First Detection of Bluetongue Virus Type 3 in Poland in 2024—A Case Study in European Bison (Bison bonasus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study and Clinical Diagnosis
2.2. Culicoides Monitoring
2.3. Surveillance Sampling
2.4. Virological Testing
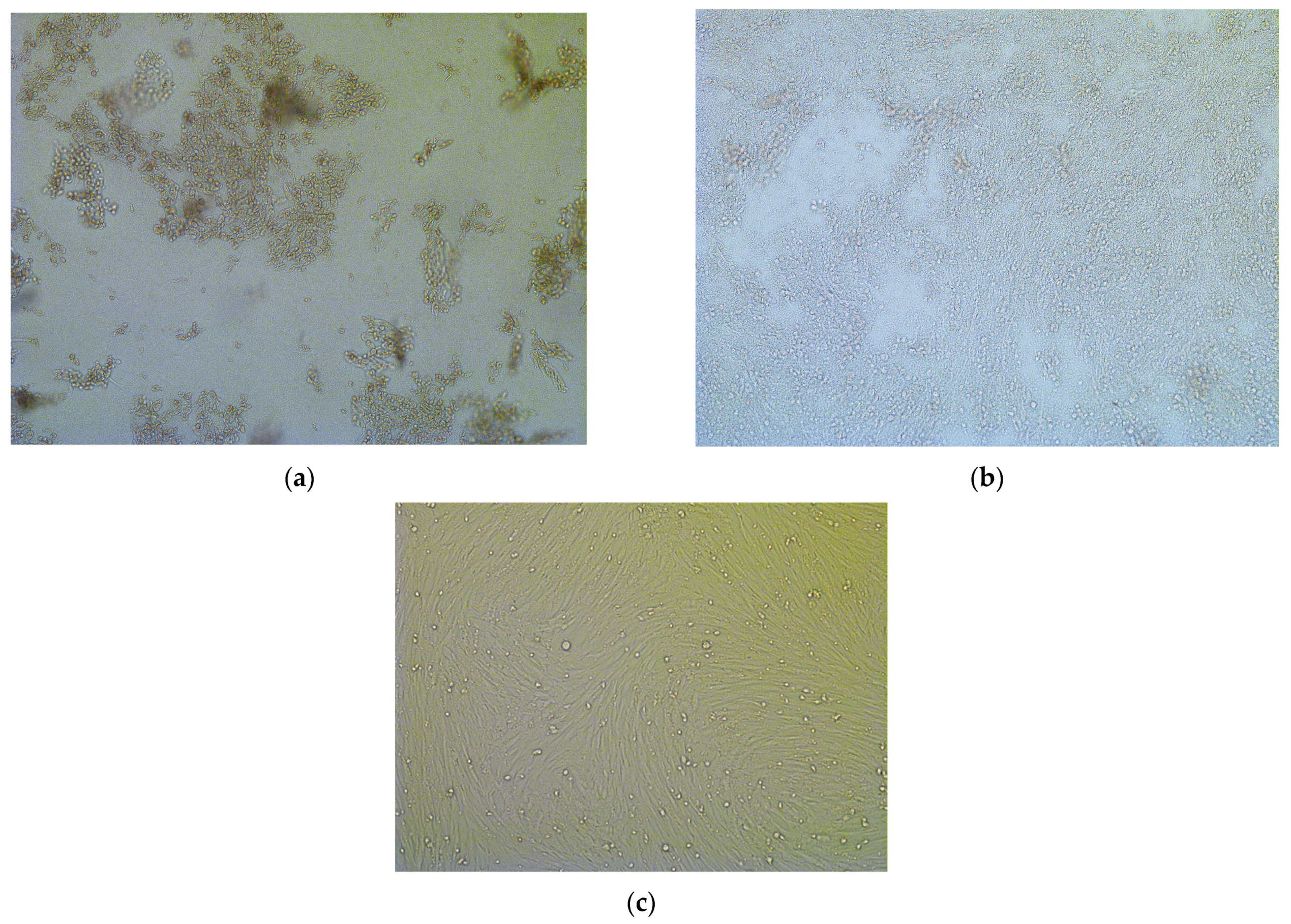
Virus Isolation
2.5. Molecular Testing
2.5.1. Nucleic Acid Extraction
2.5.2. RT-PCR
2.5.3. Phylogenetic Studies
2.6. Serological Testing
2.7. Additional Testing
3. Results
3.1. Case Study
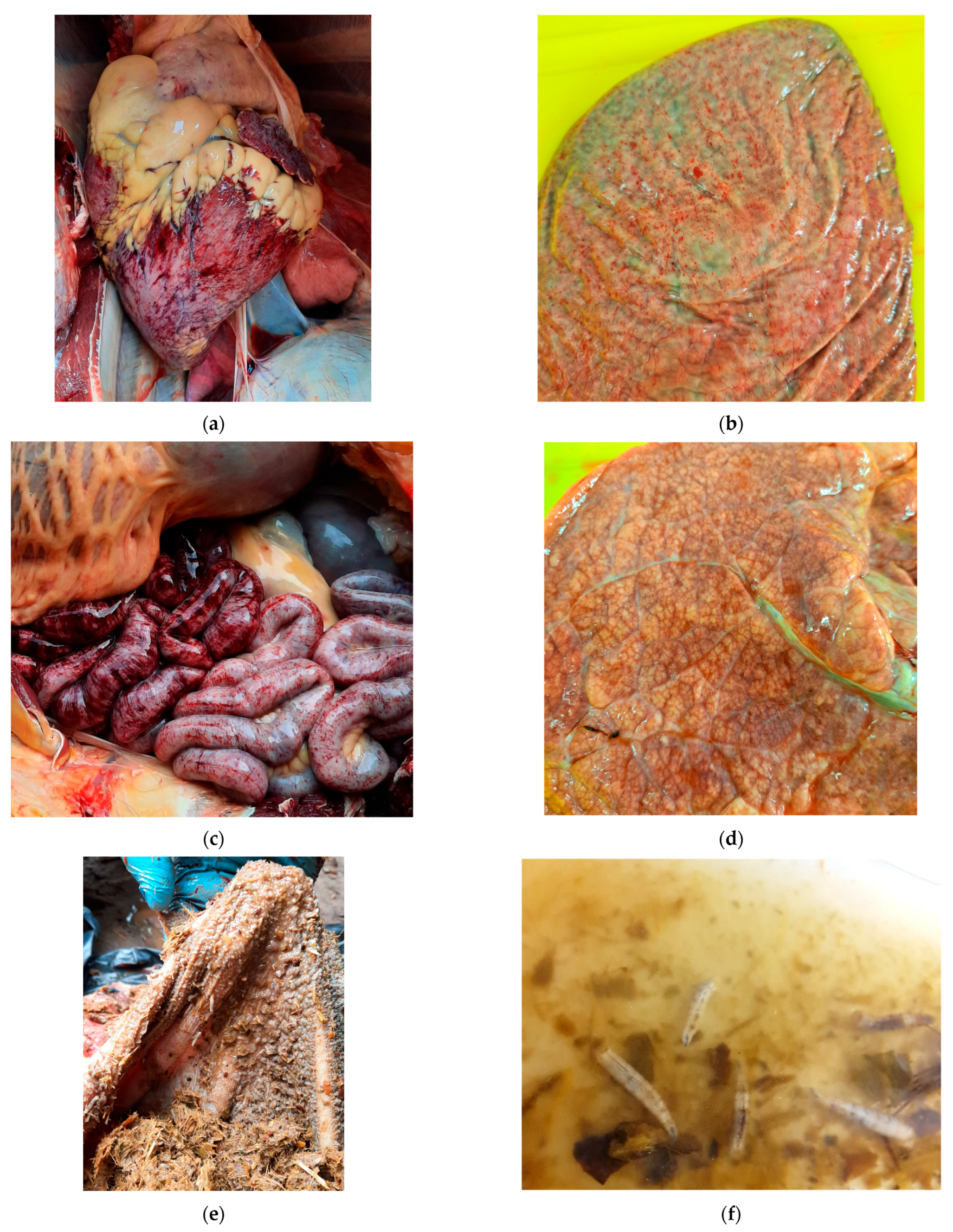
3.1.1. Necropsy Findings
3.1.2. BTV Detection
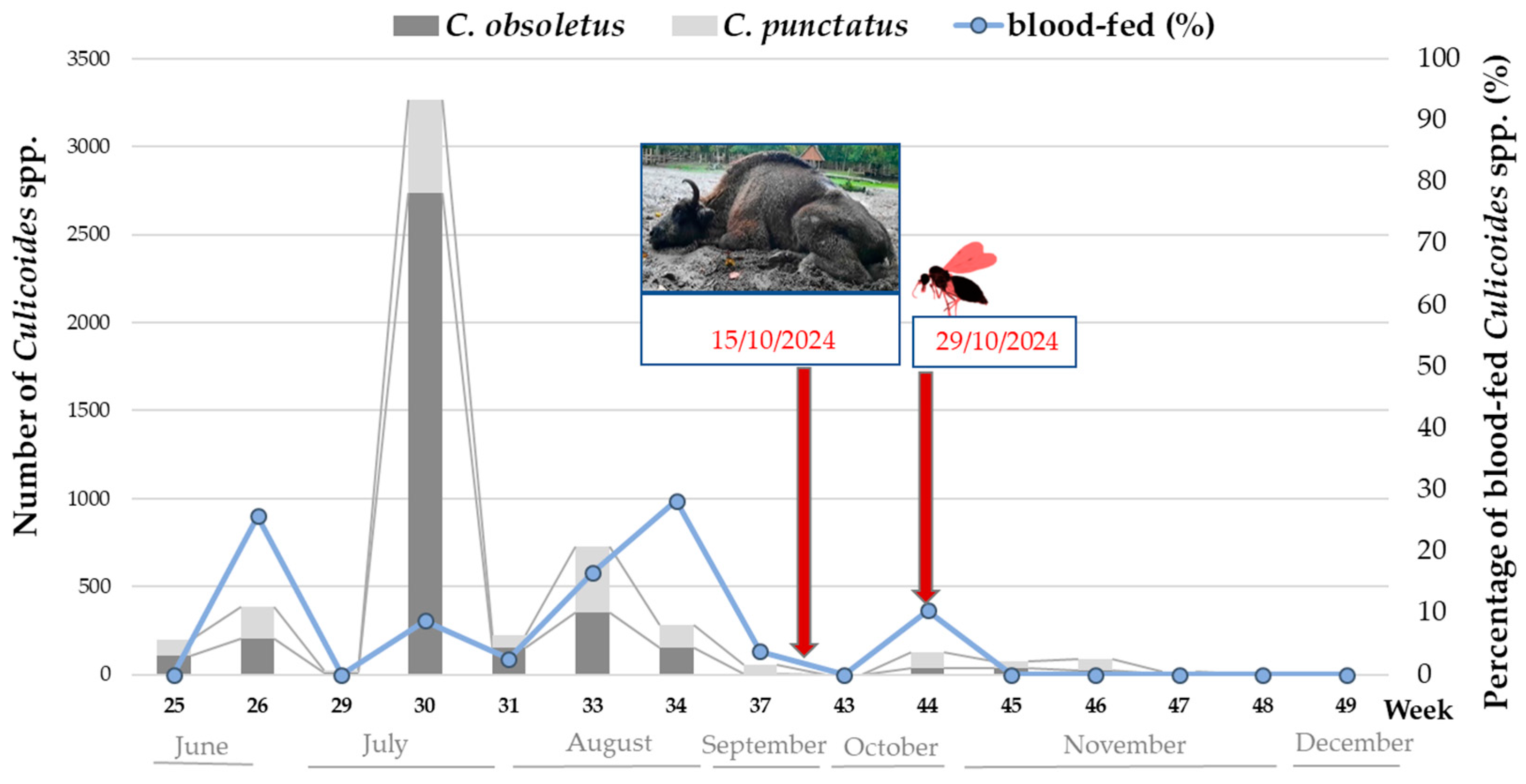
3.1.3. Culicoides spp. Activity and BTV Detection
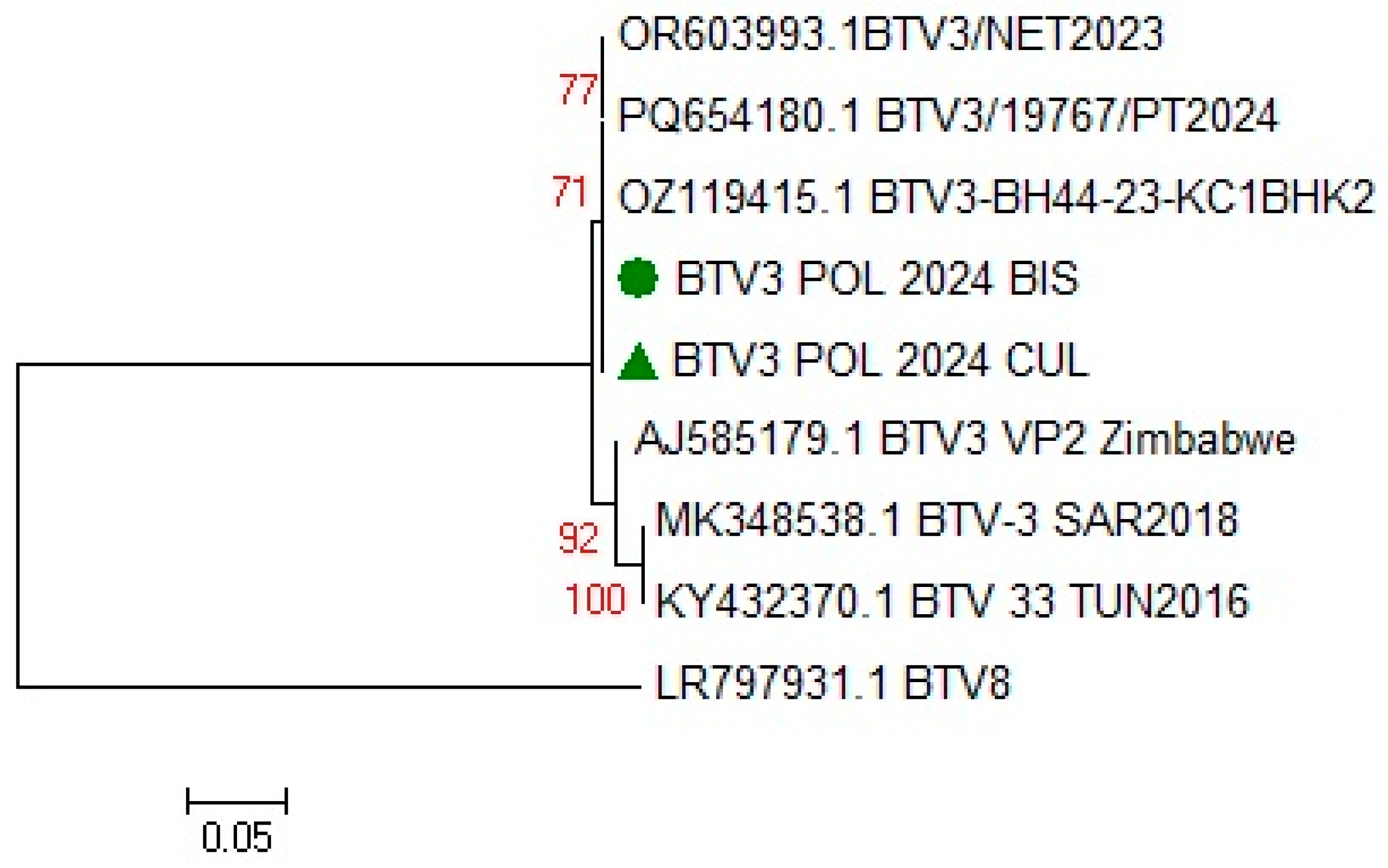
3.1.4. BTV Strain Characterization
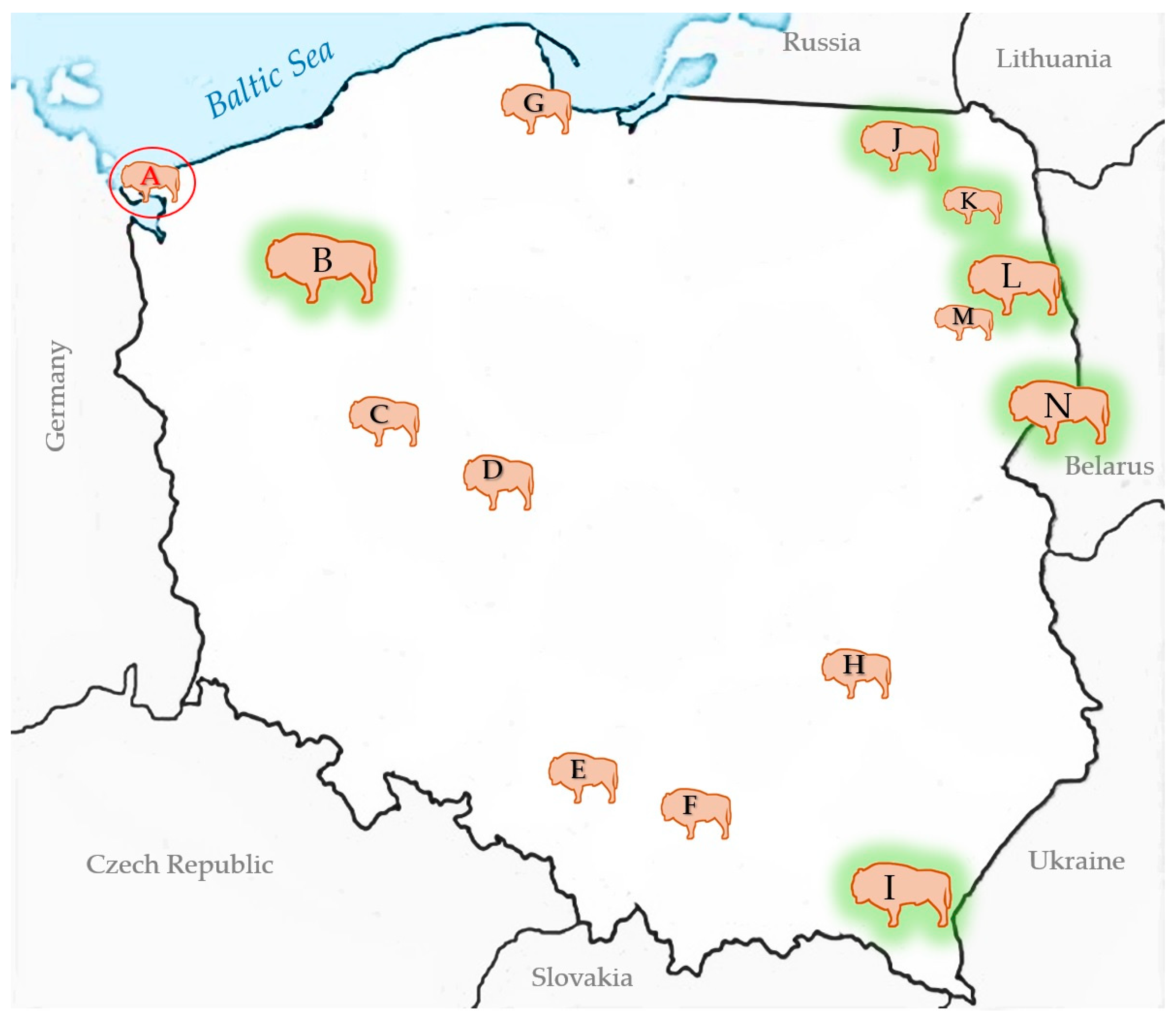
3.2. BTV Survey of European Bison in Poland
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, T.E. The history of bluetongue and a current global overview. Vet. Ital. 2004, 40, 31–38. [Google Scholar]
