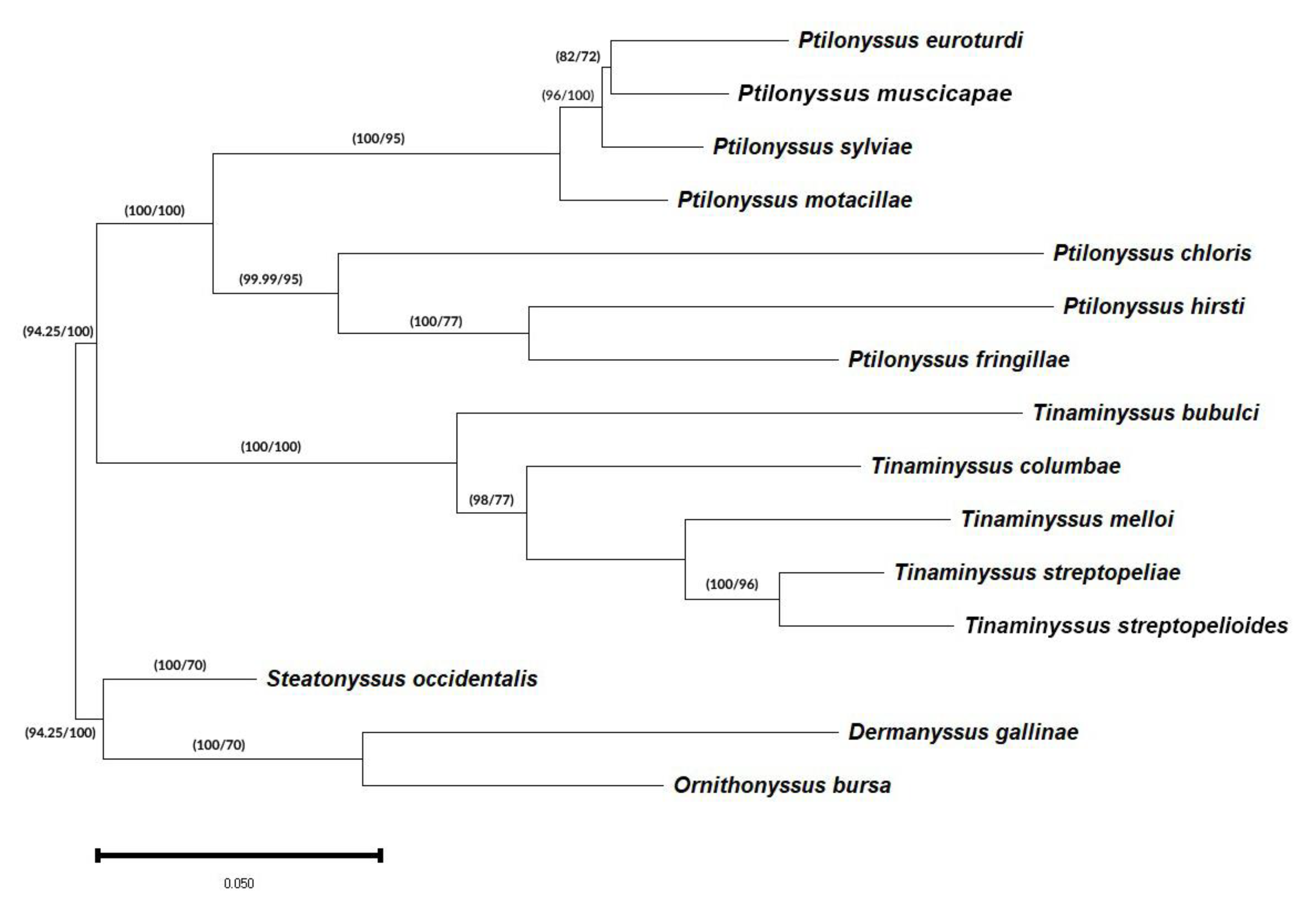
3.3. Phylogenetic and Genetic Distance Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimov, I. Rhinonyssidosis avium. Vetpharma 2011, 3, 88–90. [Google Scholar]
- Fain, A. Essai de clasiffication des Rhinonyssidae (Acari: Mesostigmata) avec description de deux genres nouveaux. Ann. Parasitol. Hum. Comp. 1957, 31, 145–157. [Google Scholar]
- Fain, A. Comparative Morphology of Rhinonyssidae. Bull. Ann. Soc. Roy. Ent. Belg. 1960, 96, 303–313. [Google Scholar]
- Strandtmann, R.W. The mesostigmatic nasal mites of birds. II. New and poorly known species of Rhinonyssidae. J. Parasitol. 1951, 37, 129. [Google Scholar] [CrossRef] [PubMed]
- Feider, Z.; Mironescu, I. Deux Rhinonyssidaes parasites sur Fulica atra de Roumanie. Ana. Sti. Univ. Lasi. 1973, 19, 159–169. [Google Scholar]
- Pence, D.B. Keys, species and host list, and bibliography for nasal mites of North American birds (Acarina: Rhinonyssinae, Turbinoptinae, Speleognathinae, and Cytoditidae). Spec. Publ. Mus. Tex. Tech Univ. 1975, 8, 1–148. [Google Scholar]
- Fain, A. Les acariens parasites nasicoles des oiseaux de Belgique. I. Deux especes nouvelles de Rhinonyssidae (Mesostigmata) avec une liste des especes connues de Belgique. Bull. Ann. Soc. R. Belge Ent. 1962, 98, 252–270. [Google Scholar]
- Sánchez-Carrión, S.A.; Dimov, I.; Márquez Jiménez, F.J.; de Rojas Álvarez, M. Morphometrical Identification and Phylogenetic Analysis of Rhinonyssidae (Acari: Mesostigmata) Parasitizing Avian Hosts: New Molecular Data. Microorganisms 2023, 11, 1783. [Google Scholar] [CrossRef]
- Domrow, R. The Nasal Mites of Queensland Birds (Acari: Dermanyssidae, Ereynetidae, and Epidermoptidae). Proc. Linn. Soc. 1969, 93, 297–426. [Google Scholar]
- Pence, D.B.; Casto, S. Two New Species and New Records of Nasal Mites of the Genus Sternostoma (Acarina: Rhinonyssinae) from Birds in Texas. J. Parasitol. 1975, 61, 360–368. [Google Scholar] [CrossRef]
- Butenko, O.M. Rhinonyssid Mites of Non-Passerine Birds of the USSR; Izdatel’stvo Moskovskogo Universiteta: Moscow, Russia, 1984. [Google Scholar]
- Kadosaka, T.K.; Kaneko, K.A. A new species and new records (Acarina: Rhinonyssidae) of avian nasal mites from Japan. Jpn. J. Sanit. Zool. 1987, 3, 33–43. [Google Scholar] [CrossRef]
- Knee, W. Five New Species of Rhinonyssidae (Mesostigmata) and One New Species of Dermanyssus (Mesostigmata: Dermanyssidae) from Birds of Alberta and Manitoba, Canada. J. Parasitol. 2008, 94, 348–374. [Google Scholar] [CrossRef] [PubMed]
- Dimov, I. Kleshchi-Rinonissidy Ptic Severo-Zapada Rossii; LLC Zhigulin.: Saint Petersburg, Russia, 2018. [Google Scholar]
- Trouessart, E.L. Note sur les Acariens Parasites des Fosses Nasales des Oiseaux. C. R. Soc. Biol. Ide. Ser. 1894, 1, 723–724. [Google Scholar]
