Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection
Abstract
1. Introduction
2. Material and Methods
2.1. Bioinformatic Analysis
2.2. Parasite Maintenance and Growth Curve
2.3. Molecular Cloning and In Vitro Transcription
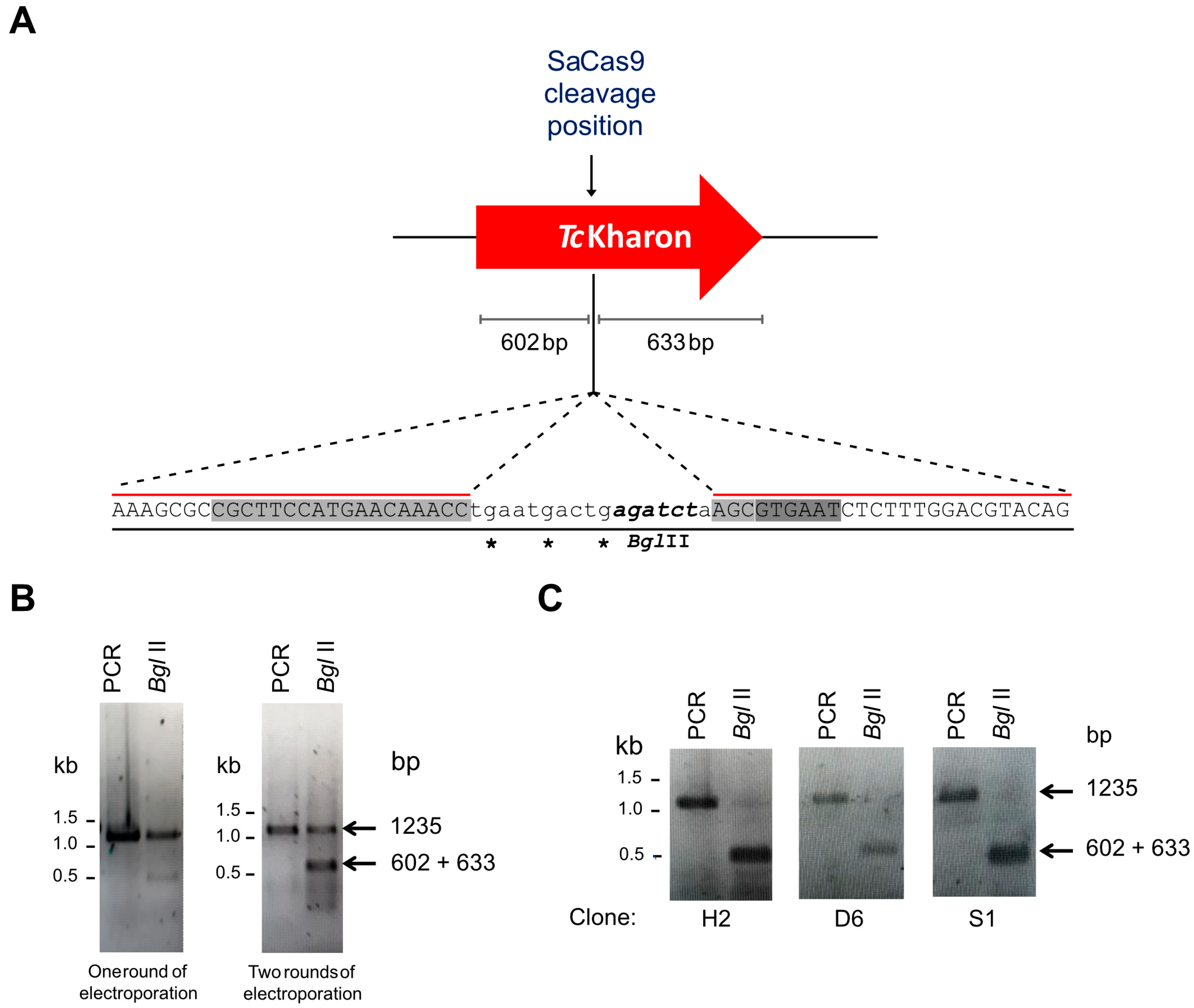
2.4. CRISPR/Cas9 Editing and Genotyping
2.5. Scanning and Transmission Electron Microscopy
2.6. Immunofluorescence Assay and Localization of GFP-Tagged TcKharon
2.7. Western Blot
2.8. Cell Infection Assays and Tissue-Cultured Derived Trypomastigote Counts
2.9. Statistical Analysis
3. Results
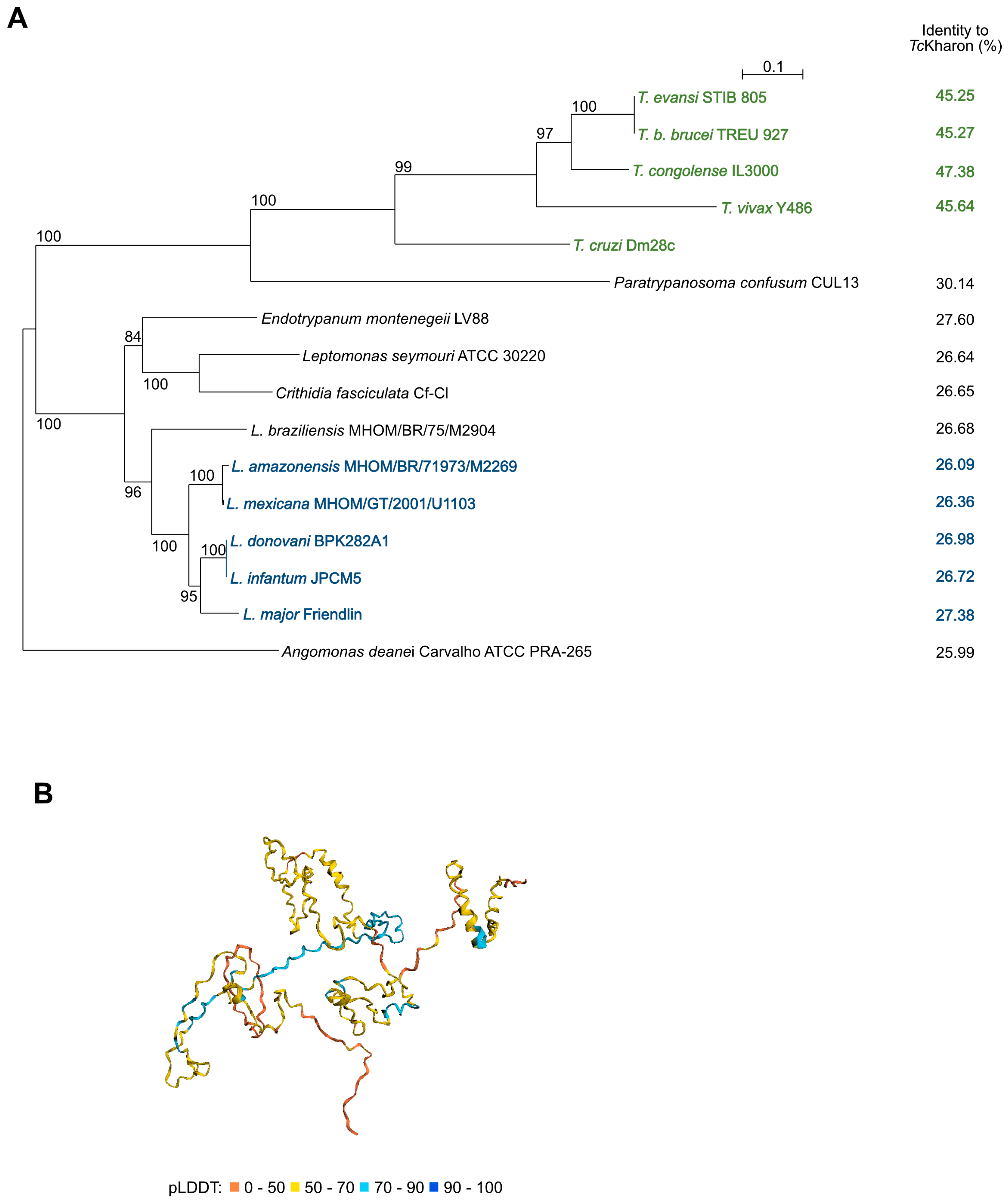
3.1. Kharon Is an Intrinsically Disordered Protein Based on the 3D Structure Prediction
3.2. TcKharon::GFP Is Localized at the Subpellicular Cytoskeleton
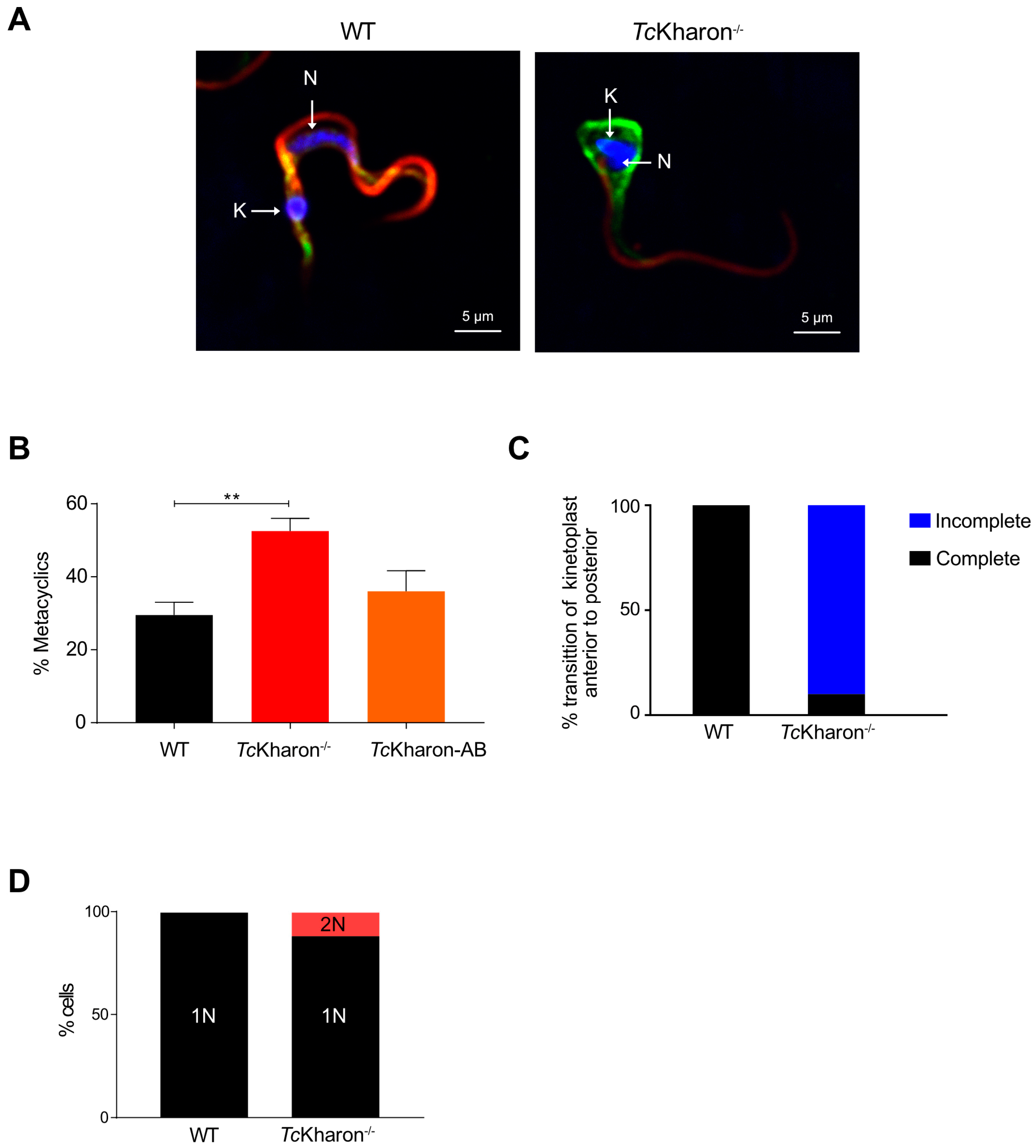
3.3. TcKharon Disruption Affects Cell Growth and Cytokinesis of Epimastigotes
3.4. Morphology of TcKharon-Null Epimastigotes Strongly Affects the Posterior Cell Region
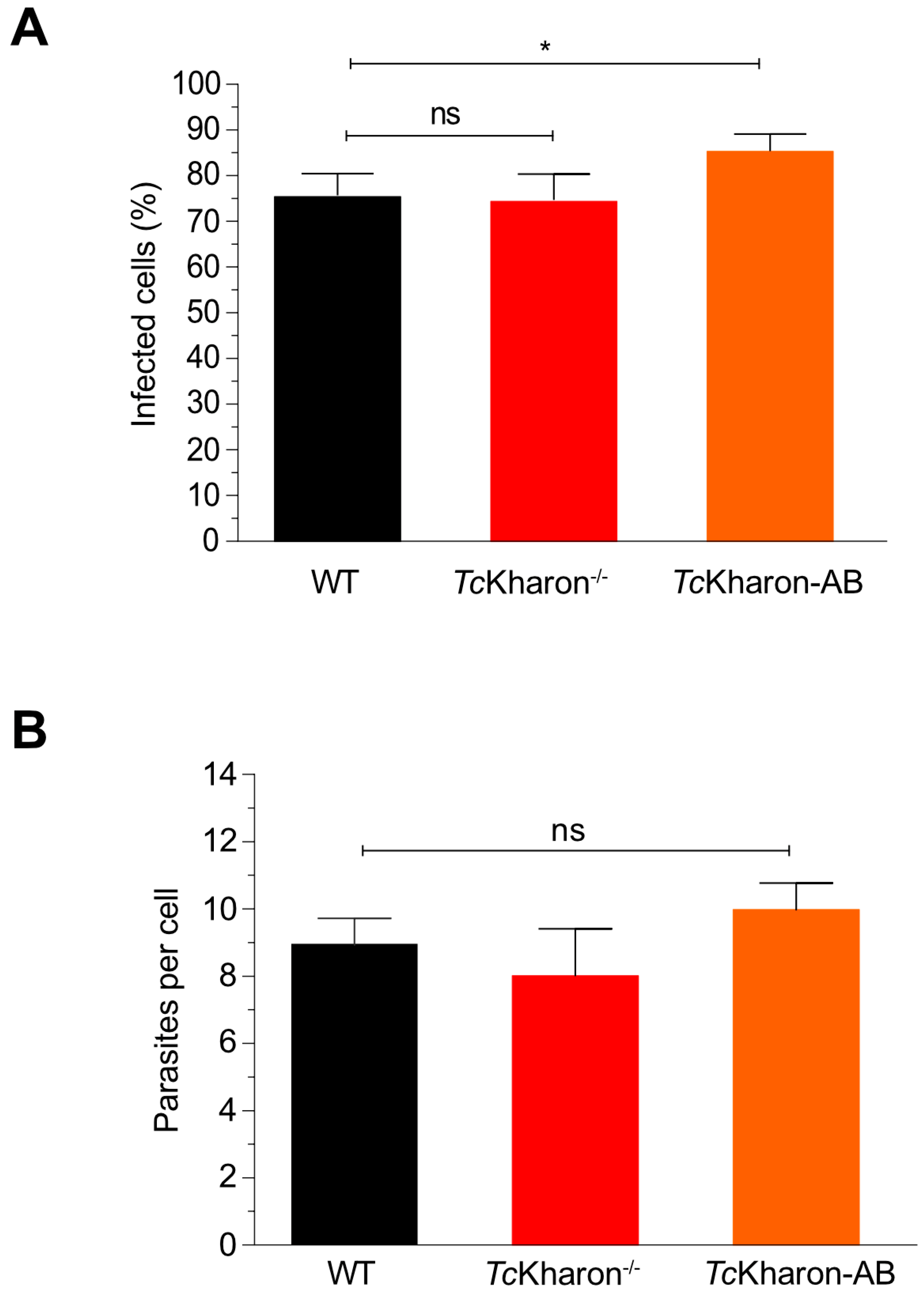
3.5. Metacyclogenesis and Cell Invasion of the TcKharon−/− Mutant Are Not Affected
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob. Heart 2022, 17, 59. [Google Scholar] [CrossRef]
- Kratz, J.M.; Garcia Bournissen, F.; Forsyth, C.J.; Sosa-Estani, S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev. Clin. Pharmacol. 2018, 11, 943–957. [Google Scholar] [CrossRef]
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165591. [Google Scholar] [CrossRef]