- Purse, B.V.; Brown, H.E.; Harrup, L.; Mertens, P.P.; Rogers, D.J. Invasion of bluetongue and other orbivirus infections into Europe: The role of biological and climatic processes. Rev. Sci. Tech. 2008, 27, 427–442. [Google Scholar] [CrossRef]
- Boender, G.J.; Hagenaars, T.J.; Holwerda, M.; Spierenburg, M.A.H.; van Rijn, P.A.; van der Spek, A.N.; Elbers, A.R.W. Spatial Transmission Characteristics of the Bluetongue Virus Serotype 3 Epidemic in The Netherlands, 2023. Viruses 2024, 16, 625. [Google Scholar] [CrossRef]
- van den Brink, K.M.J.A.; Santman-Berends, I.M.G.A.; Harkema, L.; Scherpenzeel, C.G.M.; Dijkstra, E.; Bisschop, P.I.H.; Peterson, K.; van de Burgwal, N.S.; Waldeck, H.W.F.; Dijkstra, T.; et al. Bluetongue virus serotype 3 in ruminants in the Netherlands: Clinical signs, seroprevalence and pathological findings. Vet. Rec. 2024, 195, e4533. [Google Scholar] [CrossRef]
- Voigt, A.; Kampen, H.; Heuser, E.; Zeiske, S.; Hoffmann, B.; Höper, D.; Holsteg, M.; Sick, F.; Ziegler, S.; Wernike, K.; et al. Bluetongue Virus Serotype 3 and Schmallenberg Virus in Culicoides Biting Midges, Western Germany, 2023. Emerg. Infect. Dis. 2024, 30, 1438–1441. [Google Scholar] [CrossRef]
- Newbrook, K.; Obishakin, E.; Jones, L.A.; Waters, R.; Ashby, M.; Batten, C.; Sanders, C. Clinical disease in British sheep infected with an emerging strain of bluetongue virus serotype 3. Vet. Rec. 2025, 196, e4910. [Google Scholar] [CrossRef]
- Barros, S.C.; Henriques, A.M.; Ramos, F.; Luís, T.; Fagulha, T.; Magalhães, A.; Caetano, I.; Abade Dos Santos, F.; Correia, F.O.; Santana, C.C.; et al. Emergence of Bluetongue Virus Serotype 3 in Portugal. Viruses 2024, 16, 1845. [Google Scholar] [CrossRef]
