Abstract
This study investigated the effects of dietary supplementation with Mentha piperita (MP) on growth, immune enhancement, and disease resistance in Nile tilapia (Oreochromis niloticus) over a 90-day period, particularly against Aeromonas hydrophila. MP was incorporated into the diets at concentrations of 0.0%, 0.2%, 0.4%, and 0.6%. Analysis of the essential oil composition of MP identified menthol derivatives as the primary components, along with other bioactive compounds. The results revealed that MP supplementation significantly enhanced growth performance, with fish receiving the 0.6% MP diet achieving the highest weight gain, growth rate, and feed efficiency. Additionally, MP significantly enhanced the fish’s resistance to A. hydrophila infection, with the highest survival rate observed in the 0.6% MP group. Further analyses revealed that MP positively influenced blood parameters, improving RBC and WBC counts, hemoglobin levels, as well as serum immunoglobulin M and phagocytic activity. MP also mitigated oxidative stress by increasing antioxidant enzyme activity and reducing malondialdehyde levels. Moreover, MP supplementation at the concentration of 0.6% maintained intestinal integrity against bacterial damage. Gene expression analysis showed that MP upregulated insulin-like growth factor 1, suggesting a potential mechanism for improved growth. Interestingly, MP downregulated the expression of the inflammatory gene nuclear factor kappa B before the bacterial challenge, while its expression remained more downregulated post-challenge compared to control. These findings highlight the potential of MP as an effective feed additive that enhances growth rates in Nile tilapia, boosts immunity against diseases, and improves their overall health.
1. Introduction
The farming of aquatic organisms, including water-accommodating animals and plants, is generally termed aquaculture, which occurs in both marine and freshwater systems [1]. While marine aquaculture often receives more attention for its role in seafood and aquaculture production, freshwater aquaculture is crucial for global food security and economic growth [2]. Freshwater aquaculture involves farming various aquatic species such as fish, crustaceans, mollusks, and aquatic plants in inland water bodies, including fishponds, natural or man-made lakes, rivers, and recirculation systems [3]. This sector is an important source of income, protein, and other nutrients, especially in areas with restricted access to marine products [4]. Species, such as tilapias, catfish, carps, and trout, commonly cultured in freshwater systems, are easier to obtain and cheaper than marine species, playing a crucial role in the distribution chain for local communities and small-scale farmers [1].
The tilapia, a group of cichlid fish mainly found in Africa, is among the most common species in aquaculture [5]. It reproduces quickly, can be raised in various conditions, and its production is relatively inexpensive, making it a popular choice in fish farming worldwide [6]. Tilapia is an essential source of protein and other nutrients for humans, particularly in the middle- and low-income countries. It has a relatively good taste, and its affordability makes it accessible to many consumers [7]. However, diseases remain a significant concern in tilapia farming. Bacterial diseases such as Aeromonas and Streptococcus species cause high mortality rates and economic losses [8]. In addition, parasitic infestations—such as those caused by Diplostomum and Contracaecum species—and viral infections, including Tilapia lake virus and Tilapia larvae encephalitis virus, also negatively affect tilapia health and overall productivity [9,10].
Aeromonas hydrophila is an opportunistic pathogen commonly found in freshwater environments. It can affect a wide range of freshwater fish, as well as other aquatic animals such as soft-shelled turtles. When infections occur, they can result in high mortality rates in both wild and farmed species, including catfish and tilapia [11,12]. A. hydrophila employs various mechanisms to evade the host immune system. For example, it produces specific nucleases (e.g., ahn) that have been shown to influence its susceptibility to being killed by fish macrophages [13]. However, an overactive host immune response can exacerbate the damage caused by A. hydrophila. For instance, excessive expression of tumor necrosis factor α (tnfα) amplifies the pathogen’s harmful effects on the liver—such as necrosis, cell swelling, nuclear displacement, and blurred cellular boundaries. It also intensifies the inflammatory response in the midgut, leading to increased villi rupture, vacuolization, fusion, and a marked reduction in goblet cell numbers. These changes collectively result in severe intestinal barrier damage [14].
Additionally, antibiotic resistance is frequently observed in bacteria isolated from fish, including A. hydrophila [11,15]. This has prompted research into alternative medicinal strategies to enhance immune responses, improve the antibacterial activity of antibiotics—potentially through synergistic mechanisms—and strengthen overall disease resistance [16,17]. Studies have shown that medicinal herbs play a positive modulatory role in fish, supporting growth, immune function, and reproductive health [18]. These herbs influence key effectors of growth (e.g., growth hormone (Gh) and insulin-like growth factor 1 (Igf1)), immunity (e.g., mucin-like protein, interleukin (Il) 1β, Tnfα, Il6, and interferon γ), and reproduction (e.g., vitellogenins, androgen receptors, estrogen receptors, and follicle-stimulating hormone β) [18].
Studies indicate that natural immunostimulants, including certain medicinal plants, probiotics, and other compounds, can enhance both the innate and adaptive immunity of fish against infections [19,20,21]. Plants that are rich in bioactive compounds—such as essential oils, polysaccharides, and polyphenols—serve as effective natural immunostimulants [22]. Dietary peppermint (Mentha piperita; MP) can improve tilapia resistance to bacterial and parasitic infections [23,24]. Plant-derived immunostimulants are eco-friendly and help reduce antibiotic use, controlling the emergence of antibiotic-resistant bacteria.
MP, known for its stimulating aroma and taste, has been shown to function as a natural immunostimulant in tilapia aquaculture, enhancing disease resistance against various pathogens, including bacteria (e.g., Vibrio species) [23,25]. MP has also demonstrated effective antiviral activity and notable free-radical scavenging properties, attributed to its high content of phenolic acids and flavonoids [26]. It can modulate the levels of key pro-inflammatory mediators and cytokines, including nitric oxide, TNFα, IL6, and prostaglandin E2 [26].
MP contains bioactive compounds such as menthol, menthofuran, menthyl acetate, menthone, and phenolic compounds with antioxidant and anti-inflammatory properties [27,28]. These components improve the fish’s immune system in multiple ways: they increase phagocytosis, activating cells that patrol and eliminate pathogens [29]; they stimulate antibody synthesis, helping identify and neutralize pathogens [30]; and they regulate cytokine production, orchestrating the immune response [29]. Additionally, peppermint’s antioxidative properties protect immune cells from oxidation, maintaining their functionality [31]. Tilapia fed with peppermint-supplemented diets gained better protection against bacterial infections, such as Vibrio alginolyticus, improving their immunity and other qualities that enhance their ability to fight infections [23]. Moreover, peppermint can elevate the fish’s blood parameters, including red and white blood cell counts, further fortifying their overall health and ability to combat infections [32]. Peppermint’s eco-friendliness compared to synthetic antibiotics, which are expensive and environmentally harmful, makes it a preferred immunostimulant [33].
Several studies have investigated the effects of MP on Nile tilapia (Oreochromis niloticus), focusing on its impact against parasites [24], Vibrio alginolyticus [23], Streptococcus agalactiae [34], as well as its role as a stress mitigator during fish transportation [35]. Additionally, MP has been studied in combination with probiotics to enhance resistance against Aeromonas hydrophila in Catla catla (Hamilton, 1822) [36]. These studies examined various parameters to understand the mechanisms by which MP influences fish physiological systems.
Previous studies have often tested MP leaves in high doses, ranging from 1% to 8% of the diet in various fish species [23,25,36,37]. In Nile tilapia, experiments using MP leaf concentrations of 0%, 2%, 3%, and 4% found that 2% was the most suitable level [23]. However, these concentrations may be considered feed raw materials rather than feed additives. Additionally, high doses of MP could pose potential risks [38]. For example, pulegone, a component of peppermint oil, should not exceed a 1% concentration in external preparations due to its potential toxic effects. High doses can also lead to hepatotoxicity [39]. In fish, the optimal supplementation levels remain unclear, and excessive concentrations may cause adverse health effects, including stress and digestive issues [27]. Therefore, lower concentrations of MP should be tested as a feed additive to evaluate both its production and health benefits. Moreover, lower doses may offer greater potential for long-term inclusion compared to higher doses.
Therefore, further research is needed to clarify the mechanisms of MP at relatively lower concentrations in different fish species and to explore its potential benefits against other, yet unstudied, pathogens. This study aimed to evaluate the effects of incorporating MP into Nile tilapia diets at low doses (0.0%, 0.2%, 0.4%, and 0.6%). Key areas of investigation included growth performance, survival rates, blood parameters, immune responses, oxidative stress markers, and intestinal health, particularly following an A. hydrophila challenge, a significant pathogen in aquaculture. Additionally, the study analyzed the composition of M. piperita essential oils.
2. Materials and Methods
2.1. Gas Chromatography and Mass Spectrometry (GC-MS) of Mentha Piperita
The Mentha piperita (MP) leaves were subjected to extraction using solid-phase microextraction (SPME) at 50 °C for 20 min before being injected into the GC. The GC-MS system (Agilent Technologies, Inc., Santa Clara, CA, USA) consisted of a 7890B gas chromatograph coupled with a 5977A mass spectrometer detector. The gas chromatograph utilized an HP-5MS column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness). Hydrogen was used as the carrier gas at a flow rate of 1.1 mL/min in splitless injection mode. The temperature program was as follows: an initial temperature of 50 °C (held for 0 min), increasing at a rate of 5 °C/min to 200 °C (held for 0 min), followed by a rise of 20 °C/min to 280 °C, which was maintained for 6 min. The injector and detector temperatures were set at 250 °C and 320 °C, respectively. Mass spectra were obtained using electron ionization (EI) at 70 eV, with a scan range of m/z 50–600 and no solvent delay. The ion source temperature was maintained at 230 °C, while the quadrupole temperature was set at 150 °C. Identification of constituents was performed by comparing spectral fragmentation patterns with those in the Wiley and NIST Mass Spectral Library databases.
2.2. Experimental Diets and Fish Grouping
Peppermint was purchased from a local market in the Kafrelsheikh district, Egypt, and was identified as MP in the botanical laboratory of the Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt. The leaves of MP were shade-dried, crushed into powder using an electric grinder, and mixed directly with fish feed to achieve three concentrations at 0.2%, 0.4%, and 0.6% of feed. The control diet was prepared without MP (Table 1). The produced pellets were air-dried and kept at 4 °C until use. The chemical composition of the formulated diets was determined according to AOAC [40].

Table 1.
Feed ingredients and proximate analysis of diets.
Initially, 180 all-male Nile tilapia (Oreochromis niloticus) were procured from a privately owned aquafarm in the Kafrelsheikh district, Egypt. An additional group of fish was reared under the same experimental conditions to determine the challenge adjustment dose and serve as a negative control during the challenge procedures. All fish underwent a week-long acclimatization period in holding containers equipped with adequate oxygenation and submerged filters.
Water parameters were continuously monitored to ensure optimal rearing conditions. Thereafter, uniform-sized fish averaging 10.85 ± 0.096 g were randomly assigned to twelve glass aquariums (four clusters separated into triplicate sets), each housing them in 60 L volumes (15 organisms per container). Every aquarium received consistent airflow, with partial water replacements executed daily using dechlorinated water. Prepared feeds were distributed equally among the tanks at a dose equivalent to 5% of fish weights, administered three times daily (09:00 am, 12:00 pm, and 03:00 pm) for ninety consecutive days (12 h light cycle balanced against 12 h darkness). Body weights were documented routinely every fourteen days to readjust food consumption rates while monitoring the general well-being of the population. Throughout the experiment, water quality and rearing conditions were closely monitored. The water indices had optimal levels of dissolved oxygen at 6.08 ± 0.18 mg/L, temperature at approximately 27 ± 1.18 °C, pH at 7.2 ± 0.16, and ammonia levels ranging from 0.03 to 0.42 mg/L.