- Junqueira, C.; Santos, L.I.; Galvão-Filho, B.; Teixeira, S.M.; Rodrigues, F.G.; DaRocha, W.D.; Chiari, E.; Jungbluth, A.A.; Ritter, G.; Gnjatic, S.; et al. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc. Natl. Acad. Sci. USA 2011, 108, 19695–19700. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.M.M.; Machado, L.F.M.; Doro, D.; Nascimento, F.C.; Damasceno, L.; Gazzinelli, R.T.; Fernandes, A.P.; Junqueira, C. New vaccine formulations containing a modified version of the Amastigote 2 antigen and the non-virulent Trypanosoma cruzi CL-14 strain are highly antigenic and protective against Leishmania infantum challenge. Front. Immunol. 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef]
- Burle-Caldas, G.A.; Dos Santos, N.S.A.; de Castro, J.T.; Mugge, F.L.B.; Grazielle-Silva, V.; Oliveira, A.E.R.; Pereira, M.C.A.; Reis-Cunha, J.L.; dos Santos, A.C.; Gomes, D.A.; et al. Disruption of active Trans-sialidase genes impairs egress from mammalian host cells and generates highly attenuated Trypanosoma cruzi parasites. mBio 2022, 13, e0347821. [Google Scholar] [CrossRef]
- Perez Brandan, C.; Padilla, A.M.; Xu, D.; Tarleton, R.L.; Basombrio, M.A. Knockout of the DHFR-TS gene in Trypanosoma cruzi generates attenuated parasites able to confer protection against a virulent challenge. PLoS Negl. Trop. Dis. 2011, 5, e1418. [Google Scholar] [CrossRef]
- Rooholamin, Z.; Dianat-Moghadam, H.; Esmaeilifallah, M.; Khanahmad, H. From classical approaches to new developments in genetic engineering of live attenuated vaccine against cutaneous leishmaniasis: Potential and immunization. Front. Public Health 2024, 12, 1382996. [Google Scholar] [CrossRef]
- Moreira, P.O.L.; Nogueira, P.M.; Monte-Neto, R.L. Next-generation leishmanization: Revisiting molecular targets for selecting genetically engineered live-attenuated Leishmania. Microorganisms 2023, 11, 1043. [Google Scholar] [CrossRef]