- Barua, S.; Rana, E.A.; Prodhan, M.A.; Akter, S.H.; Gogoi-Tiwari, J.; Sarker, S.; Annandale, H.; Eagles, D.; Abraham, S.; Uddin, J.M. The Global Burden of Emerging and Re-Emerging Orbiviruses in Livestock: An Emphasis on Bluetongue Virus and Epizootic Hemorrhagic Disease Virus. Viruses 2024, 17, 20. [Google Scholar] [CrossRef]
- Mayo, C.; Mullens, B.; Gibbs, E.P.; MacLachlan, N.J. Overwintering of Bluetongue virus in temperate zones. Vet. Ital. 2016, 52, 243–246. [Google Scholar]
- Wilson, A.; Darpel, K.; Mellor, P.S. Where does bluetongue virus sleep in the winter? PLoS Biol. 2008, 6, e210. [Google Scholar] [CrossRef]
- Kęsik-Maliszewska, J.; Larska, M.; Collins, Á.B.; Rola, J. Post-Epidemic Distribution of Schmallenberg Virus in Culicoides Arbovirus Vectors in Poland. Viruses 2019, 11, 447. [Google Scholar] [CrossRef]
- Falconi, C.; López-Olvera, J.R.; Gortázar, C. BTV infection in wild ruminants, with emphasis on red deer: A review. Vet. Microbiol. 2011, 151, 209–219. [Google Scholar] [CrossRef]