2.3. Growth Performance and Feed Utilization
At the end of the experiment, fish were anesthetized with tricaine methane sulfonate (MS222, 25 mg/L, Argent Laboratories, Redmond, Washington) to measure the individual weight and length (L) of each fish. Fish growth performance and somatic indices were estimated [42,43]. The other growth performance parameters and feed utilization were calculated as follows:
where ln = natural log, W1 = final weight at the end of the experiment (g), W0 = initial weight (g), Lt = final length of fish (cm), L0 = initial fish length, and t = experimental period (days).
Weight gain ratio (WG%) = (W1 − W0)/W0 × 100
Feed conversion ratio (FCR) = feed intake (g)/BWG (g)
Specific growth rate (SGR%/day) = 100 × (lnW1 − lnW0)/t
Survival rate (%) = (total number of fish at the end of the experiment/total number of fish at the start of the experiment) × 100
Average daily gain (ADG; g fish−1 day−1) = Wt − W0/days
Length gain (cm fish−1) = Lt − L0
Condition factor (K) = 100 × (W1/Lt3)
2.4. Experimental Bacterial Challenge
Following the 90-day feeding trial, a health assessment was conducted to check for active systemic infections, including those caused by Aeromonas spp. For the microbiological examination, blood and tissue samples were collected from three fish per container (liver, spleen, and kidneys). The sampling was performed using the procedures described in Section 2.5: Tissue Sampling and Blood Collection. The collected samples were promptly cultured on general nutrient and selective media to detect bacterial growth. If bacterial growth was detected, biochemical testing, including the VITEK system, was used for identification. Based on the microbiological analysis, the absence of external clinical signs of disease, there being no observed mortalities during the feeding trial, and the healthy appearance of the fish, it was concluded that the fish were in good health and showed no signs of active systemic infection.
After this assessment, the fish were subjected to a bacterial challenge with Aeromonas hydrophila (ATCC-13037), obtained from the Microbiological Resources Centre (Cairo Mircen). Before the challenge, an LD50 trial was conducted using fish from the same batch as those used in the 90-day feeding trial. These fish were maintained under identical rearing conditions. For the LD50 assay, fish were injected intraperitoneally with bacterial suspensions at concentrations of 104, 105, 106, 107, 108, 109, and 1010 CFU/mL. Mortality was recorded over a 96-h period, and the LD50 value was calculated using probit analysis, yielding an LD50 of 1 × 109 CFU/mL. Based on these results, a bacterial concentration of 1 × 108 CFU/mL (equivalent to 1/10 of the LD50, or sublethal dose) was selected for the subsequent challenge experiment. This sublethal concentration was selected to monitor physiological changes across groups while avoiding high mortality rates that could obscure infection mechanisms, enabling the study of host responses without overwhelming the body’s systems.
The bacterial cells were harvested by centrifugation at 3000× g for 10 min, washed twice with sterile phosphate-buffered saline (PBS) to remove residual culture media, and resuspended in PBS before injection. The different experimental groups (in three replicates) then received an intraperitoneal injection of 0.2 mL of a suspension containing 1 × 10⁸ CFU/mL of A. hydrophila, as described by Moustafa et al. [44]. Mortality was monitored over a 14-day observation period according to the methods outlined by Naiel et al. [45]. The fish continued to receive their designated diets during this period. Cumulative survival rate was calculated using the following formula:
At the onset of the challenge, each group consisted of 10 fish per replicate, totaling 30 fish per group.
2.5. Tissue Sampling and Blood Collection
Pre- and post-challenge samples were obtained from three fish per tank (nine per group) after 90 days of experimental feeding and 15 days after bacterial challenge. Whole blood was collected from the caudal vein using sterile syringes containing heparin. For serum collection, blood was drawn into plain tubes (without anticoagulants). The collected sera were then stored at −20 °C until further analysis. Liver samples were also taken and kept at −80 °C for RNA extraction. Additionally, intestine samples—including the anterior (immediately following the stomach), middle (central intestinal region), and posterior (preceding the anus) sections—were collected for histopathological examination, as described by Olsson [46] and Okuthe and Bhomela [47].
2.6. Hematology and Blood Biochemical Analyses
An automated blood cell counter provided measurements for red blood cells (RBCs), hemoglobin, and packed cell volume (PCV). White blood cell (WBC) counts were determined using a combination of blood smear analysis and hemocytometer data [48]. Differential WBC counts involved preparing and staining blood smears with a modified Wright’s stain. Under high magnification (×100 oil immersion), 100 cells were counted to differentiate percentages of heterophils, lymphocytes, and monocytes. Further analyses included total protein using a commercial kit, albumin using the bromocresol green binding method, calculated globulin, creatinine (colorimetric method as per Heinegård and Tiderström [49]), activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (colorimetric method at 540 nm as described by Diab, et al. [50]), and serum triglycerides and total cholesterol measured using the kits by BioDiagnostic Co., Cairo, Egypt.
2.7. Immune and Oxidative Stress Responses
Phagocytic activity and the phagocytic index, as described by Abo-Al-Ela et al. [51], were determined. Briefly, fresh blood samples were incubated with Candida albicans at 37 °C for 1 h. Blood smears were prepared, stained with Giemsa, and examined under a microscope. Phagocytic activity represents the percentage of phagocytic cells containing yeast, while the phagocytic index indicates the average number of yeast particles per phagocytic cell.
ELISA, a technique described by Demers and Bayne [52], was used to measure serum lysozyme activity and immunoglobulin M (IgM) levels. A commercially available ELISA kit (BioDiagnostic Co., Egypt) was employed to assess the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), along with malondialdehyde (MDA) concentration. Measurements were performed at a wavelength of 450 nm using a microplate ELISA reader as detailed in Ren et al. [53].
2.8. Histomorphological Examination
Following a 15-day bacterial challenge, fish intestine samples were fixed in 10% formalin for 48 h, dehydrated in graded ethanol, cleared in xylene, and paraffin-embedded. Five-micron sections were then cut using a microtome and stained with hematoxylin and eosin [54] for histological examination.
2.9. cDNA Synthesis and Real-Time PCR
Total RNA was extracted from the liver using QIAzol Lysis Reagent (QIAGEN, Hilden, Germany). The quality and quantity of the extracted RNA were confirmed by agarose gel electrophoresis and spectrophotometry, respectively. cDNA synthesis was performed using 2 µg of RNA, following the protocol of the FastLane Cell cDNA Kit (QIAGEN, Germany).
The expression of insulin-like growth factor 1 (igf1) and nuclear factor kappa B (nf-κB) was analyzed in the liver. Specific primers for these genes were designed using mRNA sequences available on the NCBI website. Details regarding primer sequences and corresponding gene bank accession numbers can be found in Table 2. To determine changes in gene expression levels between experimental groups, real-time PCR was performed using a Bio-RAD device (Milpitas, CA, USA) and SYBR green master mix (Enzynomics, Daejeon, Republic of Korea). The cycling conditions included 40 cycles of denaturation at 95 °C for 15 s, primer annealing at 60 °C for 1 min, and an extension step at 72 °C for 30 s. Amplification efficiencies were assessed using standard curve analysis. Based on these efficiencies, the modified 2–ΔΔCt method was used to calculate relative changes in mRNA expression based on cycle threshold (Ct) values obtained during PCR. β-actin was used as the internal reference gene, following the method developed by Pfaffl [55] and briefly described by Elbialy et al. [56].

Table 2.
Primers used for real-time PCR.
2.10. Statistical Analysis
After verifying data for normality and homogeneity of variance, statistical analysis was conducted using GraphPad Prism (version 8.01) software. One-way and two-way ANOVA tests were used to evaluate group differences, followed by Tukey’s post-hoc test for specific variations. Cumulative mortalities and relative protection were assessed using Kaplan–Meier survival analysis, with results interpreted by the log-rank (Mantel–Cox) test. Statistical significance was set at p < 0.05, indicated by different superscript letters. All results are presented as means ± SEM.
3. Results
3.1. Chemical Composition of MP Essential Oils
The major compounds in Mentha piperita (MP) were menthol and its derivative, levomenthol (Table 3). Levomenthol was the most abundant, accounting for 46.79% of the total detected compounds, followed by (-)-neomenthyl acetate (12.96%), L-menthone (5.77%), 2-cyclohexen-1-one, 3-methyl-6-(1-methylethyl)- (4.01%), and D-carvone (3.66%). Other notable compounds included cis-calamenene (3.22%), caryophyllene (3.02%), pulegone (1.25%), and eucalyptol (0.76%). The remaining compounds each contributed less than 2% to the total composition (Table 3).

Table 3.
GC/Mass of M. piperita essential oils.
3.2. Growth Performance, Feed Utilization, and Survivability
Fish fed diets supplemented with MP exhibited the highest growth performance compared to the control group after a 90-day experimental period (Table 4). Statistically significant improvements were observed across various growth metrics, including final body weight (FBW), weight gain (WG), average daily gain (ADG), specific growth rate (SGR), relative growth rate (RGR), final length, and length gain. The survival rates showed no significant differences among the groups, with no mortalities recorded during the 90-day experimental period. Interestingly, the 0.6% MP diet group achieved the highest numerical values for FBW, WG, ADG, SGR, RGR, final length, and length gain, while demonstrating the lowest FCR (Table 4).

Table 4.
Growth performance parameters of Nile tilapia fed different dietary levels of Mentha piperita (peppermint)-supplemented diets for 90 days.
3.3. Survivability
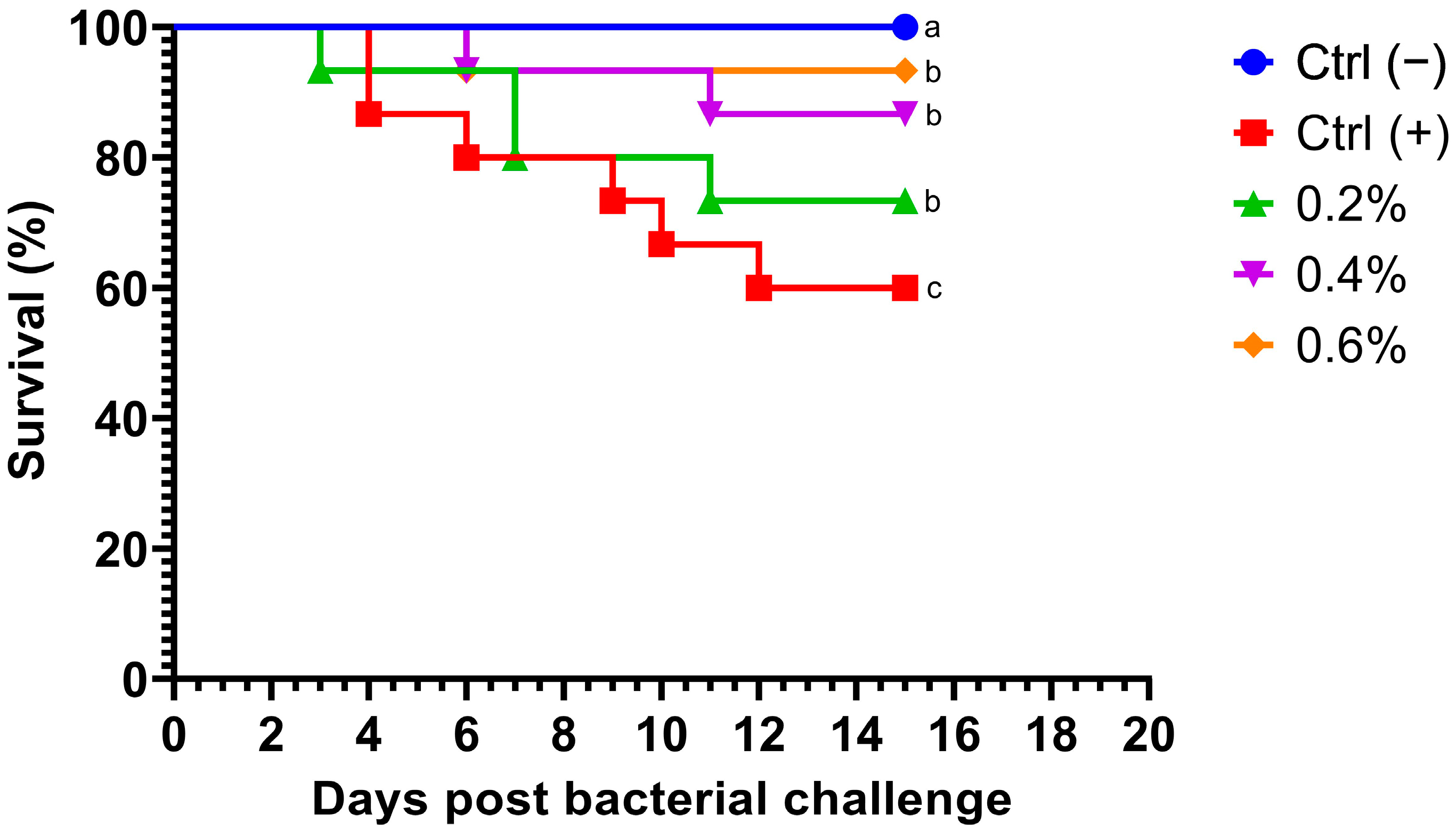
Fish fed MP at different concentrations exhibited a dramatic decrease in mortality after being challenged with A. hydrophila. Notably, no deaths occurred within the first 70 h post-challenge (Figure 1). Survival rates in fish challenged with the bacteria were significantly higher (p = 0.0254) in all treatment groups compared to the control group. Additionally, no mortalities were observed in the negative control group. Fish fed with the highest concentration of MP (0.6%) displayed the most significant cumulative survival rate compared to other treatments.
