Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity
Abstract
1. Introduction
2. Case Description
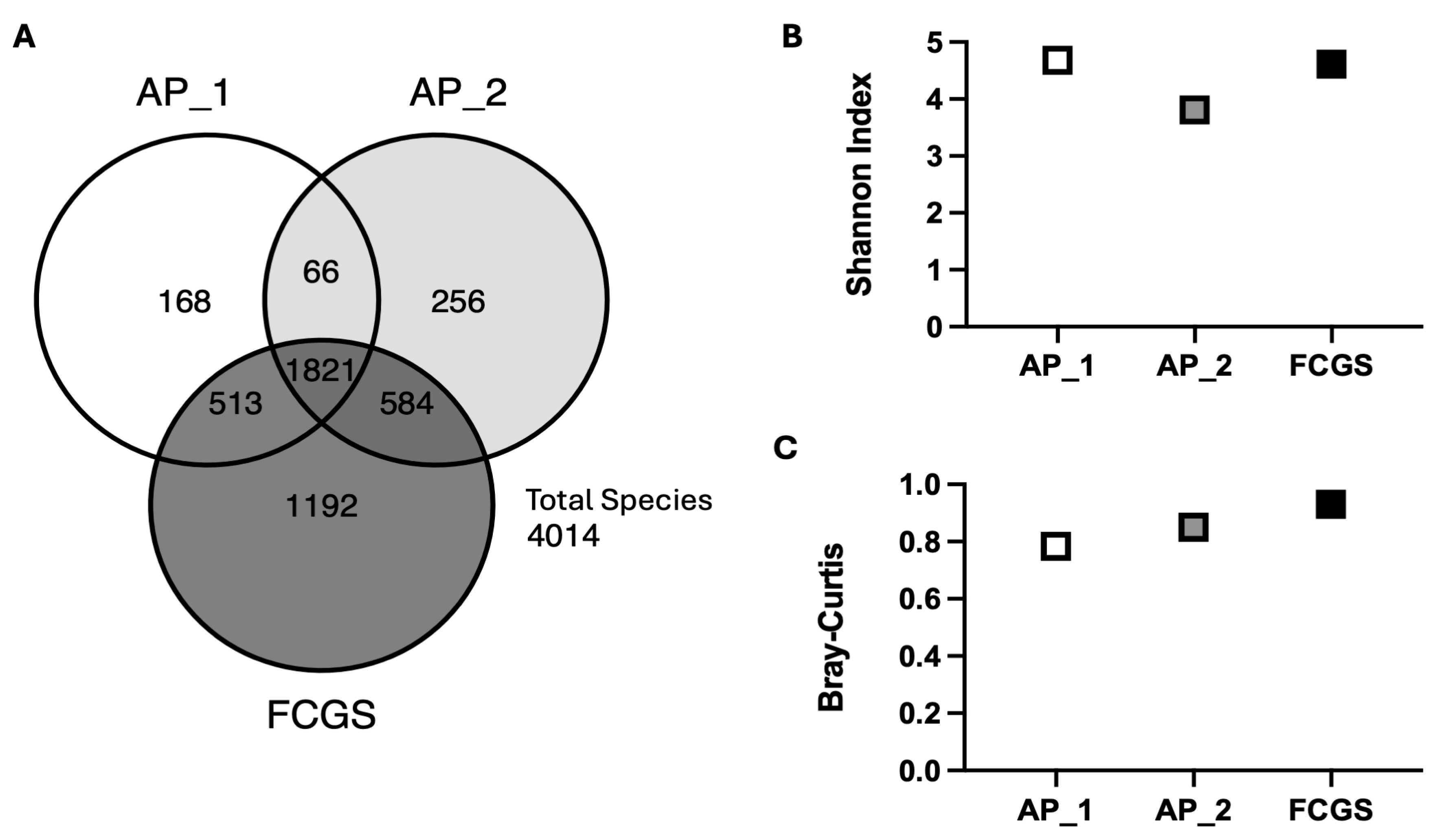
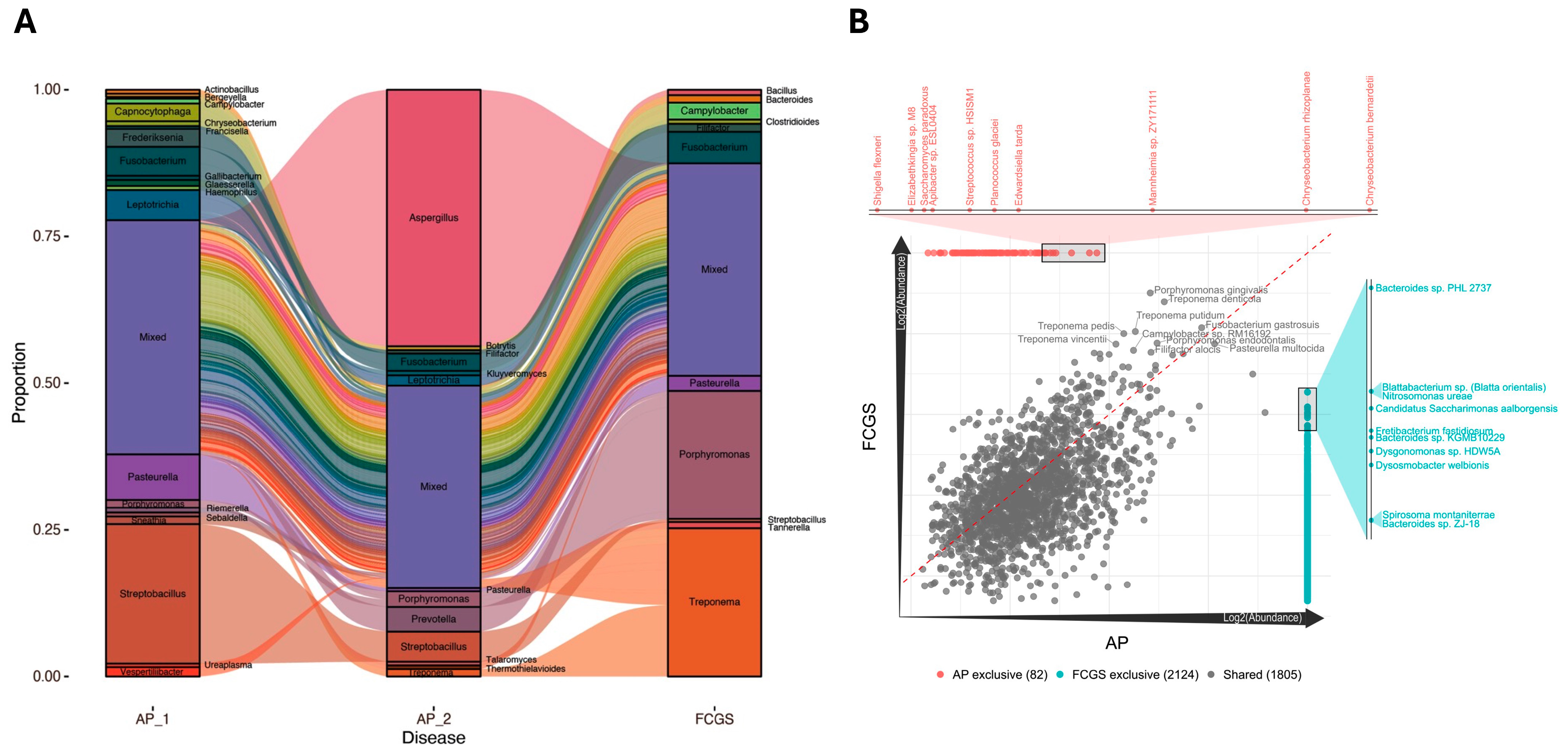
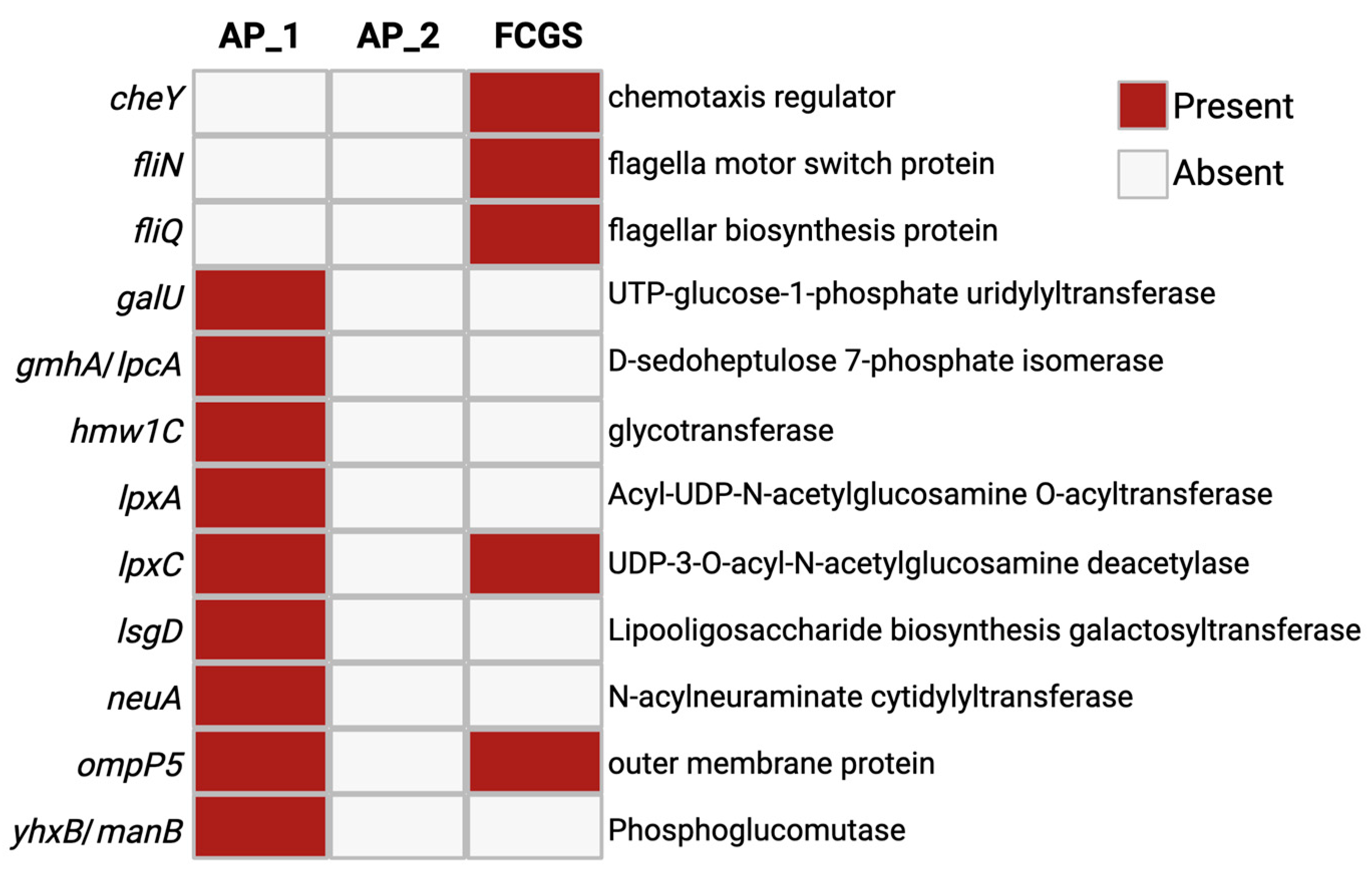
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soltero-Rivera, M.; Vapniarsky, N.; Rivas, I.L.; Arzi, B. Clinical, radiographic and histopathologic features of early-onset gingivitis and periodontitis in cats (1997–2022). J. Feline Med. Surg. 2023, 25, 1098612X221148577. [Google Scholar] [CrossRef] [PubMed]
- Soltero-Rivera, M.; Goldschmidt, S.; Arzi, B. Feline chronic gingivostomatitis current concepts in clinical management. J. Feline Med. Surg. 2023, 25, 1098612X231186834. [Google Scholar] [CrossRef] [PubMed]
- Dolieslager, S.M.; Lappin, D.F.; Bennett, D.; Graham, L.; Johnston, N.; Riggio, M.P. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet. Immunol. Immunopathol. 2013, 151, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Dolieslager, S.M.; Riggio, M.P.; Lennon, A.; Lappin, D.F.; Johnston, N.; Taylor, D.; Bennett, D. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet. Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Older, C.E.; Gomes, M.O.S.; Hoffmann, A.R.; Policano, M.D.; Reis, C.; Carregaro, A.B.; Ambrosio, C.E.; Carregaro, V.M.L. Influence of the FIV Status and Chronic Gingivitis on Feline Oral Microbiota. Pathogens 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.; Grenier, J.K.; Webb, S.M.; Miller, A.D.; Miranda, I.C.; Parker, J.S.L. Transcriptomic signatures of feline chronic gingivostomatitis are influenced by upregulated IL6. Sci. Rep. 2023, 13, 13437. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Tutt, C. Periodontal disease in cats: Back to basics–with an eye on the future. J. Feline Med. Surg. 2015, 17, 45–65. [Google Scholar] [CrossRef]