- Souza, W. Structural organization of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 2009, 104 (Suppl. 1), 89–100. [Google Scholar] [CrossRef]
- Billington, K.; Halliday, C.; Madden, R.; Dyer, P.; Barker, A.R.; Moreira-Leite, F.F.; Carrington, M.; Vaughan, S.; Hertz-Fowler, C.; Dean, S.; et al. Genome-wide subcellular protein map for the flagellate parasite Trypanosoma brucei. Nat. Microbiol. 2023, 8, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.N.; Huynh, C.T.; Sladewski, T.E.; Zuromski, J.L.; Ruiz, A.E.; de Graffenried, C.L. The Trypanosoma brucei subpellicular microtubule array is organized into functionally discrete subdomains defined by microtubule associated proteins. PLoS Pathog. 2021, 17, e1009588. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.M.M.; Lanza, J.S.; Tunes, L.G.; Fiuza, J.A.; Roy, G.; Orfanó, A.D.S.; De Carvalho, A.T.; Frézard, F.; De Barros, A.L.B.; Murta, S.M.F.; et al. Growth arrested live-attenuated Leishmania infantum KHARON1 null mutants display cytokinesis defect and protective immunity in mice. Sci. Rep. 2018, 8, 11627. [Google Scholar] [CrossRef]
- Kelly, F.D.; Tran, K.D.; Hatfield, J.; Schmidt, K.; Sanchez, M.A.; Landfear, S.M. A cytoskeletal protein complex is essential for division of intracellular amastigotes of Leishmania mexicana. J. Biol. Chem. 2020, 295, 13106–13122. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.D.; Rodriguez-Contreras, D.; Vieira, D.P.; Yates, P.A.; David, L.; Beatty, W.; Elferich, J.; Landfear, S.M. KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J. Biol. Chem. 2013, 288, 22721–22733. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Tran, K.D.; Valli, J.; Hobbs, S.; Johnson, E.; Gluenz, E.; Landfear, S.M. KHARON is an essential cytoskeletal protein involved in the trafficking of flagellar membrane proteins and cell division in african trypanosomes. J. Biol. Chem. 2016, 291, 19760–19773. [Google Scholar] [CrossRef]