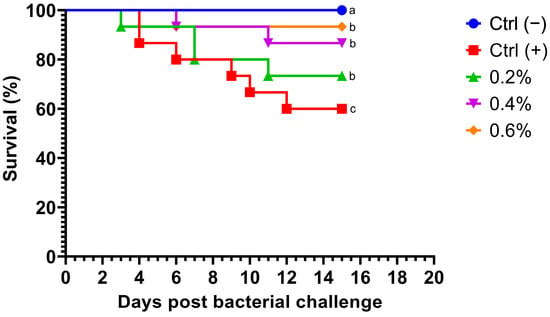
Figure 1.
Kaplan–Meier survival curve of Nile tilapia (Oreochromis niloticus) fed diets supplemented with 0%, 0.2%, 0.4%, and 0.6% Mentha piperita (peppermint) for 90 days and subsequently challenged with Aeromonas hydrophila. Survival curves with different letters indicate significant differences between groups.
3.4. Hematology Results
Table 5 shows that dietary MP significantly impacted hematological parameters in fish both pre- and post-challenge assay. Fish fed MP diets exhibited slight but higher RBC and WBC counts compared to the control group, both before and after the challenge. Notably, the highest RBC and WBC counts were observed in the group fed 0.6% MP feed post-challenge. Hemoglobin (Hb) levels in both pre- and post-challenge groups also showed a significant increase in all MP-fed groups compared to the control. Importantly, all MP-fed groups demonstrated significantly higher packed cell volume (PCV) and mean corpuscular hemoglobin (MCH) compared to the control group.

Table 5.
Hematological indices (mean ± SEM) of Nile tilapia fed different dietary levels of Mentha piperita (peppermint)-supplemented diets for 90 days.
Fish fed MP diets displayed altered proportions of lymphocytes, monocytes, neutrophils, and eosinophils compared to the control group. Although slight decreases in neutrophil levels were observed in the MP-fed groups prior to the challenge, this trend was reversed following the challenge. Lymphocyte levels increased both before and after the challenge in fish fed MP diets compared to the control. In contrast, monocytes and eosinophils showed minimal changes across all groups. The diet containing 0.6% MP demonstrated the most pronounced effect.
3.5. Biochemical Analyses
Table 6 highlights the impact of the MP diets on various biochemical parameters. Fish fed the MP diet, particularly at a 0.6% concentration, showed significant decreases in liver enzymes (ALT and AST) compared to the control group, both before and after the challenge. These enzymes increased post-challenge across all groups. Total protein and albumin content remained unaffected by the dietary treatments. Interestingly, serum globulin and triglycerides were significantly higher in the MP diet groups, especially at 0.6%, compared to the control, pre- and post-challenge. Additionally, the MP diet significantly reduced cholesterol, urea, and creatinine levels in fish both before and after the challenge.

Table 6.
Biochemical analysis (mean ± SEM) of Nile tilapia fed different dietary levels of Mentha piperita (peppermint)-supplemented diets for 90 days.
3.6. Immune Responses
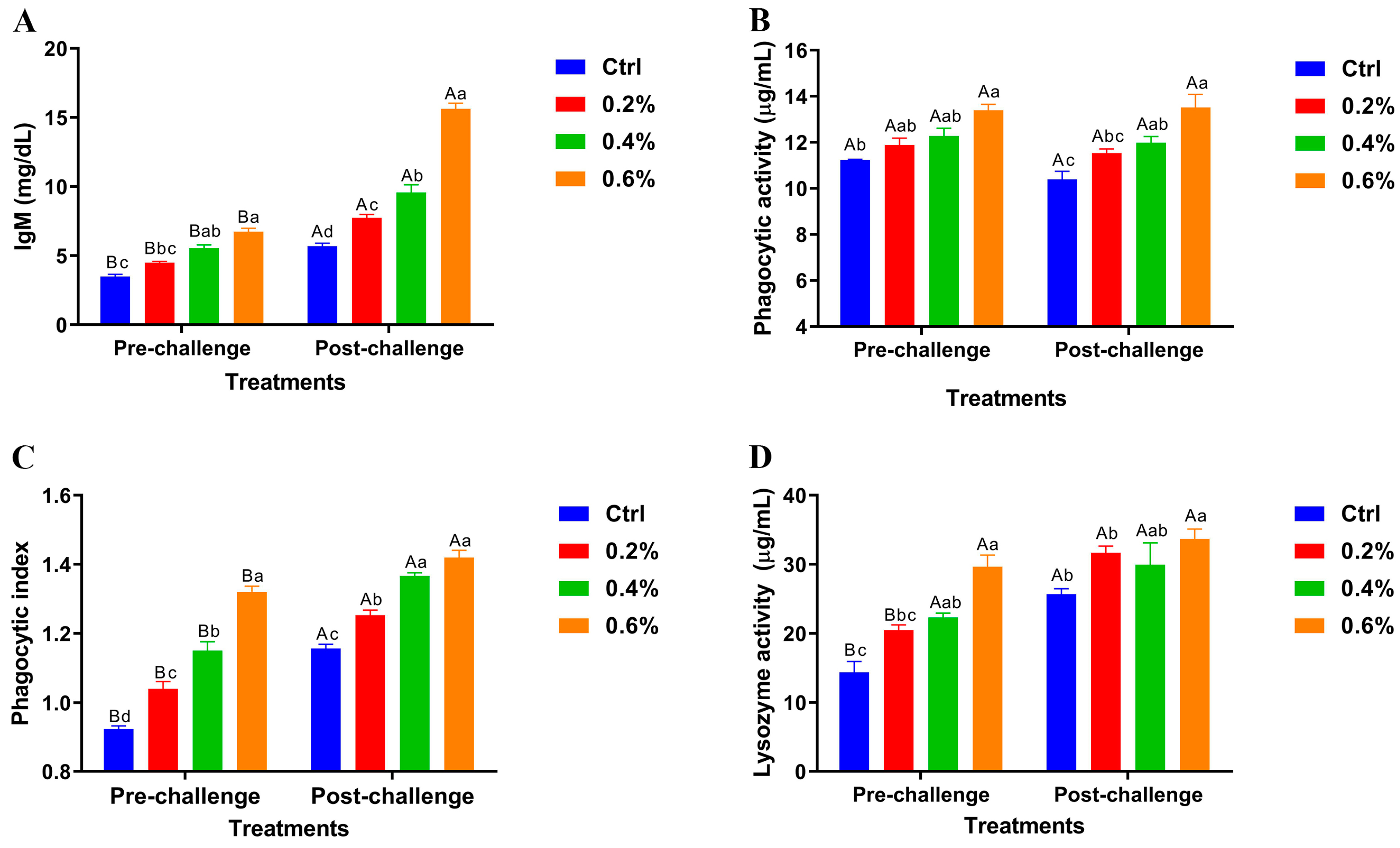
The results revealed significant variations in pre- and post-challenge levels of serum immunoglobulin M (IgM), phagocytic activity, phagocytic index, and lysozyme activity across the dietary groups shown in Figure 2A–D. Importantly, the highest MP concentration (0.6%) demonstrated highly significant differences in these immune parameters compared to the control and other treatment groups, both before and after the challenge.
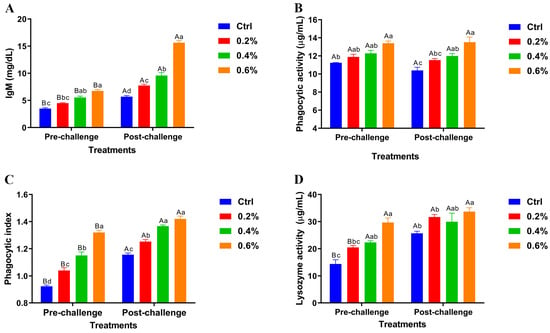
Figure 2.
Pre- and post-challenge serum immunoglobulin M (IgM) levels (A), phagocytic activity (B), phagocytic index (C), and lysozyme activity (D) in Nile tilapia fed diets supplemented with 0%, 0.2%, 0.4%, and 0.6% Mentha piperita (peppermint) for 90 days and challenged with Aeromonas hydrophila. Data are expressed as the mean ± SEM. Values with different letter superscripts are significantly different between groups.
3.7. Oxidative Stress Responses
The findings presented in Table 7 reveal a statistically significant increase in the antioxidant enzymes glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) in fish fed diets supplemented with MP compared to the control, both before and after the challenge. Furthermore, the post-challenge readings for GPx, SOD, and CAT showed higher values compared to their respective pre-challenge levels. Conversely, the concentration of the oxidative stress marker malondialdehyde (MDA) was substantially lower in fish receiving MP supplementation, both pre- and post-challenge. Notably, the post-challenge MDA levels were lower than their pre-challenge levels in all groups, with 0.6% MP showing the lowest MDA activities.

Table 7.
Antioxidant analysis (mean ± SEM; nmol/mL) of Nile tilapia fed different dietary levels of Mentha piperita (peppermint)-supplemented diets for 90 days.