- Shaw, C.A.; Soltero-Rivera, M.; Profeta, R.; Weimer, B.C. Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis. Bacteria 2025, 4, 1. [Google Scholar] [CrossRef]
- Soltero-Rivera, M.; Shaw, C.; Arzi, B.; Lommer, M.; Weimer, B.C. Feline Chronic Gingivostomatitis Diagnosis and Treatment through Transcriptomic Insights. Pathogens 2024, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.N.; Arzi, B.; Verstraete, F.J. Therapeutic Management of Feline Chronic Gingivostomatitis: A Systematic Review of the Literature. Front. Vet. Sci. 2016, 3, 54. [Google Scholar] [CrossRef]
- Davis, E.M. Gene Sequence Analyses of the Healthy Oral Microbiome in Humans and Companion Animals. J. Vet. Dent. 2016, 33, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microbes Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Weese, J.S. Oral Microbiome in Dogs and Cats: Dysbiosis and the Utility of Antimicrobial Therapy in the Treatment of Periodontal Disease. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Croft, J.; O’Flynn, C.; Deusch, O.; Colyer, A.; Allsopp, J.; Milella, L.; Davis, I.J. A Pyrosequencing Investigation of Differences in the Feline Subgingival Microbiota in Health, Gingivitis and Mild Periodontitis. PLoS ONE 2015, 10, e0136986. [Google Scholar] [CrossRef]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: Characterization and comparison between diseased and healthy cats. Sci. Rep. 2019, 9, 12340. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Elebyary, O.; Fine, N.; Oveisi, M.; Glogauer, M. Metabolites of the oral microbiome: Important mediators of multikingdom interactions. FEMS Microbiol. Rev. 2022, 46, fuab039. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; He, F.; Si, M.; Sun, P.; Chen, Q. Effects of Antibiotic Use on Saliva Antibody Content and Oral Microbiota in Sprague Dawley Rats. Front. Cell Infect. Microbiol. 2022, 12, 721691. [Google Scholar] [CrossRef]