- Tran, K.D.; Vieira, D.P.; Sanchez, M.A.; Valli, J.; Gluenz, E.; Landfear, S.M. Kharon1 null mutants of Leishmania mexicana are avirulent in mice and exhibit a cytokinesis defect within macrophages. PLoS ONE 2015, 10, e0134432. [Google Scholar] [CrossRef]
- Affolter, M.; Hemphill, A.; Roditi, I.; Müller, N.; Seebeck, T. The repetitive microtubule-associated proteins MARP-1 and MARP-2 of Trypanosoma brucei. J. Struct. Biol. 1994, 112, 241–251. [Google Scholar] [CrossRef]
- Schock, M.; Schmidt, S.; Ersfeld, K. Novel cytoskeleton-associated proteins in Trypanosoma brucei are essential for cell morphogenesis and cytokinesis. Microorganisms 2021, 9, 2234. [Google Scholar] [CrossRef]
- Cooper, R.; de Jesus, A.R.; Cross, G.A. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J. Cell Biol. 1993, 122, 149–156. [Google Scholar] [PubMed]
- LaCount, D.J.; Barrett, B.; Donelson, J.E. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J. Biol. Chem. 2002, 277, 17580–17588. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.P. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar] [PubMed]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef]
- Saenz-Garcia, J.L.; Borges, B.S.; Souza-Melo, N.; Machado, L.V.; Miranda, J.S.; Pacheco-Lugo, L.A.; Moretti, N.S.; Wheleer, R.; Medeiros, L.C.S.; DaRocha, W.D. Trypanin disruption affects the motility and infectivity of the protozoan Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2022, 11, 807236. [Google Scholar] [CrossRef]