3.8. Histomorphology Features
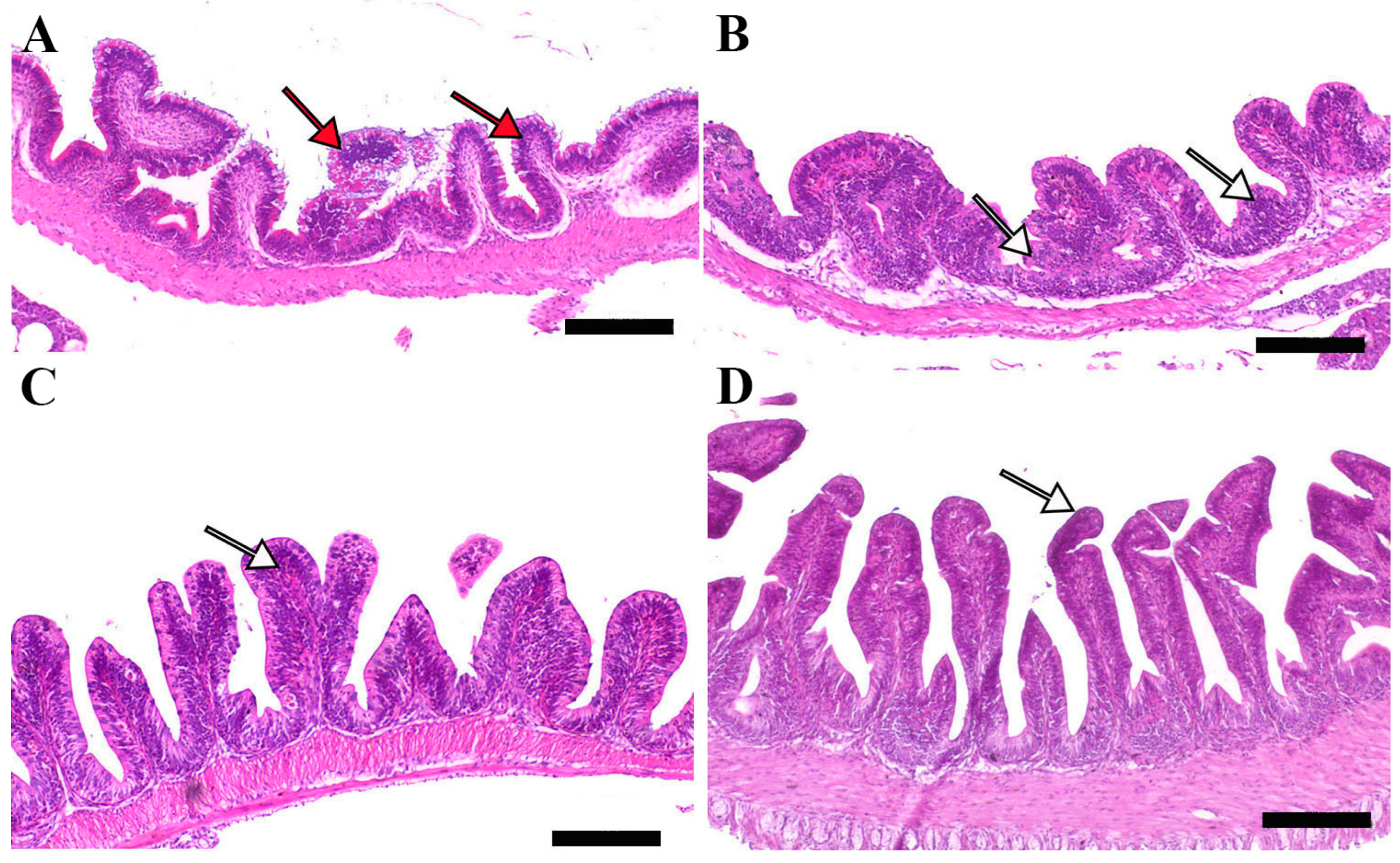
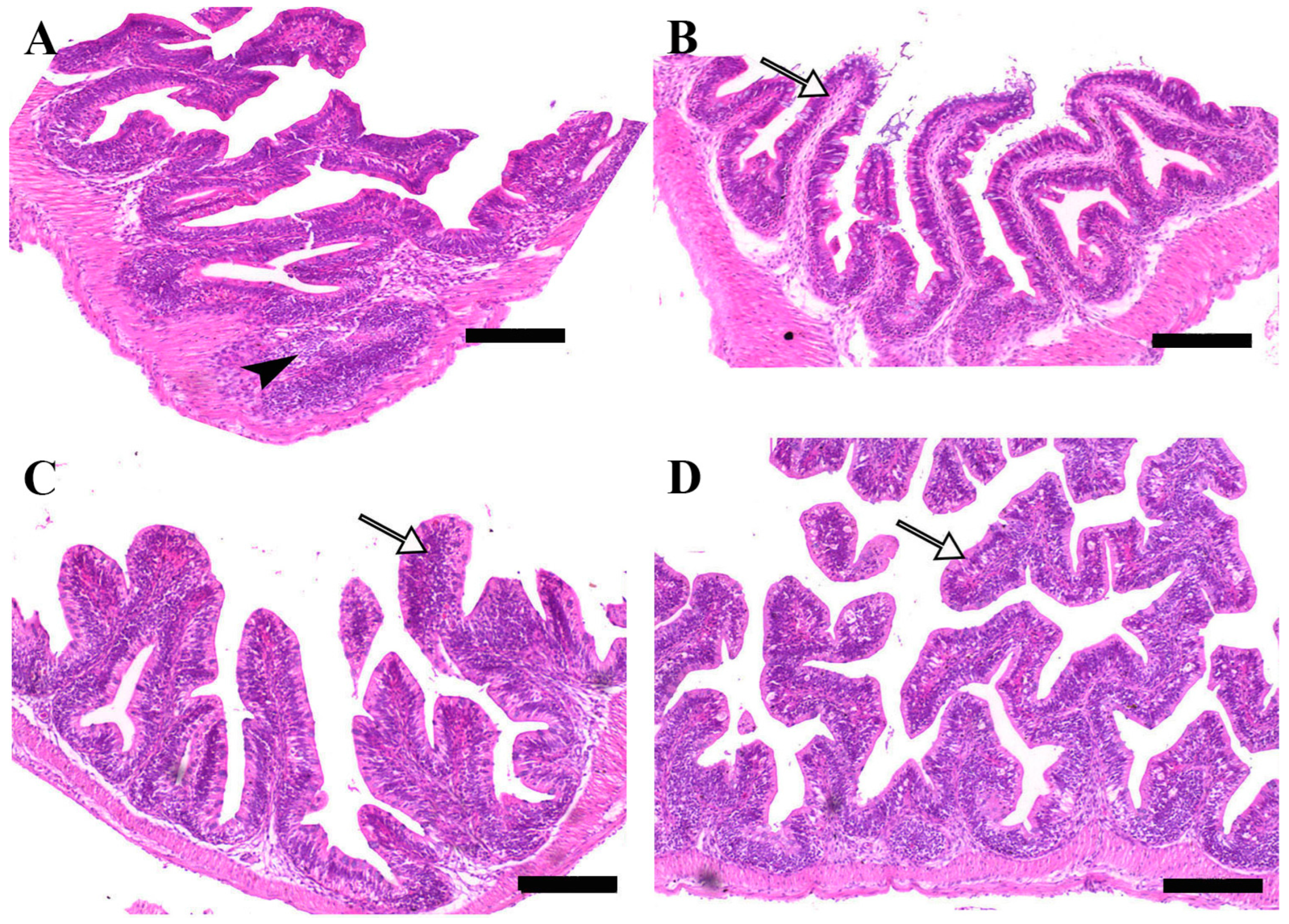
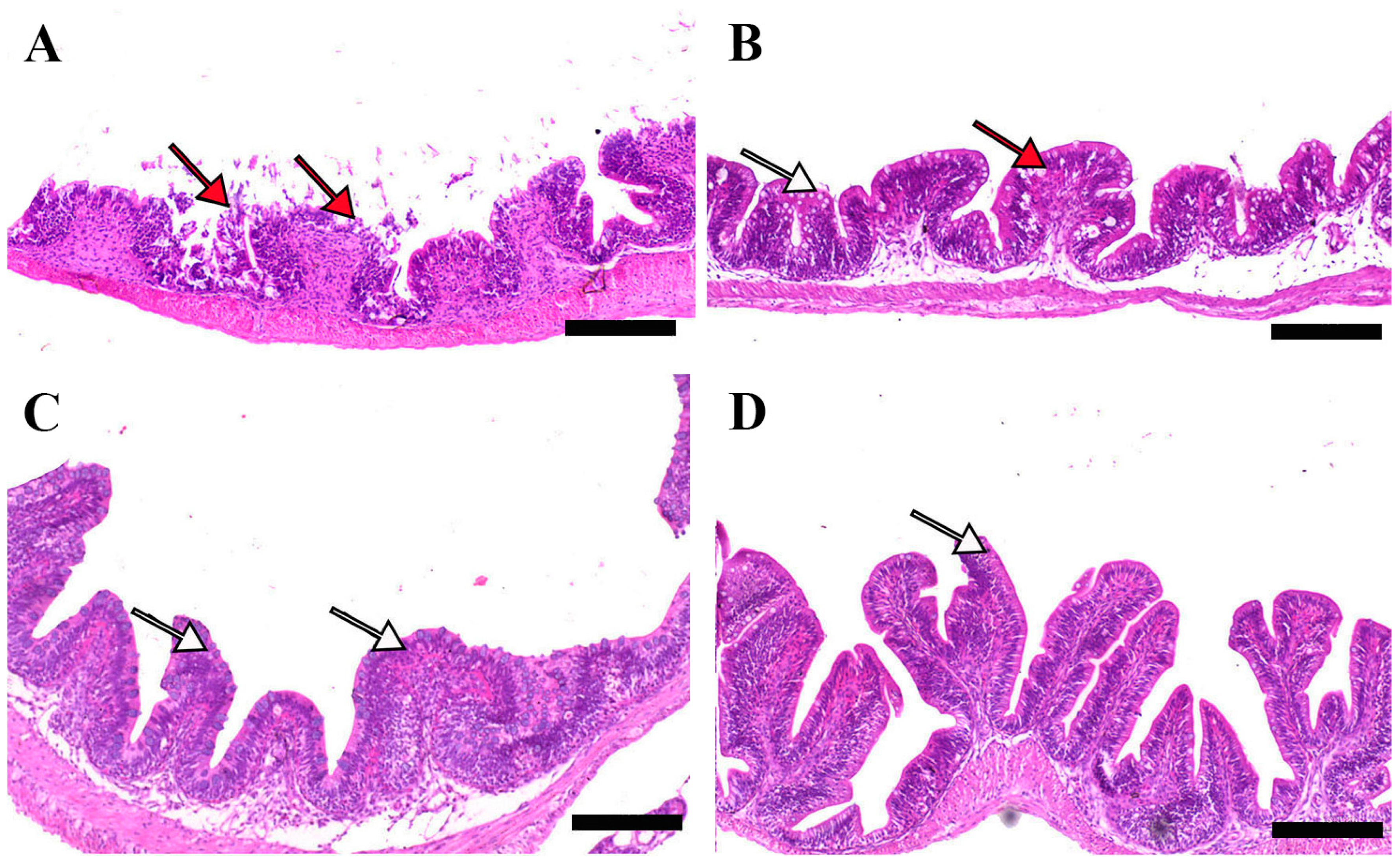
Microscopic examination of intestinal tissue sections after the challenge revealed atrophy, blunting of the mucosal folds, and signs of enteritis, including desquamation of the intestinal lining epithelium, in challenged fish receiving 0.0% MP (control). In contrast, fish supplemented with 0.2% and 0.4% dietary MP showed a reduction in these pathological changes. Notably, fish supplemented with 0.6% MP exhibited improved intestinal structural integrity and an increase in mucosal fold length, enhancing tissue stability against the bacterial challenge caused by A. hydrophila (Figure 3, Figure 4 and Figure 5).
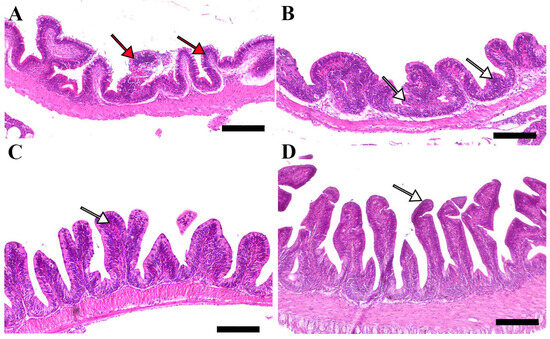
Figure 3.