- Talavera, S.; Muñoz-Muñoz, F.; Verdún, M.; Pujol, N.; Pagès, N. Revealing potential bridge vectors for BTV and SBV: A study on Culicoides blood feeding preferences in natural ecosystems in Spain. Med. Vet. Entomol. 2018, 32, 35–40. [Google Scholar] [CrossRef]
- Plumb, G.; Kowalczyk, R.; Hernandez-Blanco, J.A. Bison bonasus. The IUCN Red List of Threatened Species 2020: e.T2814A45156279. Available online: https://www.iucnredlist.org/species/2814/45156279 (accessed on 27 February 2025).
- Raczyński, J. European Bison Pedigree Book 2023; Białowieża National Park: Białowieża, Poland, 2024; p. 9. Available online: https://bpn.gov.pl/pliki-do-pobrania/pobierz/7cc95262-0985-45df-9b39-493b293e87da.pdf (accessed on 27 February 2025).
- Nores, C.; Álvarez-Laó, D.; Navarro, A.; Pérez-Barbería, F.J.; Castaños, P.M.; Castaños de la Fuente, J.; Morales Muñiz, A.; Azorit, C.; Muñoz-Cobo, J.; Fernández Delgado, C.; et al. Rewilding through inappropriate species introduction: The case of European bison in Spain. Conserv. Sci. Pract. 2024, 6, e13221. [Google Scholar] [CrossRef]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasites Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Larska, M.; Tomana, J.; Socha, W.; Rola, J.; Kubiś, P.; Olech, W.; Krzysiak, M.K. Learn the Past and Present to Teach the Future—Role of Active Surveillance of Exposure to Endemic and Emerging Viruses in the Approach of European Bison Health Protection. Diversity 2023, 15, 535. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K. Infectious Disease Monitoring of European Bison (Bison Bonasus). In Wildlife Population Monitoring; Ferretti, M., Ed.; IntechOpen Limited: London, UK, 2019; pp. 1–21. [Google Scholar]
- WOAH Terrestrial Manual 2021, Bluetongue (Infection with Bluetongue Virus), Chapter 3.1.3. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.03_BLUETONGUE.pdf (accessed on 20 January 2025).
