Chemical Composition, Antifungal Activity, and Plant-Protective Potential of Rosa damascena Mill. Essential Oil Against Fusarium graminearum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
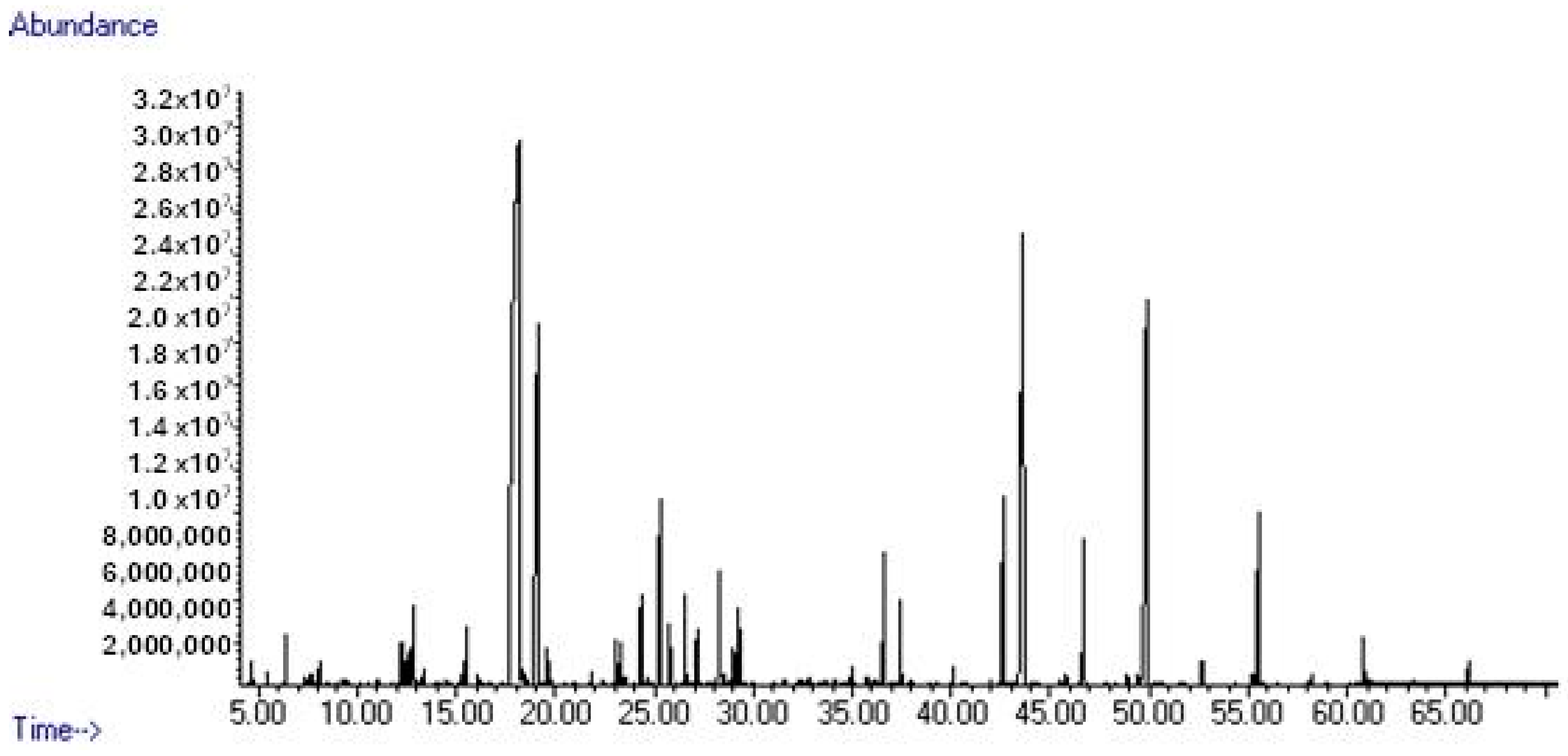
2.2. Gas Chromatography/Mass Spectrometry Analysis
2.3. Evaluation of Antifungal Activity
2.4. Proliferation Analysis
2.5. Fluorescence Microscopy Assay
2.6. Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
2.7. Infection Tests for Fusarium Crown Rot (FCR) Disease
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Evaluation