Representative histomorphological features (post-challenge) of H&E-stained sections from the anterior intestine of Nile tilapia fed diets supplemented with (A) 0%, (B) 0.2%, (C) 0.4%, or (D) 0.6% Mentha piperita (peppermint) for 90 days, followed by challenge with Aeromonas hydrophila. Red arrows indicate atrophic degenerative changes in the intestinal folds, while white arrows highlight normal intestinal folds. Scale bars = 100 μm.
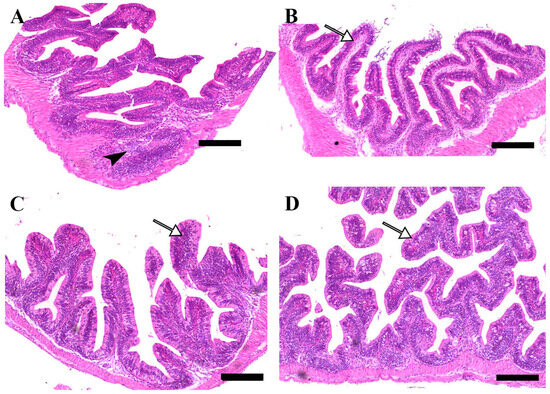
Figure 4.
Representative histomorphological features of H&E-stained sections from the middle intestine of Nile tilapia fed diets supplemented with (A) 0%, (B) 0.2%, (C) 0.4%, or (D) 0.6% Mentha piperita (peppermint) for 90 days, followed by challenge with Aeromonas hydrophila. Black arrowheads indicate focal infiltration of inflammatory cells, while white arrows point to normal intestinal folds. Scale bars = 100 μm.
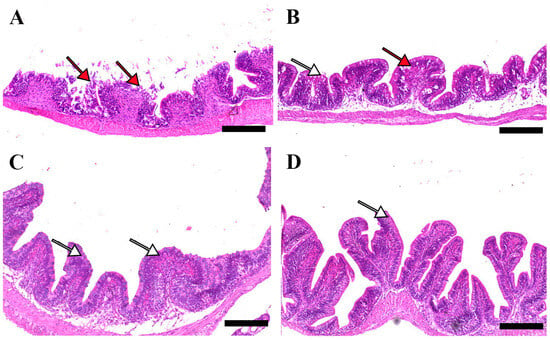
Figure 5.
Representative histomorphological features of H&E-stained sections from the posterior intestine of Nile tilapia fed diets supplemented with (A) 0%, (B) 0.2%, (C) 0.4%, or (D) 0.6% Mentha piperita (peppermint) for 90 days, followed by challenge with Aeromonas hydrophila. Red arrows indicate atrophic degenerative changes in the intestinal folds, while white arrows highlight normal intestinal folds. Scale bars = 100 μm.
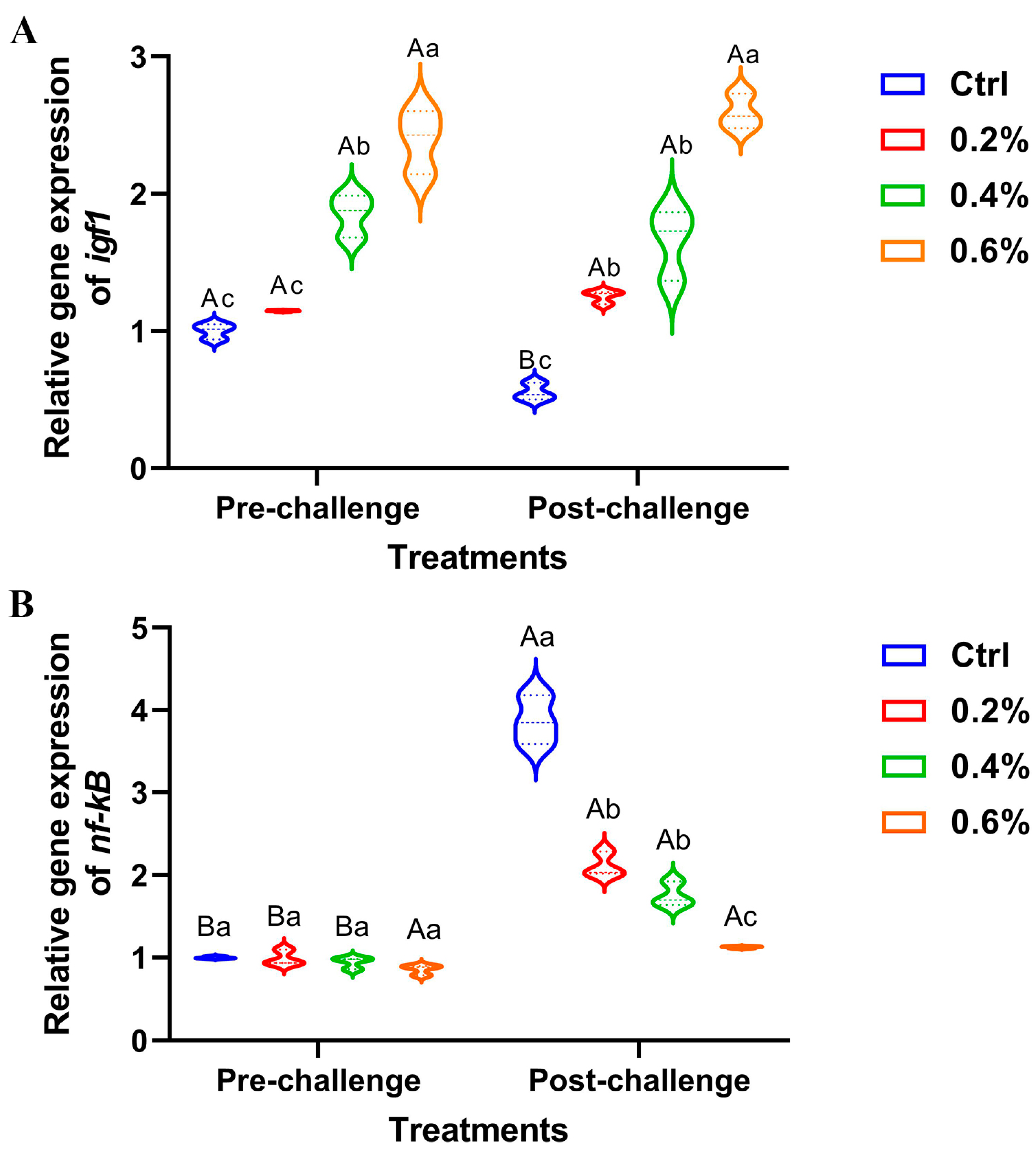
3.9. Gene Expression Results
The data presented in Figure 6A illustrates a significant upregulation of hepatic igf1 expression, both pre- and post-challenge, in fish supplemented with MP, with the most pronounced effect observed in fish receiving 0.6%. There were no changes between the pre- and post-challenge stages for hepatic igf1 expression. Conversely, the expression of the hepatic nf-κB gene exhibited non-significant downregulation in the pre-challenge stage, while significant upregulation was observed post-challenge, particularly in the control group compared to the other treatment groups (Figure 6B).
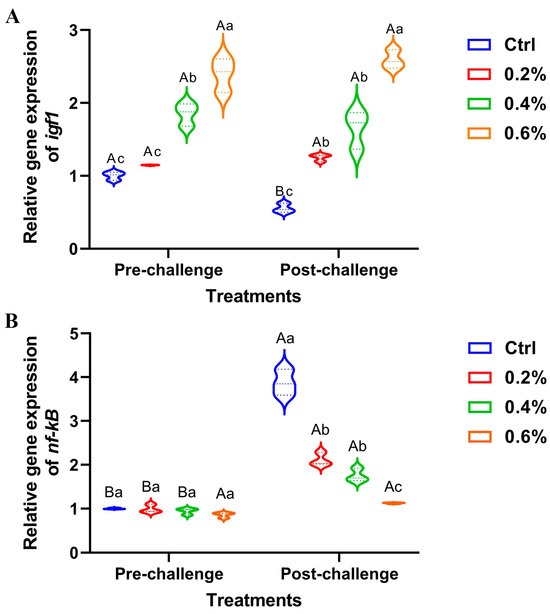
Figure 6.
The expression of (A) insulin-like growth factor 1 (igf1) gene and (B) nuclear factor kappa B (nf-κB) gene in Nile tilapia fed diets supplemented with 0%, 0.2%, 0.4%, and 0.6% Mentha piperita (peppermint) for 90 days and challenged with Aeromonas hydrophila. Gene expression was normalized using β-actin as a reference gene. Data are expressed as the mean ± SEM. Values with distinct letter superscripts exhibit significant differences across groups.
4. Discussion
MP is a versatile medicinal plant that offers numerous benefits in aquaculture [57]. It contains several potent properties and natural compounds that show a significant role in promoting the health and well-being of aquatic organisms [58]. MP, which contains 40–55% menthol, has antimicrobial effects and stress-reducing capabilities. It also has the potential to enhance the overall productivity and sustainability of aquaculture practice [59].
Our findings showed that Nile tilapia given diets containing MP for 90 days exhibited improved growth outcomes compared to the control group. The group with the MP concentration of 0.6% demonstrated favorable growth performance and the lowest FCR. These findings align with studies by Magouz, et al. [32] and Dawood, et al. [60], which highlighted how menthol essential oil extracted from peppermint plants improved the growth performance of Nile tilapia when included in their diet at ratios ranging from 0.2% to 0.3%.
The enhanced growth could be attributed to several factors. The results showed that MP upregulated the hepatic expression of igf1—an important gene in fish growth [61,62]. The igf1 gene plays a critical role in regulating protein synthesis and muscle development in fish [63,64]. It stimulates muscle growth by suppressing protein breakdown and the expression of atrophy-related ubiquitin ligases, such as atrogin-1 and muscle ring finger 1 [65]. Phytogenic feed additives derived from herbs have been documented to enhance the expression of growth- and immune-related genes, including gh and igf1 [18]. These additives may influence growth and other physiological pathways either by directly stimulating gene expression or by enhancing other growth-related factors such as intestinal health, nutrient absorption, and feed utilization. Additionally, they help minimize environmental stress, creating conditions conducive to normal physiological functioning, which in turn promotes growth and overall health [66,67].
The observed enhancement in the growth performance of Nile tilapia fed MP-supplemented diets can be attributed to MP’s ability to boost the activity of digestive enzymes, thereby improving digestion and absorption, as suggested by Aguiar, et al. [27]. Dietary phytogenic additives have consistently been shown to increase digestive enzyme activity in fish [68,69]. This improved feed utilization translates into increased growth rates and reduced FCR [70]. Additionally, mint plants consist of substances such as menthol, which may improve the taste of the feed, encouraging higher consumption and ultimately better growth [71]. Thus, the mint plant could act as a potential natural growth enhancer in Nile tilapia farming, supporting output and financial gains.
Regarding the effect on fish health, A. hydrophila is a major pathogen causing severe economic strain in tilapia aquaculture, leading to high mortality rates [72,73]. Evaluating a fish’s tolerance to such infections is essential for understanding the benefits of dietary additives [74]. Similar to the established effectiveness of MP supplementation as an antimicrobial agent [25,75], this study found that fish fed MP-supplemented diets exhibited significantly higher survival rates compared to the control group after bacterial challenge. Notably, no mortalities were observed in the negative control group, and fish fed the highest MP concentration (0.6%) displayed the highest cumulative survival rate.