- Vitzthum, H.G. Milben aus der Nasenhöhle von Vögeln. J. Ornithol. 1935, 83, 563–587. [Google Scholar] [CrossRef]
- Crossley, D.A. A new species of nasal mite, Neonyssus (Neonyssus) columbae, from the pigeon (Acarina, Mesostigmata, Rhinonyssidae). Proc. Entomol. Soc. Wash. 1950, 52, 309–313. [Google Scholar]
- George, J.E. The nasal mites of the genus Ptilonyssus (Acarina Rhinonyssidae) occurring on some North American passeriformbirds. J. Kans. Entomol. Soc. 1961, 34, 105–132. [Google Scholar]
- Wilson, N. New records and descriptions of Rhinonyssidae mostly from New Guinea. Pac. Insects 1964, 6, 357–388. [Google Scholar]
- Dimov, I. Taxonomic Diversity and Morphology of Mites of the Family Rhinonyssidae of the Northwest of Russia; ZIN RAS: St. Petersburg, Russia, 2000; 214p, ISBN 978-5-6044687-1-5. [Google Scholar]
- Bregetova, N.G. Mites from the nasal cavity of Fly carchers of the genus Muscicapa. Parazitologiya 1970, 4, 59–62. [Google Scholar]
- Fain, A. Les acariens parasites nasicoles des oiseaux de Belgique. IV. Notes sur quelques Rhinonyssidae avec description de deux especes nouvelles. Bull. Ann. De La Soc. R. Belgue D’entomol. 1964, 100, 55–61. [Google Scholar]
- Fain, A.; Aitken, T. Ácaros parásitos nasales de aves de Trinidad (Indias Occidentales). I. Rhinonyssidae: Mesostigmata. Bull. Inst. R. Sci. Nat. Belg. 1967, 43, 1–44. [Google Scholar]
- Dimov, I. Three New Species of Nasal Mites Ptilonyssus (Mesostigmata, Rhinonyssidae) from Russia. Arhimed-J. Sci. Pract. 2020, 9, 41–52. [Google Scholar]
- Huyse, T.; Poulin, R.; Théron, A. Speciation in Parasites: A Population Genetics Approach. Trends Parasitol. 2005, 21, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Mora, M.D.; Ubeda, J.M.; Cutillas, C.; Navajas, M.; Guevara, D. Phylogenetic relationships in rhinonyssid mites (Acari: Rhinonyssidae) based on mitochondrial 16S rDNA sequences. Exp. Appl. Acarol. 2001, 25, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.; Oconnor, B.M. Phylogeny of Dermanyssoidea (Acari: Parasitiformes) suggests multiple origins of parasitism. Acarologia 2010, 50, 113–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.-Y.; Wang, R.-L.; Niu, D.-L. Divergent Domains of 28S Ribosomal RNA Gene: DNA Barcodes for Molecular Classification and Identification of Mites. Parasit. Vectors 2020, 13, 251. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Xia, X. DAMBE5: A Comprehensive Software Package for Data Analysis in Molecular Biology and Evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate max-imum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. FigTree v1.3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 22 November 2024).