3.2. Antifungal Treatment Analysis
3.3. Fluorescence Microscopy Analysis
3.4. Gene Expression Analysis
3.5. Disease Severity Analysis
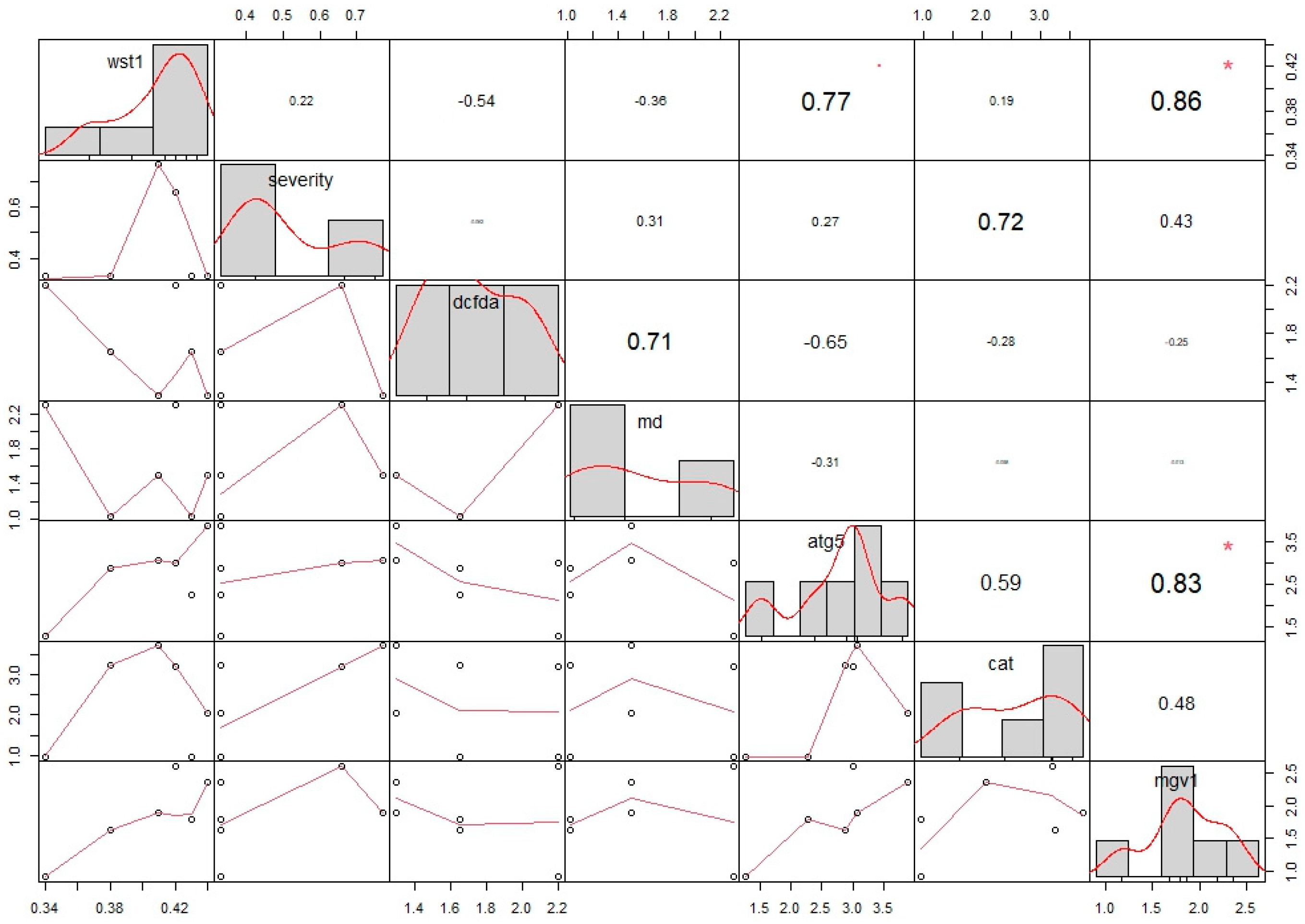
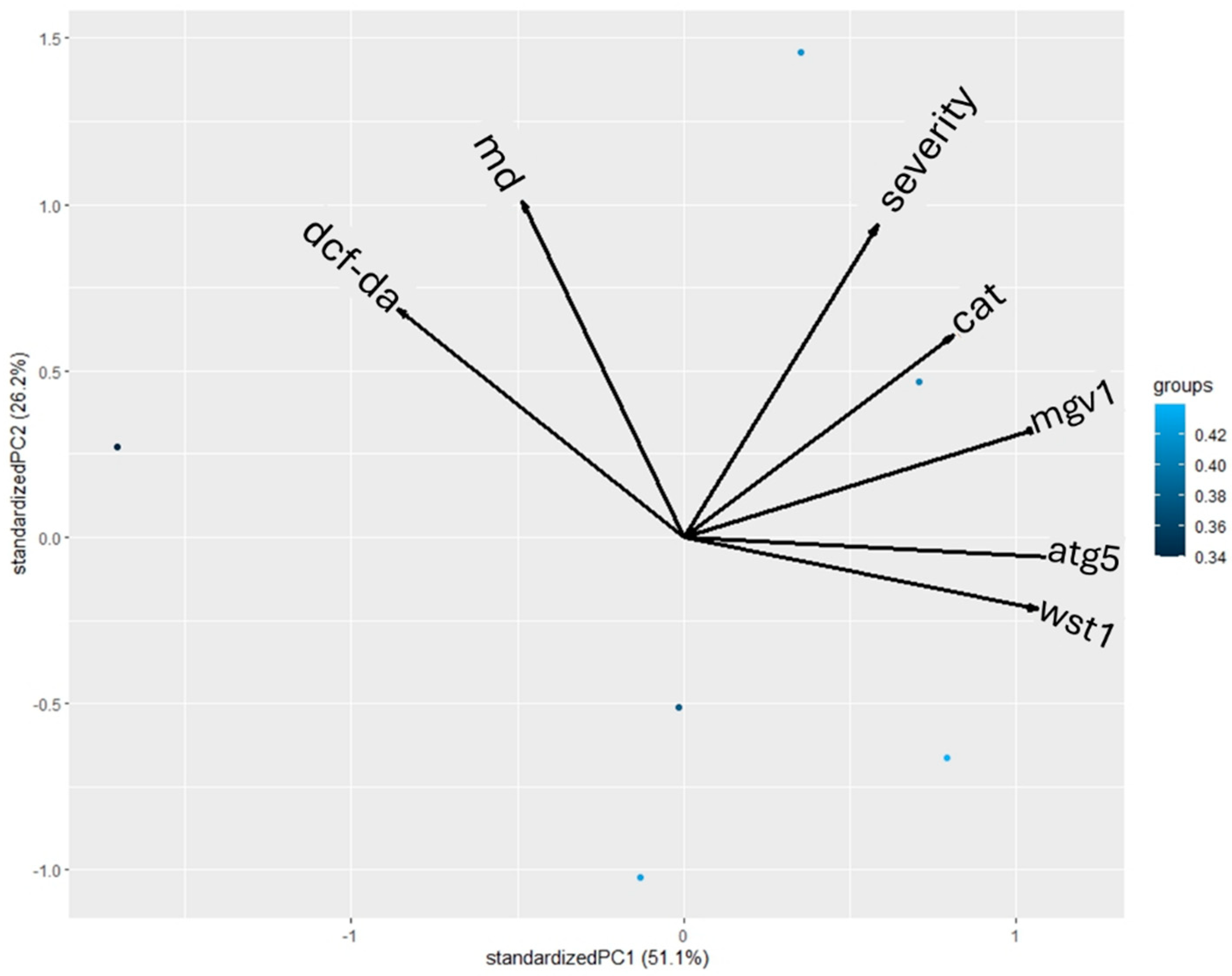
3.6. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alisaac, E.; Mahlein, A.K. Fusarium head blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium head blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Shude, S.; Yobo, K.S.; Mbili, N.C. Progress in the management of Fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.D.; Madden, L.V.; Paul, P.A. Efficacy and Economics of Integrating In-Field and Harvesting Strategies to Manage Fusarium Head Blight of Wheat. Plant Dis. 2014, 98, 1407–1421. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Proctor, R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef]
- Foroud, N.A.; Eudes, F. Trichothecenes in cereal grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Lavkor, I. Fungal Species Causing Ear Rot in Corn and Occurred Mycotoxins in Corn. CPN-2001. 2019, pp. 1197–1209. Available online: https://cropprotectionnetwork.org/publications/an-overview-of-ear-rots (accessed on 11 December 2024).