- Peng, D.; Tarleton, R. EuPaGDT: A web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb Genom. 2015, 1, e000033. [Google Scholar] [CrossRef]
- Kangussu-Marcolino, M.M.; Cunha, A.P.; Avila, A.R.; Herman, J.P.; DaRocha, W.D. Conditional removal of selectable markers in Trypanosoma cruzi using a site-specific recombination tool: Proof of concept. Mol. Biochem. Parasitol. 2014, 198, 71–74. [Google Scholar] [CrossRef]
- Pacheco-Lugo, L.; Díaz-Olmos, Y.; Sáenz-García, J.; Probst, C.M.; DaRocha, W.D. Effective gene delivery to Trypanosoma cruzi epimastigotes through nucleofection. Parasitol. Int. 2017, 66, 236–239. [Google Scholar] [CrossRef]
- Souza-Melo, N.; Alcantara, C.L.; Vidal, J.C.; Rocha, G.M.; de Souza, W. Implications of flagellar attachment zone proteins TcGP72 and TcFLA-1BP in morphology, proliferation, and intracellular dynamics in Trypanosoma cruzi. Pathogens 2023, 12, 1367. [Google Scholar] [CrossRef]
- de Almeida, J.M.; Nunes, F.O.; Ceole, L.F.; Klimeck, T.D.F.; da Cruz, L.A.; Tófoli, D.; Borges, B.S.; Garcez, W.S.; Tozetti, I.A.; Medeiros, L.C.S.; et al. Synergistic effect and ultrastructural changes in Trypanosoma cruzi caused by isoobtusilactone A in short exposure of time. PLoS ONE 2021, 16, e0245882. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.C.P.; Freymüller-Haapalainen, E.; Schenkman, S. Three-dimensional reconstruction of Trypanosoma cruzi epimastigotes and organelle distribution along the cell division cycle: 3D Electron Microscopy of Trypanosoma cruzi. Cytom. Part A 2011, 79A, 538–544. [Google Scholar] [CrossRef]