The resistance of Nile tilapia fed MP could be attributed to the various bioactive components present in the plant. Peppermint contains essential oils (e.g., menthol, levomenthol and menthone) and phenolic compounds with known antimicrobial and immunomodulatory properties [76,77]. Menthol disrupts the bacterial communication system (quorum sensing; QS) in various Gram-negative pathogens, including A. hydrophila [78]. These pathogens utilize diverse acyl homoserine lactone (AHL) molecules for QS. Menthol’s effect was observed through a reduction in AHL-dependent production of violacein (a pigment), virulence factors, and biofilm formation, suggesting broad-spectrum anti-QS activity [78].
Fish fed MP-supplemented diets significantly improved blood parameters compared to the control group in both pre- and post-bacterial challenge groups. These improvements included higher RBC and WBC counts, increased Hb percentage, PCV, and MCH. Additionally, MP influenced the proportions of WBC populations, including lymphocytes, monocytes, neutrophils, and eosinophils. Similarly, Nile tilapia fed 2% MP showed significantly enhanced hematological parameters compared to those fed 3% and 4% MP [23]. Moreover, a concentration of 0.5% MP exhibited a higher antibacterial effect after infection by Streptococcus agalactiae in red tilapia [79]. The composition of MP (e.g., menthol, menthone, and menthofuran) may promote the production of red and white blood cells in the fish. Increased Hb levels suggest better oxygen delivery to tissues, indicating the ability of MP to improve oxygen transport capacity [80,81]. In this way, MP shows a mitigating function against the degenerative effects that may follow infection.
Fish fed MP-supplemented diets, particularly at a 0.6% concentration, exhibited significant reductions in liver enzymes (ALT and AST) as well as cholesterol, urea, and creatinine levels, both before and after a bacterial challenge. This is noteworthy, as Aeromonas hydrophila infection is known to cause hepatic and renal damage in fish [73,82], leading to elevated liver enzymes, urea, and creatinine levels [83]. The reductions observed in these parameters in MP-fed fish indicate improved hepatic and renal function, as well as overall health.
Menthol, the major component of MP, has been found to protect against sepsis-induced hepatic injury [84]. It significantly reduces serum liver enzyme levels and hepatic concentrations of TNF-α, MDA, and cleaved caspase-3 while maintaining balanced hepatic SOD and GSH levels. Additionally, menthol enhances biomarkers associated with regeneration and survival, such as B-cell lymphoma 2 (an anti-apoptotic factor) and proliferating cell nuclear antigen, following sepsis-induced liver injury. These effects contribute to improved hepatic histopathological changes [84]. Moreover, menthol can modulate inflammatory molecules, including Toll-like receptor 4, myeloid differentiation primary response 88, and NF-κB, to protect against liver and brain injuries [85]. MP also exhibits renal protective effects by reducing lipid peroxidation, as well as urea and creatinine levels, in injured kidneys [86].
Furthermore, menthone, a component of MP, possesses both local and systemic anti-inflammatory properties [87]. It regulates type-I interferon signaling through Tyk2 ubiquitination to modulate local inflammation [88], influences T-cell subtypes, and reduces pro-inflammatory cytokines [89]. Additionally, menthone has demonstrated protective effects against DNA damage [90]. Both menthol and menthone exhibit protective and anti-inflammatory properties against parasite-induced injury in the liver and intestine [91]. Consequently, menthone may offer protective benefits for the gastrointestinal tract and other internal organs.
Furthermore, MP-fed groups displayed higher serum globulin and triglyceride levels, while total protein and albumin levels remained unchanged. Similar findings have been reported in rohu (Labeo rohita) fingerlings, where lower cholesterol and glucose levels were observed alongside enhanced resistance to A. hydrophila [37]. Serum globulin primarily consists of immunoglobulins [92], and its increase suggests enhanced humoral immune activity and elevated immunoglobulin levels in MP-fed fish. Additionally, reduced cholesterol levels in MP-fed fish may have further supported their immune response, as elevated cholesterol is known to impair normal immune function in fish [93]. Dietary cholesterol has been shown to induce inflammation by upregulating pro-inflammatory cytokine expression while suppressing anti-inflammatory cytokines, partly through the modulation of NF-κB and TOR signaling pathways in fish immune organs [93].
MP contains various concentrations of limonene, pulegone, carvone, and eucalyptol (1,8-cineole), which contribute to its beneficial biological activities [94]. Limonene has anti-inflammatory properties by inhibiting the NF-κB/AP-1 pathway [95]. Carvone has potent antipathogenic effects (e.g., antibacterial, antifungal, antiparasitic) as well as antistress effects [96].
Other active compounds detected in MP, such as caryophyllene, pulegone, and eucalyptol, also exhibit significant biological activity. These compounds demonstrate antibacterial effects by altering membrane permeability and integrity in various bacteria, such as Bacillus cereus, leading to membrane damage [97,98,99]. Caryophyllene additionally possesses antioxidant and anti-inflammatory properties [100]. Pulegone suppresses the expression of biofilm-formation-related genes in bacteria such as Escherichia coli [101]. Eucalyptol has antioxidant, antimicrobial, and pro-apoptotic effects, and exerts its anti-inflammatory activity by suppressing NF-κB p65 [102].
Consistent with these characteristics, the results indicate that MP significantly downregulates hepatic nf-κB expression. Furthermore, reductions in ALT and AST levels suggest that MP may provide hepatoprotective effects through its anti-inflammatory and antioxidant properties [103]. Additionally, the observed decrease in urea and creatinine levels implies that MP may support kidney function by enhancing waste product removal [35]. These findings suggest that dietary MP can positively influence fish health and metabolism by improving their biochemical parameters.
Dietary MP improved serum IgM levels, phagocytic, and lysozyme activities in a dose-dependent manner, with the best performance at the concentration of 0.6%. These improvements were observed both before and after the bacterial challenge. The bactericidal activity of MP stems from the presence of flavonoids, tannins, and other bioactive compounds. MP is also a good source of vitamins A and C, as well as minerals like potassium and calcium, which are important for enhancing the immune system [104,105]. MP’s bioactive components help scavenge free radicals, mitigating oxidative stress and improving overall health [106,107]. MP significantly enhances antioxidant defenses—as evidenced by increased GPx, SOD, and CAT levels—while decreasing MDA, suggesting advanced resilience towards oxidative stress and protection against infections.
This study revealed that dietary supplementation with MP, particularly at the concentration of 0.6%, protected the intestinal integrity of fish challenged with A. hydrophila. MP promoted beneficial bacteria, such as Lactobacillus and Bifidobacterium [108], suggesting its contribution to achieving a balanced gut microbiota, thereby improving gut and immune health. Furthermore, MP can act as a prebiotic compound [109], providing nourishment for beneficial gut bacteria and supporting their proliferation.
These findings suggest that MP can be a promising natural strategy to improve gut health and protect against intestinal damage caused by bacterial infections in fish. However, further research is needed to explore the underlying mechanisms, including the expression of additional immune- and growth-related genes, changes in immune effectors such as complement activity, as well as transcriptomic and microbiota alterations.
5. Conclusions
Mentha piperita (MP) can contribute to improved fish health and performance in aquaculture. This study provides compelling evidence for the potential of MP as a valuable medicinal plant in aquaculture, specifically as a dietary supplement for Nile tilapia. Using MP could be environmentally friendly, minimizing reliance on antibiotics. Future research should aim to determine the optimal dose and methods of delivering MP, provide a more detailed description of the mechanisms by which it works, and evaluate the effectiveness of MP against different pathogens and in different fish species. Additionally, investigating possible interactions with other natural feed additives could help develop multi-faceted feeding plans to enhance fish health and output in aquaculture.
Author Contributions
Conceptualization: A.A.A.Z., A.M.E.-G. and E.E.H.; Data curation: A.E.E. and H.G.A.-A.-E.; Formal analysis: H.G.A.-A.-E., N.H.M., A.E.E., H.G.A.-A.-E. and A.A.A.Z.; Investigation: H.G.A.-A.-E., A.A.A.Z., N.H.M., A.E.E., E.E.H. and A.M.E.-G.; Methodology: N.H.M. and A.E.E.; Funding acquisition: H.G.A.-A.-E.; Visualization: H.G.A.-A.-E. and A.E.E.; Writing—original draft: A.E.E., N.H.M. and H.G.A.-A.-E.; Writing—review and editing: H.G.A.-A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The current study was approved and performed in accordance with the guidelines and regulations of the Institutional Committee on Aquatic Animal Care and Use in Research, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University (Egypt IAACUC-KSU-025-2021, approved on 5 February 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to Ahmed E. Elshafey (ahmed_alshafei@fsh.kfs.edu.eg) upon reasonable request.