- Hofmann, M.; Griot, C.; Chaignat, V.P.; Erler, L.; Thür, B. Bluetongue disease reaches Switzerland. Schweiz. Arch. Tierheilkd. 2008, 150, 49–56. (In German) [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Videvall, E.; Bensch, S.; Ander, M.; Chirico, J.; Sigvald, R.; Ignell, R. Molecular identification of bloodmeals and species composition in Culicoides biting midges. Med. Vet. Entomol. 2013, 27, 104–112. [Google Scholar] [CrossRef]
- Friedrich-Loeffler-Institut (FLI) Webpage on the Bluetongue Situation. Available online: https://www.fli.de/de/aktuelles/tierseuchengeschehen/blauzungenkrankheit/ (accessed on 1 March 2025).
- Rodrigues, E.; (PWN Waterleidingbedrijf Noord-Holland, Velserbroek, The Netherlands); Rasmussen, T.B.; (Statens Serum Institut, Copenhagen, Denmark). Personal communication, 2024.
- Krzysiak, M.K.; Iwaniak, W.; Kęsik-Maliszewska, J.; Olech, W.; Larska, M. Serological study of exposure to selected arthropod-borne pathogens in European bison (Bison bonasus) in Poland. Transbound. Emerg. Dis. 2017, 64, 1411–1423. [Google Scholar] [CrossRef]
- Orłowska, A.; Trębas, P.; Smreczak, M.; Marzec, A.; Żmudziński, J.F. First detection of bluetongue virus serotype 14 in Poland. Arch. Virol. 2016, 161, 1969–1972. [Google Scholar] [CrossRef]
- Koltsov, A.; Tsybanov, S.; Gogin, A.; Kolbasov, D.; Koltsova, G. Identification and characterization of bluetongue virus serotype 14 in Russia. Front. Vet. Sci. 2020, 7, 26. [Google Scholar] [CrossRef]
- Flannery, J.; Frost, L.; Fay, P.; Hicks, H.; Henstock, M.; Smreczak, M.; Orłowska, A.; Rajko-Nenow, P.; Darpel, K.; Batten, C. BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms. Microorganisms 2020, 8, 892. [Google Scholar] [CrossRef]
- Krzysiak, M.K.; Anusz, K.; Konieczny, A.; Rola, J.; Salat, J.; Strakova, P.; Olech, W.; Larska, M. The European bison (Bison bonasus) as an indicatory species for the circulation of tick-borne encephalitis virus (TBEV) in natural foci in Poland. Ticks Tick Borne Dis. 2021, 12, 101799. [Google Scholar] [CrossRef]
- Juszczyk, A.; Larska, M.; Krzysiak, M.K. Frequency of tick infestation in European bison species in the context of changing environmental conditions as an indicator of potential risk of pathogen exposure. In Proceedings of the Conference 100 Years of European Bison Restitution, Niepołomice, Poland, 6–8 March 2023; pp. 37–38. [Google Scholar]
- Foxi, C.; Delrio, G.; Falchi, G.; Marche, M.G.; Satta, G.; Ruiu, L. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasites Vectors 2016, 9, 440. [Google Scholar] [CrossRef]
- Goffredo, M.; Catalani, M.; Federici, V.; Portanti, O.; Marini, V.; Mancini, G.; Quaglia, M.; Santilli, A.; Teodori, L.; Savini, G. Vector species of Culicoides midges implicated in the 2012–2014 Bluetongue epidemics in Italy. Vet. Ital. 2015, 51, 131–138. [Google Scholar]
- Yavru, S.; Dik, B.; Bulut, O.; Uslu, U.; Yapici, O.; Kale, M.; Avci, O. New Culicoides vector species for BTV transmission in Central and Central West of Anatolia. Ann. Res. Rev. Biol. 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, B.; Bauer, B.; Bauer, C.; Bätza, H.J.; Beer, M.; Clausen, P.H.; Geier, M.; Gethmann, J.M.; Kiel, E.; Liebisch, G.; et al. Monitoring of putative vectors of bluetongue virus serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 1481–1484. [Google Scholar] [CrossRef]