- Pence, D.B. Congruent Inter-Relationships of the Rhinonyssinae (Dermanyssidae) with Their Avian Hosts. In Recent Advances in Acarology; Elsevier: Amsterdam, The Netherlands, 1979; pp. 371–377. ISBN 9780125922029. [Google Scholar]
- Fain, A. Les Rhinonyssides Parasites Des Pigeons. Rev. Zool. Bot. Afr. 1962, 65, 305–324. [Google Scholar]
- Rojas, M.; Doña, J.; Jovani, R.; Dimov, I.; Zurita, A.; Callejon, R. Evidence of Cryptic Species in the Genus Tinaminyssus (Acari: Rhinonyssidae) Based on Morphometrical and Molecular Data. Exp. Appl. Acarol. 2018, 75, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Stålstedt, J.; Wohltmann, A.; Bergsten, J.; Mąkol, J. Towards Resolving the Double Classification in Erythraeus (Actinotrichida: Erythraeidae): Matching Larvae with Adults Using 28S Sequence Data and Experimental Rearing. Org. Divers. Evol. 2016, 16, 761–790. [Google Scholar] [CrossRef]
- Lehmitz, R.; Decker, P. The Nuclear 28S Gene Fragment D3 as Species Marker in Oribatid Mites (Acari, Oribatida) from German Peatlands. Exp. Appl. Acarol. 2017, 71, 259–276. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Dantas-Torres, F.; Annoscia, G.; Cantacessi, C.; Otranto, D. Comparative Analyses of Mitochondrial and Nuclear Genetic Markers for the Molecular Identification of Rhipicephalus spp. Infect. Genet. Evol. 2013, 20, 422–427. [Google Scholar] [CrossRef]
- Antonovskaia, A.A. Using DNA Markers in Studies of Chigger Mites (Acariformes, Trombiculidae). Entomol. Rev. 2018, 98, 1351–1368. [Google Scholar] [CrossRef]
| Mite Species | Host Birds | Geographic Location | Genbank Accession Number |
|---|---|---|---|
| Ptilonyssus euroturdi | Turdus merula | Spain | PQ726434 |
| Ptilonyssus muscicapae | Muscicapa striata | Spain: Cádiz | PQ726436 |
| Ptilonyssus sylviae | Curruca melanocephala | Spain: Montellano: Sevilla | PQ726435 |
| Ptilonyssus motacillae | Motacilla alba | Spain: Montellano: Sevilla | PQ726433 |
| Ptilonyssus chloris | Chloris chloris | Spain: Montellano: Sevilla | PQ726430 |
| Ptilonyssus hirsti | Passer domesticus | Spain: Sevilla | PQ726432 |
| Ptilonyssus fringillae | Fringilla coelebs | Spain: Montellano: Sevilla | PQ726431 |
| Tinaminyssus bubulci | Bubulcis ibis | Spain: Montellano: Sevilla | PQ726429 |
| Tinaminyssus columbae | Columba livia | Spain: Zaragoza | PQ726426 |
| Tinaminyssus melloi | Columba livia | Spain: Zaragoza | PQ726425 |
| Tinaminyssus streptopeliae | Streptopelia decaocto | Spain: Sevilla | PQ726427 |
| Tinaminyssus streptopelioides | Streptopelia turtur | Spain | PQ726428 |
| Steatonyssus occidentalis | - | GU440594.1 | |
| Dermanyssus gallinae | - | FJ911771.1 | |
| Ornithonyssus bursa | - | FJ911789.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Carrión, S.A.; Márquez, F.J.; de Rojas, M. Utility of 28S Ribosomal RNA Gene Domains for Molecular Classification and Phylogeny of Rhinonyssid Mites. Pathogens 2025, 14, 156. https://doi.org/10.3390/pathogens14020156
Sánchez-Carrión SA, Márquez FJ, de Rojas M. Utility of 28S Ribosomal RNA Gene Domains for Molecular Classification and Phylogeny of Rhinonyssid Mites. Pathogens. 2025; 14(2):156. https://doi.org/10.3390/pathogens14020156
Chicago/Turabian StyleSánchez-Carrión, Susana A., Francisco J. Márquez, and Manuel de Rojas. 2025. "Utility of 28S Ribosomal RNA Gene Domains for Molecular Classification and Phylogeny of Rhinonyssid Mites" Pathogens 14, no. 2: 156. https://doi.org/10.3390/pathogens14020156
APA StyleSánchez-Carrión, S. A., Márquez, F. J., & de Rojas, M. (2025). Utility of 28S Ribosomal RNA Gene Domains for Molecular Classification and Phylogeny of Rhinonyssid Mites. Pathogens, 14(2), 156. https://doi.org/10.3390/pathogens14020156