- DaRocha, W.D.; Silva, R.A.; Bartholomeu, D.C.; Pires, S.F.; Freitas, J.M.; Macedo, A.M.; Vazquez, M.P.; Levin, M.J.; Teixeira, S.M.R. Expression of exogenous genes in Trypanosoma cruzi: Improving vectors and electroporation protocols. Parasitol. Res. 2004, 92, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.M.; Pappu, R.V. AlphaFold and implications for intrinsically disordered proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef]
- Wheeler, R.J. A resource for improved predictions of Trypanosoma and Leishmania protein three-dimensional structure. PLoS ONE 2021, 16, e0259871. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Gadelha, C.; Wickstead, B.; de Souza, W.; Gull, K.; Cunha-e-Silva, N. Cryptic paraflagellar rod in endosymbiont-containing kinetoplastid protozoa. Eukaryot. Cell. 2005, 4, 516–525. [Google Scholar] [CrossRef]
- Sousa, M.A.D. A simple method to purify biologically and antigenically preserved bloodstream trypomastigotes of Trypanosoma cruzi using Deae-cellulose columns. Mem. Inst. Oswaldo Cruz 1983, 78, 317–333. [Google Scholar] [CrossRef]
- Elias, M.C.; da Cunha, J.P.; de Faria, F.P.; Mortara, R.A.; Freymüller, E.; Schenkman, S.; de Faria, F.P. Morphological events during the Trypanosoma cruzi cell cycle. Protist 2007, 158, 147–157. [Google Scholar] [CrossRef]
- Wheeler, R.J.; Gull, K.; Sunter, J.D. Coordination of the cell cycle in Trypanosomes. Annu. Rev. Microbiol. 2019, 73, 133–154. [Google Scholar] [CrossRef]
- Robinson, D.R.; Sherwin, T.; Ploubidou, A.; Byard, E.H.; Gull, K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 1995, 128, 1163–1172. [Google Scholar] [CrossRef]
- Sinclair, A.N.; de Graffenried, C.L. More than microtubules: The structure and function of the subpellicular array in Trypanosomatids. Trends Parasitol. 