| Variable | BTV Seropositive | Pan-BTV RT-PCR | ||
|---|---|---|---|---|
| n/N 1 | % (CI 2) | n/N 1 | Strain | |
| Origin (total population size 3) | ||||
| A—Wolin National Park (9) | 1 4/3 | 33.3 (0.8–90.6) | 1 4/1 | BTV-3 |
| B—Zachodniopomorskie herds (349) | 0/4 | 0 (0–60.2) | 0/0 | |
| I—Bieszczady (750) | 0/10 | 0 (0–30.8) | 0/0 | |
| C—Poznań Zoo (9) | 0/1 | 0 (0–97.5) | 0/0 | |
| D—Gołuchów (7) | 0/2 | 0 (0–84.1) | 0/0 | |
| E—Pszczyna (53) | 1/51 | 2 (0.05–10.5) | 0/1 | |
| F—Niepołomice (16) | 0/1 | 0 (0–97.5) | 0/0 | |
| G—Gdańsk Zoo (8) | 0/2 | 0 (0–84.1) | 0/0 | |
| H—Bałtów (9) | 0/4 | 0 (0–60.2) | 0/0 | |
| J—Borecka Forest (127) | 0/3 | 0 (0–70.8) | 0/0 | |
| K—Augustowska Forest (23) | 0/1 | 0 (0–97.5) | 0/0 | |
| L—Knyszyńska Forest (298) | 3/16 | 18.8 (4.0–45.6) | 0/3 | |
| M—Kopna Góra | 0/1 | 0 (0–97.5) | 0/0 | |
| N—Białowieża Forest (829) | 33/62 | 53.2 (40.1–66.0) | 0/33 | |
| Population type | ||||
| Free-ranging | 35/82 | 42.7 (31.8–54.1) | ||
| captive | 3/79 | 3.8 (0.7–10.7) | ||
| Age group | ||||
| ≤1 year old | 2/26 | 7.7 (1.0–25.1) | 0/1 | |
| 2–3 years old | 1/38 | 2.6 (0.056–13.8) | 0/1 | |
| ≥4 years old | 34/97 | 35.0 (25.9–45.8) | 1 4/34 | BTV-3 |
| Gender | ||||
| Female | 22/72 | 30.6 (20.5–43.0) | 1 4/22 | BTV-3 |
| Male | 15/89 | 16.8 (9.7–26.3) | 0/15 | |
| Health status | ||||
| Immobilized (healthy) | 7/84 | 8.3 (3.4–16.4) | 0/7 | |
| Eliminated | 17/40 | 42.5 (27.0–59.1) | 0/17 | |
| Fallen | 11/26 | 42.3 (23.4–63.1) | 1 4/11 | BTV-3 |
| Dead in traffic accident | 2/7 | 28.6 (3.7–71.0) | 0/2 | |
| Missing data | 1/4 | 25.0 (0.6–80.6) | 0/1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larska, M.; Orłowska, A.; Łopuszyński, W.; Skurka, Ł.; Nowakowska, A.; Trębas, P.; Krzysiak, M.K.; Rola, J.; Smreczak, M. First Detection of Bluetongue Virus Type 3 in Poland in 2024—A Case Study in European Bison (Bison bonasus). Pathogens 2025, 14, 377. https://doi.org/10.3390/pathogens14040377
Larska M, Orłowska A, Łopuszyński W, Skurka Ł, Nowakowska A, Trębas P, Krzysiak MK, Rola J, Smreczak M. First Detection of Bluetongue Virus Type 3 in Poland in 2024—A Case Study in European Bison (Bison bonasus). Pathogens. 2025; 14(4):377. https://doi.org/10.3390/pathogens14040377
Chicago/Turabian StyleLarska, Magdalena, Anna Orłowska, Wojciech Łopuszyński, Łukasz Skurka, Agnieszka Nowakowska, Paweł Trębas, Michał K. Krzysiak, Jerzy Rola, and Marcin Smreczak. 2025. "First Detection of Bluetongue Virus Type 3 in Poland in 2024—A Case Study in European Bison (Bison bonasus)" Pathogens 14, no. 4: 377. https://doi.org/10.3390/pathogens14040377
APA StyleLarska, M., Orłowska, A., Łopuszyński, W., Skurka, Ł., Nowakowska, A., Trębas, P., Krzysiak, M. K., Rola, J., & Smreczak, M. (2025). First Detection of Bluetongue Virus Type 3 in Poland in 2024—A Case Study in European Bison (Bison bonasus). Pathogens, 14(4), 377. https://doi.org/10.3390/pathogens14040377