- Adler, C.J.; Malik, R.; Browne, G.V.; Norris, J.M. Diet may influence the oral microbiome composition in cats. Microbiome 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef] [PubMed]
- Meuric, V.; Martin, B.; Guyodo, H.; Rouillon, A.; Tamanai-Shacoori, Z.; Barloy-Hubler, F.; Bonnaure-Mallet, M. Treponema denticola improves adhesive capacities of Porphyromonas gingivalis. Mol. Oral. Microbiol. 2013, 28, 40–53. [Google Scholar] [CrossRef]
- Nibali, L. Aggressive Periodontitis: Microbes and host response, who to blame? Virulence 2015, 6, 223–228. [Google Scholar] [CrossRef]
- Kong, N.; Ng, W.; Lee, V.; Kelly, L.; Weimer, B. Production and Analysis of High Molecular Weight Genomic DNA for NGS Pipelines Using Agilent DNA Extraction Kit (p/n 200600); Agilent Technologies: Santa Clara, CA, USA, 2013; Volume 10. [Google Scholar]
- Basbas, C.; Garzon, A.; Schlesener, C.; van Heule, M.; Profeta, R.; Weimer, B.C.; Silva-Del-Rio, N.; Byrne, B.A.; Karle, B.; Aly, S.S.; et al. Unveiling the microbiome during post-partum uterine infection: A deep shotgun sequencing approach to characterize the dairy cow uterine microbiome. Anim. Microbiome 2023, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Garzon, A.; Basbas, C.; Schlesener, C.; Silva-Del-Rio, N.; Karle, B.M.; Lima, F.S.; Weimer, B.C.; Pereira, R.V. WGS of intrauterine E. coli from cows with early postpartum uterine infection reveals a non-uterine specific genotype and virulence factors. mBio 2024, 15, e0102724. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Haiminen, N.; Chambliss, D.; Edlund, S.; Kunitomi, M.; Huang, B.C.; Kong, N.; Ganesan, B.; Baker, R.; Markwell, P.; et al. Monitoring the microbiome for food safety and quality using deep shotgun sequencing. npj Sci. Food 2021, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.; Sun, L.; Yu, J.; Jin, Q. VFDB 2008 release: An enhanced web-based resource for comparative pathogenomics. Nucleic Acids Res. 2008, 36, D539–D542. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Faller, L.L.; Klitgord, N.; Mazumdar, V.; Ghodsi, M.; Sommer, D.D.; Gibbons, T.R.; Treangen, T.J.; Chang, Y.C.; Li, S.; et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE 2012, 7, e37919. [Google Scholar] [CrossRef] [PubMed]
- Lenartova, M.; Tesinska, B.; Janatova, T.; Hrebicek, O.; Mysak, J.; Janata, J.; Najmanova, L. The Oral Microbiome in Periodontal Health. Front. Cell Infect. Microbiol. 2021, 11, 629723. [Google Scholar] [CrossRef] [PubMed]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef]
- Munoz Navarro, C.; Del Carmen Sanchez Beltran, M.; Arriagada Vargas, C.; Batalla Vazquez, P.; Diniz Freitas, M.; Limeres Posse, J.; Diz Dios, P.; Garcia Mato, E. Analysis of the Oral Microbiome in a Patient with Cardiofaciocutaneous Syndrome and Severe Periodontal Disease: Impact of Systemic Antibiotic Therapy. Antibiotics 2022, 11, 1754. [Google Scholar] [CrossRef]
- Stone, A.E.S. Feline oral inflammation: Diagnosis and options for treatment. Companion Anim. 2024, 29, 112–118. [Google Scholar] [CrossRef]
- Antezack, A.; Etchecopar-Etchart, D.; La Scola, B.; Monnet-Corti, V. New putative periodontopathogens and periodontal health-associated species: A systematic review and meta-analysis. J. Periodontal Res. 2023, 58, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The increasing importance of the oral microbiome in periodontal health and disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red complex: Polymicrobial conglomerate in oral flora: A review. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.R.; Henker, L.C.; Rodrigues Hoffmann, A. The feline cutaneous and oral microbiota are influenced by breed and environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Kuroda, Y.; Kikuchi, Y.; Kokubu, E.; Ishihara, K. Porphyromonas gingivalis diffusible signaling molecules enhance Fusobacterium nucleatum biofilm formation via gene expression modulation. J. Oral. Microbiol. 2023, 15, 2165001. [Google Scholar] [CrossRef]
- Cools, F.; Torfs, E.; Vanhoutte, B.; de Macedo, M.B.; Bonofiglio, L.; Mollerach, M.; Maes, L.; Caljon, G.; Delputte, P.; Cappoen, D.; et al. Streptococcus pneumoniae galU gene mutation has a direct effect on biofilm growth, adherence and phagocytosis in vitro and pathogenicity in vivo. Pathog. Dis. 2018, 76, fty069. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Shi, M.; Wei, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The Subgingival Microbiome of Periodontal Pockets With Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front. Cell Infect. Microbiol. 2018, 8, 124. [Google Scholar] [CrossRef]
- Johnston, W.; Rosier, B.T.; Artacho, A.; Paterson, M.; Piela, K.; Delaney, C.; Brown, J.L.; Ramage, G.; Mira, A.; Culshaw, S. Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci. Rep. 2021, 11, 9796. [Google Scholar] [CrossRef] [PubMed]
- Ingendoh-Tsakmakidis, A.; Mikolai, C.; Winkel, A.; Szafranski, S.P.; Falk, C.S.; Rossi, A.; Walles, H.; Stiesch, M. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell Microbiol. 2019, 21, e13078. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chis, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.-I.; Minamino, T. Chapter 6—Flagella. In Molecular Medical Microbiology, 3rd ed.; Tang, Y.-W., Hindiyeh, M.Y., Liu, D., Sails, A., Spearman, P., Zhang, J.-R., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 97–126. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Kornman, K.S.; Coykendall, A.L. Observation of fimbriae and flagella in dispersed subgingival dental plaque and fresh bacterial isolates from periodontal disease. J. Periodontal Res. 1983, 18, 620–633. [Google Scholar] [CrossRef]