Acknowledgments
The authors would like to thank the Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt for their technical assistance during the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADG | average daily gain |
| AHL | acyl homoserine lactone |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CAT | catalase |
| FBW | final body weight |
| FCR | feed conversion ratio |
| GPx | glutathione peroxidase |
| Hb | hemoglobin |
| igf1 | insulin-like growth factor 1 |
| IgM | immunoglobulin M |
| K | condition factor |
| MCH | mean corpuscular hemoglobin |
| MDA | malondialdehyde |
| MP | mentha piperita |
| nf-κB | nuclear factor kappa B |
| PCV | packed cell volume |
| RBCs | red blood cells |
| RGR | relative growth rate |
| SGR | specific growth rate |
| SOD | superoxide dismutase |
| WBC | white blood cell |
| WG | weight gain ratio |
References
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Troell, M.; Costa-Pierce, B.; Stead, S.; Cottrell, R.S.; Brugere, C.; Farmery, A.K.; Little, D.C.; Strand, Å.; Pullin, R.; Soto, D.; et al. Perspectives on aquaculture’s contribution to the sustainable development goals for improved human and planetary health. J. World Aquacult. Soc. 2023, 54, 251–342. [Google Scholar] [CrossRef]
- Bandyopadhyay, B.K. Freshwater Aquaculture: A Functional Approach; CRC Press: London, UK, 2022. [Google Scholar]
- Norman, R.; Crumlish, M.; Stetkiewicz, S. The importance of fisheries and aquaculture production for nutrition and food security. Rev. Sci. Tech. Off. Int. Epizoot. 2019, 38, 395–407. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M.; Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Maundu, A.M. Digestibility, Growth and Economic Performance of Nile tilapia (Oreochromis niloticus) Fed on a Mixture of Plant Protein Diets in Cages. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2020. [Google Scholar]
- Haenen, O.L.M.; Dong, H.T.; Hoai, T.D.; Crumlish, M.; Karunasagar, I.; Barkham, T.; Chen, S.L.; Zadoks, R.; Kiermeier, A.; Wang, B.; et al. Bacterial diseases of tilapia, their zoonotic potential and risk of antimicrobial resistance. Rev. Aquac. 2023, 15, 154–185. [Google Scholar] [CrossRef]
- Tesfay, S.; Mekonen, T.; Tadesse, D.; Tsegaluel, A.; Hiluf, G. A survey of ecto and endoparasites of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fingerlings in Midmar reservoir, Adwa, northern Ethiopia. J. Appl. Anim. Res. 2024, 52, 2310158. [Google Scholar] [CrossRef]
- Clyde, C.W.; Tan, J.P.; Yeap, S.K.; Yong, C.Y. Current updates on viral infections affecting tilapia. Aquac. Fish, 2024; in press. [Google Scholar] [CrossRef]
- Sherif, A.H.; Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 2023, 23, 80. [Google Scholar] [CrossRef]
- Dubey, S.; Maiti, B.; Girisha, S.K.; Das, R.; Lamkhannat, M.; Mutoloki, S.; Chen, S.-C.; Karunasagar, I.; Evensen, Ø.; Munang’andu, H.M. Aeromonas species obtained from different farmed aquatic species in India and Taiwan show high phenotypic relatedness despite species diversity. BMC Res. Notes 2021, 14, 313. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.; Qin, Z.; Li, A.; Gu, Z.; Liu, X.; Lin, L.; Zhou, Y. Contribution of nuclease to the pathogenesis of Aeromonas hydrophila. Virulence 2015, 6, 515–522. [Google Scholar] [CrossRef]
- Xiong, N.-X.; Wang, F.; Luo, W.-S.; Ou, J.; Qin, Z.-L.; Huang, M.-Z.; Luo, S.-W. Tumor necrosis factor α2 (TNFα2) facilitates gut barrier breach by Aeromonas hydrophila and exacerbates liver injury in hybrid fish. Aquaculture 2023, 577, 739995. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, H.; Kaur, B.; Naveen Kumar, B.T.; Tyagi, A.; Singh, P.; Tanuj; Dubey, S.; Munang’andu, H.M. Isolating pathogenic multidrug-resistant Aeromonas hydrophila from diseased fish and assessing the effectiveness of a novel lytic Aeromonas veronii bacteriophage (AVP3) for biocontrol. Microb Pathog 2024, 196, 106914. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Herbal supplementation diets on hematology and innate immunity in goldfish against Aeromonas hydrophila. Fish Shellfish. Immunol. 2010, 28, 354–361. [Google Scholar] [CrossRef]
- Rehan, M.M.; Abouzaid, A.A.; Abo-Al-Ela, H.G.; Abdou, M.S.; Elsaidy, N.R. Utilization of Origanum oil as a health promoter in Nile tilapia (Oreochromis niloticus) challenged with Pseudomonas aeruginosa. Aquaculture 2024, 584, 740683. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.O.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H.V. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Atef, S.; Ahmed, O.M.; Said, M.M.; Abo-Al-Ela, H.G. Dietary Bacillus species modulate lipid metabolism-related parameters, growth, water quality, and bacterial load in Nile tilapia (Oreochromis niloticus). Anim. Feed. Sci. Technol. 2024, 310, 115943. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G. Does vitamin C mitigate the detrimental effect of androgens on immunity? Res. Vet. Sci. 2019, 125, 43–44. [Google Scholar] [CrossRef]
- Mariappan, B.; Kaliyamurthi, V.; Binesh, A. Chapter 8—Medicinal plants or plant derived compounds used in aquaculture. In Recent Advances in Aquaculture Microbial Technology; Mathew, J., Jose, M.S., Radhakrishnan, E.K., Kumar, A., Eds.; Academic Press: New Delhi, India, 2023; pp. 153–207. [Google Scholar]
- Abu-Zahra, N.I.S.; ElShenawy, A.M.; Ali, G.I.E.; Al-sokary, E.T.; Mousa, M.A.; El-Hady, H.A.M.A. Mentha piperita powder enhances the biological response, growth performance, disease resistance, and survival of Oreochromis niloticus infected with Vibrio alginolyticus. Aquac. Int. 2024, 32, 6353–6379. [Google Scholar] [CrossRef]
- de Oliveira Hashimoto, G.S.; Neto, F.M.; Ruiz, M.L.; Acchile, M.; Chagas, E.C.; Chaves, F.C.M.; Martins, M.L. Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 2016, 450, 182–186. [Google Scholar] [CrossRef]
- Talpur, A.D. Mentha piperita (Peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquaculture 2014, 420–421, 71–78. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Aguiar, G.A.C.C.d.; Carneiro, C.L.d.S.; Campelo, D.A.V.; Rusth, R.C.T.; Maciel, J.F.R.; Baldisserotto, B.; Zuanon, J.A.S.; Oliveira, A.V.d.; Oliveira, M.G.d.A.; Freitas, M.B.D.d.; et al. Effects of dietary peppermint (Mentha piperita) essential oil on growth performance, plasma biochemistry, digestive enzyme activity, and oxidative stress responses in juvenile Nile tilapia (Oreochromis niloticus). Fishes 2023, 8, 374. [Google Scholar] [CrossRef]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and antimicrobial characterization of Mentha piperita L. and Rosmarinus officinalis L. essential oils and in vitro potential cytotoxic effect in human colorectal carcinoma cells. Molecules 2022, 27, 6106. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.-Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish. Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint oils: In vitro ability to perform anti-inflammatory, antioxidant, and antimicrobial activities and to enhance intestinal barrier integrity. Antioxidants 2021, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A.O. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Goudarzi, M.A.; Radfar, M.; Goudarzi, Z. Peppermint as a promising treatment agent in inflammatory conditions: A comprehensive systematic review of literature. Phytother. Res. 2024, 38, 187–195. [Google Scholar] [CrossRef]
- de Souza Silva, L.T.; de Pádua Pereira, U.; de Oliveira, H.M.; Brasil, E.M.; Pereira, S.A.; Chagas, E.C.; Jesus, G.F.A.; Cardoso, L.; Mouriño, J.L.P.; Martins, M.L. Hemato-immunological and zootechnical parameters of Nile tilapia fed essential oil of Mentha piperita after challenge with Streptococcus agalactiae. Aquaculture 2019, 506, 205–211. [Google Scholar] [CrossRef]
- Brandão, F.R.; Duncan, W.P.; Farias, C.F.S.; de Melo Souza, D.C.; de Oliveira, M.I.B.; Rocha, M.J.S.; Monteiro, P.C.; Majolo, C.; Chaves, F.C.M.; de Almeida O’Sullivan, F.L.; et al. Essential oils of Lippia sidoides and Mentha piperita as reducers of stress during the transport of Colossoma macropomum. Aquaculture 2022, 560, 738515. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Saluja, S. Synergistic effects of autochthonous probiotic bacterium and Mentha piperita diets in Catla catla (Hamilton, 1822) for enhanced growth and immune response. Fish Aquat. Sci. 2019, 22, 16. [Google Scholar] [CrossRef]
- Padala, D.; Marakini, G.N.; Kokkam Valappil, A.; Prabhakaran, P.L.; Muhammad Abdullah Al, M.; Kavalagiriyanahalli Srinivasiah, R. Effect of dietary peppermint (Mentha piperita) on growth, survival, disease resistance and haematology on fingerlings of rohu (Labeo rohita). Aquac. Res. 2021, 52, 2697–2705. [Google Scholar] [CrossRef]
- dos Anjos, A.C.P.; Isaac, A. The efficacy and dosage of Mentha piperita essential oil in the control of Monogenean parasites in Oreochromis niloticus. J. Parasit. Dis. 2020, 44, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Voigt, V.; Franke, H.; Lachenmeier, D.W. Risk assessment of pulegone in foods based on benchmark dose–response modeling. Foods 2024, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2007. [Google Scholar]
- Ashry, A.M.; Hassan, A.M.; Habiba, M.M.; El-Zayat, A.; El-Sharnouby, M.E.; Sewilam, H.; Dawood, M.A.O. The impact of dietary curcumin on the growth performance, intestinal antibacterial capacity, and haemato-biochemical parameters of gilthead seabream (Sparus aurata). Animals 2021, 11, 1779. [Google Scholar] [CrossRef]
- Elshafey, A.E.; Khalafalla, M.M.; Zaid, A.A.A.; Mohamed, R.A.; Abdel-Rahim, M.M. Source diversity of Artemia enrichment boosts goldfish (Carassius auratus) performance, β-carotene content, pigmentation, immune-physiological and transcriptomic responses. Sci. Rep. 2023, 13, 21801. [Google Scholar] [CrossRef]
- Hamed, S.; El-Kassas, S.; Abo-Al-Ela, H.G.; Abdo, S.E.; Abou-Ismail, U.A.; Mohamed, R.A. Temperature and feeding frequency: Interactions with growth, immune response, and water quality in juvenile Nile tilapia. BMC Vet. Res. 2024, 20, 520. [Google Scholar] [CrossRef]
- Moustafa, E.M.; Dawood, M.A.O.; Assar, D.H.; Omar, A.A.; Elbialy, Z.I.; Farrag, F.A.; Shukry, M.; Zayed, M.M. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 2020, 515, 734589. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Ismael, N.E.M.; Negm, S.S.; Ayyat, M.S.; Al-Sagheer, A.A. Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 526, 735370. [Google Scholar] [CrossRef]
- Olsson, C. Gut anatomy. In Encyclopedia of Fish Physiolog, 2nd ed.; Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Oxford, UK, 2024; pp. 339–347. [Google Scholar]
- Okuthe, G.E.; Bhomela, B. Morphology, histology and histochemistry of the digestive tract of the Banded tilapia, Tilapia sparrmanii (Perciformes: Cichlidae). Zoologia 2020, 37, e51043. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergün, S.; Çelik, E.Ş. Effect of dietary spice supplementations on welfare status of sea bass, Dicentrarchus labrax L. Proc. Nat. Acad. Sci. India. Sect. B-Biol. Sci. 2016, 86, 229–237. [Google Scholar] [CrossRef]
- Heinegård, D.; Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta. 1973, 43, 305–310. [Google Scholar] [CrossRef]
- Diab, A.M.; Salem, R.M.; Abeer, E.-K.M.S.; Ali, G.I.E.; El-Habashi, N. Experimental ochratoxicosis A in Nile tilapia and its amelioration by some feed additives. Int. J. Vet. Sci. Med. 2018, 6, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Abo-Al-Ela, H.G.; El-Nahas, A.F.; Mahmoud, S.; Ibrahim, E.M. Vitamin C modulates the immunotoxic effect of 17α-methyltestosterone in Nile tilapia. Biochemistry 2017, 56, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, S.; Cai, Y.; Wu, Y.; Tian, L.; Liao, J.; Wang, S.; Jiang, L.; Guo, W.; Zhou, Y. Antioxidant capacity, non-specific immunity, histopathological analysis and immune-related genes expression in Nile tilapia Oreochromis niloticus infected with Aeromonas schubertii. Aquaculture 2020, 529, 735642. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Shenzhen, China, 2018. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Salah, A.S.; Elsheshtawy, A.; Elkatatny, N.M.; Fouad, A.M.; Abo-Al-Ela, H.G. Differential tissue regulation of nrf2/keap1 crosstalk in response to Aeromonas infection in Nile tilapia: A comparative study. Aquacult. Int. 2024, 32, 545–562. [Google Scholar] [CrossRef]