2019, 35, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.; Gull, K. WCB is a C2 domain protein defining the plasma membrane-sub-pellicular microtubule corset of kinetoplastid parasites. Protist 2008, 159, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, C.; Giroud, C.; Robinson, D.R.; Besteiro, S.; Bosc, C.; Bringaud, F.; Baltz, T. Two related subpellicular cytoskeleton-associated proteins in Trypanosoma brucei stabilize microtubules. Mol. Biol. Cell 2002, 13, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenz-Garcia, J.L.; Souza-Melo, N.; Miranda, J.S.; Borges, B.; Pacheco-Lugo, L.A.; Quintero-Solano, J.M.; Moretti, N.; Wheeler, R.; Soares-Medeiros, L.C.; DaRocha, W.D. Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection. Pathogens 2025, 14, 312. https://doi.org/10.3390/pathogens14040312
Saenz-Garcia JL, Souza-Melo N, Miranda JS, Borges B, Pacheco-Lugo LA, Quintero-Solano JM, Moretti N, Wheeler R, Soares-Medeiros LC, DaRocha WD. Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection. Pathogens. 2025; 14(4):312. https://doi.org/10.3390/pathogens14040312
Chicago/Turabian StyleSaenz-Garcia, Jose Luis, Normanda Souza-Melo, Juliana Severo Miranda, Beatriz Borges, Lisandro A. Pacheco-Lugo, Jose M. Quintero-Solano, Nilmar Moretti, Richard Wheeler, Lia C. Soares-Medeiros, and Wanderson D. DaRocha. 2025. "Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection" Pathogens 14, no. 4: 312. https://doi.org/10.3390/pathogens14040312
APA StyleSaenz-Garcia, J. L., Souza-Melo, N., Miranda, J. S., Borges, B., Pacheco-Lugo, L. A., Quintero-Solano, J. M., Moretti, N., Wheeler, R., Soares-Medeiros, L. C., & DaRocha, W. D. (2025). Kharon Is Crucial for Trypanosoma cruzi Morphology but Does Not Impair In Vitro Infection. Pathogens, 14(4), 312. https://doi.org/10.3390/pathogens14040312