- Goetting-Minesky, M.P.; Godovikova, V.; Fenno, J.C. Approaches to Understanding Mechanisms of Dentilisin Protease Complex Expression in Treponema denticola. Front. Cell Infect. Microbiol. 2021, 11, 668287. [Google Scholar] [CrossRef] [PubMed]
- Lux, R.; Miller, J.N.; Park, N.H.; Shi, W. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect. Immun. 2001, 69, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.; Martin, M.; Passineau, M.J.; Godovikova, V.; Fenno, J.C.; Wu, H. Activation of the Innate Immune System by Treponema denticola Periplasmic Flagella through Toll-Like Receptor 2. Infect. Immun. 2018, 86, e00573-17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Szymanski, C.M.; Baylink, A. Bacterial chemotaxis in human diseases. Trends Microbiol. 2023, 31, 453–467. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, C.A.; Soltero-Rivera, M.; Profeta, R.; Weimer, B.C. Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity. Pathogens 2025, 14, 228. https://doi.org/10.3390/pathogens14030228
Shaw CA, Soltero-Rivera M, Profeta R, Weimer BC. Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity. Pathogens. 2025; 14(3):228. https://doi.org/10.3390/pathogens14030228
Chicago/Turabian StyleShaw, Claire A., Maria Soltero-Rivera, Rodrigo Profeta, and Bart C. Weimer. 2025. "Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity" Pathogens 14, no. 3: 228. https://doi.org/10.3390/pathogens14030228
APA StyleShaw, C. A., Soltero-Rivera, M., Profeta, R., & Weimer, B. C. (2025). Case Report: Shift from Aggressive Periodontitis to Feline Chronic Gingivostomatitis Is Linked to Increased Microbial Diversity. Pathogens, 14(3), 228. https://doi.org/10.3390/pathogens14030228