- Nazeemashahul, S.; Fawole, F.J.; AM, B.R.; Jayant, M.; Qureshi, N.; Nottanalan, H.; Deo, A.D.; Sardar, P. Herbal Immunomodulators for Aquaculture. In Immunomodulators in Aquaculture and Fish Health; CRC Press: Boca Raton, FL, USA, 2023; pp. 119–135. [Google Scholar]
- Kazemi, A.; Iraji, A.; Esmaealzadeh, N.; Salehi, M.; Hashempur, M.H. Peppermint and menthol: A review on their biochemistry, pharmacological activities, clinical applications, and safety considerations. Crit. Rev. Food Sci. Nutr. 2023, 65, 1553–1578. [Google Scholar] [CrossRef]
- Ng, J.J.Y.; Yusoff, N.A.H.; Elias, N.A.; Norhan, N.A.-S.; Harun, N.A.; Abdullah, F.; Ishak, A.N.; Hassan, M. Phytotherapy use for disease control in aquaculture: A review of the last 5 years. Aquac. Int. 2024, 32, 2687–2712. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El-Salam Metwally, A.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Abdel-Razek, M.A.S.; Soliman, A.A.; Amer, A.A.; Abdel-Razik, N.I.; Abdel-Latif, H.M.R.; et al. The impact of menthol essential oil against inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Fish Shellfish. Immunol. 2020, 102, 316–325. [Google Scholar] [CrossRef]
- Bersin, T.V.; Mapes, H.M.; Journey, M.L.; Beckman, B.R.; Lema, S.C. Insulin-like growth factor-1 (Igf1) signaling responses to food consumption after fasting in the Pacific rockfish Sebastes carnatus. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2023, 282, 111444. [Google Scholar] [CrossRef] [PubMed]
- Hack, N.L.; Strobel, J.S.; Journey, M.L.; Beckman, B.R.; Lema, S.C. Response of the insulin-like growth factor-1 (Igf1) system to nutritional status and growth rate variation in olive rockfish (Sebastes serranoides). Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2018, 224, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Duran, B.O.S.; Zanella, B.T.T.; Perez, E.S.; Mareco, E.A.; Blasco, J.; Dal-Pai-Silva, M.; Garcia de la serrana, D. Amino acids and IGF1 regulation of fish muscle growth revealed by transcriptome and microRNAome integrative analyses of pacu (Piaractus mesopotamicus) myotubes. Int. J. Mol. Sci. 2022, 23, 1180. [Google Scholar] [CrossRef]
- Hamed, S.; El-Kassas, S.; Abo-Al-Ela, H.G.; Abdo, S.E.; Al Wakeel, R.A.; Abou-Ismail, U.A.; Mohamed, R.A. Interactive effects of water temperature and dietary protein on Nile tilapia: Growth, immunity, and physiological health. BMC Vet. Res. 2024, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. -Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef]
- Kokou, F.; Gupta, S.; Kumar, V. Editorial: Understanding the interplay between diet, feed ingredients and gut microbiota for sustainable aquaculture. Front. Mar. Sci. 2022, 9, 853548. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Hussein, M.N.; Khattaby, A.E.-R.A.; Abo-Al-Ela, H.G. Impact of dietary Biocide clay on growth, physiological status, and histologicalindicators of the liver and digestive tract in Nile tilapia (Oreochromis niloticus). Sci. Rep. 2025, 15, 5311. [Google Scholar]
- Mohammady, E.Y.; Soaudy, M.R.; Mohamed, A.E.; El-Erian, M.M.A.; Farag, A.; Badr, A.M.M.; Bassuony, N.I.; Ragaza, J.A.; El-Haroun, E.R.; Hassaan, M.S. Can dietary phytogenic mixture improve performance for growth, digestive enzyme activity, blood parameters, and antioxidant and related gene expressions of Nile tilapia, Oreochromis niloticus? Anim. Feed Sci. Technol. 2022, 290, 115369. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The role of functional feed additives in enhancing aquaculture sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Paknejad, H.; Hosseini Shekarabi, S.P.; Shamsaie Mehrgan, M.; Hajimoradloo, A.; Khorshidi, Z.; Rastegari, S. Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol. Biochem. 2020, 46, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Geiger, S.; Braun, H.-S.; Aschenbach, J.R. Dietary supplementation of menthol-rich bioactive lipid compounds alters circadian eating behaviour of sheep. BMC Vet. Res. 2019, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Shirajum Monir, M.; Yusoff, S.M.; Mohamad, A.; Ina-Salwany, M.Y. Vaccination of tilapia against motile Aeromonas septicemia: A review. J. Aquat. Anim. Health 2020, 32, 65–76. [Google Scholar] [CrossRef]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Yusof, M.T.; Monir, M.S.; Mohamad, A.; Muhamad-Sofie, M.H.N.; Lee, J.Y.; Chin, Y.K.; et al. Comparative pathogenicity of Aeromonas spp. In cultured red hybrid tilapia (Oreochromis niloticus × O. mossambicus). Biology 2021, 10, 1192. [Google Scholar] [CrossRef]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 2022, 14, 816–847. [Google Scholar] [CrossRef]
- Chagas, E.C.; Majolo, C.; Monteiro, P.C.; Oliveira, M.R.d.; Gama, P.E.; Bizzo, H.R.; Chaves, F.C.M. Composition of essential oils of Mentha species and their antimicrobial activity against Aeromonas spp. J. Essent. Oil Res. 2020, 32, 209–215. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; ELsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid. -based. Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef]
- Vo, V.-T.; Tran, T.-H.; Nguyen, T.-T.-T.; Truong, V.-T.; Pham, C.-T.; Pham, T.-M.; Thuong, H.N.T. Hematological parameters of red tilapia (Oreochromis sp.) fed essential oils of Mentha piperita after challenge with Streptococcus agalactiae. Pak. J. Zool. 2023, 55, 1123. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Chakravarti, A.R.; Bhattacharjee, S. Bioactive components of peppermint (Mentha piperita L.), their pharmacological and ameliorative potential and ethnomedicinal benefits: A review. J. Pharmacogn. Phytochem. 2022, 11, 109–114. [Google Scholar] [CrossRef]
- Sellegounder, D.; Gupta, Y.R.; Murugananthkumar, R.; Senthilkumaran, B. Enterotoxic effects of Aeromonas hydrophila infection in the catfish, Clarias gariepinus: Biochemical, histological and proteome analyses. Vet. Immunol. Immunopathol. 2018, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, E.M.E.; Khoris, E.A.; Kassem, S. Assessment the efficacy of some various treatment methods, in vitro and in vivo, against Aeromonas hydrophila infection in fish with regard to side effects and residues. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022, 253, 109246. [Google Scholar] [CrossRef]
- Matouk, A.I.; El-Daly, M.; Habib, H.A.; Senousy, S.; Naguib Abdel Hafez, S.M.; Kasem, A.W.; Almalki, W.H.; Alzahrani, A.; Alshehri, A.; Ahmed, A.-S.F. Protective effects of menthol against sepsis-induced hepatic injury: Role of mediators of hepatic inflammation, apoptosis, and regeneration. Front. Pharmacol. 2022, 13, 952337. [Google Scholar] [CrossRef] [PubMed]
- Kaboutari, M.; Asle-Rousta, M.; Mahmazi, S. Protective effect of menthol against thioacetamide-induced hepatic encephalopathy by suppressing oxidative stress and inflammation, augmenting expression of BDNF and α7-nACh receptor, and improving spatial memory. Eur. J. Pharmacol. 2024, 981, 176916. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ben Hsouna, A.; Athmouni, K.; van Pelt, J.; Makni Ayadi, F.; Rebai, T.; Elfeki, A. Protective effects of Mentha piperita L. leaf essential oil against CCl4 induced hepatic oxidative damage and renal failure in rats. Lipids Health Dis. 2018, 17, 9. [Google Scholar] [CrossRef]
- Su, Y.-H.; Lin, J.-Y. Menthone inhalation alleviates local and systemic allergic inflammation in ovalbumin-sensitized and challenged asthmatic mice. Int. J. Mol. Sci. 2022, 23, 4011. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Cao, X.; Yang, Y.; Gong, Z.; Ren, T.; Du, Q.; Yuan, Y.; Zuo, Y.; Miao, Y.; et al. Menthone inhibits type-I interferon signaling by promoting Tyk2 ubiquitination to relieve local inflammation of rheumatoid arthritis. Int. Immunopharmacol. 2022, 112, 109228. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Gong, Z.; Ren, T.; Du, Q.; Yuan, Y.; Zuo, Y.; Miao, Y.; He, J.; Qiao, C.; et al. A natural plant ingredient, menthone, regulates T cell subtypes and lowers pro-inflammatory cytokines of rheumatoid arthritis. J. Nat. Prod. 2022, 85, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Toghiani, S.; Hayati Roudbari, N.; Dashti, G.R.; Rouzbehani, S. The effects of vitamin C and menthone on acyclovir induced DNA damage in rat spermatozoa: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 703–710. [Google Scholar] [PubMed]
- Zaia, M.G.; Cagnazzo, T.d.O.; Feitosa, K.A.; Soares, E.G.; Faccioli, L.H.; Allegretti, S.M.; Afonso, A.; Anibal, F.d.F. Anti-inflammatory properties of menthol and menthone in Schistosoma mansoni infection. Front. Pharmacol. 2016, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Jolles, S.; Borrell, R.; Zouwail, S.; Heaps, A.; Sharp, H.; Moody, M.; Selwood, C.; Williams, P.; Phillips, C.; Hood, K.; et al. Calculated globulin (CG) as a screening test for antibody deficiency. Clin. Exp. Immunol. 2014, 177, 671–678. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Jiang, W.-D.; Feng, L.; Wu, P.; Liu, Y.; Zeng, Y.-Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; et al. Low or excess levels of dietary cholesterol impaired immunity and aggravated inflammation response in young grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2018, 78, 202–221. [Google Scholar] [CrossRef]
- Buleandra, M.; Oprea, E.; Popa, D.E.; David, I.G.; Moldovan, Z.; Mihai, I.; Badea, I.A. Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Nat. Prod. Commun. 2016, 11, 551–555. [Google Scholar]
- Kathem, S.H.; Nasrawi, Y.S.; Mutlag, S.H.; Nauli, S.M. Limonene exerts anti-inflammatory effect on LPS-induced jejunal injury in mice by inhibiting NF-κB/AP-1 pathway. Biomolecules 2024, 14, 334. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health benefits and pharmacological properties of carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef]
- Santos, E.L.; Freitas, P.R.; Araújo, A.C.J.; Almeida, R.S.; Tintino, S.R.; Paulo, C.L.R.; Silva, A.C.A.; Silva, L.E.; do Amaral, W.; Deschamps, C.; et al. Enhanced antibacterial effect of antibiotics by the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. and its major constituent beta-caryophyllene. Phytomed. Plus 2021, 1, 100100. [Google Scholar]
- Farhanghi, A.; Aliakbarlu, J.; Tajik, H.; Mortazavi, N.; Manafi, L.; Jalilzadeh-Amin, G. Antibacterial interactions of pulegone and 1,8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci. Nutr. 2022, 10, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Sango, C.; Batovska, D. An update on microbial biosynthesis of β-caryophyllene, a sesquiterpene with multi-pharmacological properties. Fermentation 2024, 10, 60. [Google Scholar] [CrossRef]
- Gong, H.; He, L.; Zhao, Z.; Mao, X.; Zhang, C. The specific effect of (R)-(+)-pulegone on growth and biofilm formation in multi-drug resistant Escherichia coli and molecular mechanisms underlying the expression of pgaABCD genes. Biomed. Pharmacother. 2021, 134, 111149. [Google Scholar] [CrossRef] [PubMed]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Ali, M.A.M.; Sewilam, H. Menthol essential oil is a practical choice for intensifying the production of Nile tilapia (Oreochromis niloticus): Effects on the growth and health performances. Aquaculture 2021, 543, 737027. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Masouri, L.; Bagherzadeh-Kasmani, F.; Mehri, M.; Rokouei, M.; Masouri, B. Mentha piperita as a promising feed additive used to protect liver, bone, and meat of Japanese quail against aflatoxin B1. Trop. Anim. Health Prod. 2022, 54, 254. [Google Scholar] [CrossRef]
- Roy, D.; Mallick, B.; Samanta, D. Augmentation of antioxidative potential of in vitro propagated Mentha piperita L. Indian J. Exp. Biol. 2022, 58, 131–137. [Google Scholar]
- Riachi, L.G.; De Maria, C.A.B. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Marhamatizadeh, M.H.; Afrasiabi, S.; Rezazadeh, S.; Marhamati, Z. Effect of spearmint on the growth of Lactobacillus acidophilus and Bifidobacterium bifidum in probiotic milk and yogurt. Afr. J. Food Sci. 2011, 5, 747–753. [Google Scholar]
- Hussein, S.M.; M’Sadeq, S.A.; Beski, S.S.M.; Mahmood, A.L.; Frankel, T.L. Different combinations of peppermint, chamomile and a yeast prebiotic have different impacts on production and severity of intestinal and bursal abnormalities of broilers challenged with coccidiosis. Ital. J. Anim. Sci. 2021, 20, 1924–1934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).