- Lu, Q.; Luo, J.-Y.; Ruan, H.-N.; Wang, C.-J.; Yang, M.-H. Structure-toxicity relationships, toxicity mechanisms and health risk assessment of food-borne modified deoxynivalenol and zearalenone: A comprehensive review. Sci. Total Environ. 2022, 806, 151192. [Google Scholar] [CrossRef]
- Reddy, K.E.; Song, J.; Lee, H.-J.; Kim, M.; Kim, D.-W.; Jung, H.J.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.-W. Effects of high levels of deoxynivalenol and zearalenone on growth performance, and hematological and immunological parameters in pigs. Toxins 2018, 10, 114. [Google Scholar] [CrossRef]
- Gao, S.; Hanson, B.D.; Qin, R.; Wang, D.; Yates, S.R. Comparisons of Soil Surface Sealing Methods to Reduce Fumigant Emission Loss. J. Environ. Qual. 2011, 40, 1480–1487. [Google Scholar] [CrossRef]
- Qin, R.; Gao, S.; Ajwa, H.; Sullivan, D.; Wang, D.; Hanson, B.D. Field Evaluation of a New Plastic Film (Vapor Safe) to Reduce Fumigant Emissions and Improve Distribution in Soil. J. Environ. Qual. 2011, 40, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, A.; Musa, T.; Voegele, R.T.; Vogelgsang, S. The potential of antagonistic fungi for control of Fusarium graminearum and Fusarium crookwellense varies depending on the experimental approach. J. Appl. Microbiol. 2015, 118, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.G.; Chen, Y.; Voldeng, H.D.; Fedak, G.; Savard, M.E.; Längle, T.; Zhang, J.; Harman, G.E. Concentration and cultivar effects on efficacy of clo-1 biofungicide in controlling Fusarium head blight of wheat. Biol. Control 2014, 73, 2–7. [Google Scholar] [CrossRef]
- Masika, P.J.; Afolayan, A.J. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J. Ethnopharmacoogy 2002, 83, 129–134. [Google Scholar] [CrossRef]
- Fandohan, P.; Gbenou, J.D.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.; Wingfield, M.J. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J. Agric. Food Chem. 2004, 52, 6824–6829. [Google Scholar] [CrossRef]
- Abbas, A.; Wright, C.W.; El-Sawi, N.; Yli-Mattila, T.; Malinen, A.M. A methanolic extract of Zanthoxylum bungeanum modulates secondary metabolism regulator genes in Aspergillus flavus and shuts down aflatoxin production. Sci. Rep. 2022, 12, 5995. [Google Scholar] [CrossRef]
- Abbas, A.; Yli-Mattila, T. Biocontrol of Fusarium graminearum, a causal agent of Fusarium head blight of wheat, and deoxynivalenol accumulation: From in vitro to in planta. Toxins 2022, 14, 299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Aristimuño Ficoseco, M.E.; Sequin, C.J.; Aceñolaza, P.G.; Vattuone, M.A.; Catalán, C.A.N.; Sampietro, D.A. Antifungal metabolites from Schinopsis balansae Engl (Anacardiaceae): Isolation, identification and evidences of their mode of action on Fusarium graminearum Schwabe. Nat. Prod. Res. 2017, 31, 1450–1453. [Google Scholar] [CrossRef]
- Christova, P.K.; Dobreva, A.M.; Dzhurmanski, A.G.; Dincheva, I.N.; Dimkova, S.D.; Zapryanova, N.G. The impact of plant essential oils on the growth of the pathogens Botrytis cinerea, Fusarium solani, and Phytophthora pseudocryptogea. Life 2024, 14, 817. [Google Scholar] [CrossRef]
- Natu, K.N.; Tatke, P.A. Essential oils—Prospective candidates for antifungal treatment? J. Essent. Oil Res. 2019, 31, 347–360. [Google Scholar] [CrossRef]
- Barak, T.H.; Eryilmaz, M.; Karaca, B.; Servi, H.; Kara Ertekin, S.; Dinc, M.; Ustuner, H. Antimicrobial, Anti-Biofilm, Anti-Quorum Sensing and Cytotoxic Activities of Thymbra spicata L. subsp. spicata Essential Oils. Antibiotics 2025, 14, 181. [Google Scholar]
- Barak, T.H.; Bölükbaş, E.; Bardakci, H. Evaluation of marketed rosemary essential oils (Rosmarinus officinalis L.) in terms of European pharmacopoeia 10.0 criteria. Turk. J. Pharm. Sci. 2023, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- El-Banhawy, A.; Acedo, C.; Qari, S.; Elkordy, A. Molecular identification and phylogenetic placement of Rosa arabica Crép.(Rosaceae), a critically endangered plant species. Life 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, N.; Pejić, I. Taxonomic review of the genus Rosa. Agric. Conspec. Sci. 2018, 83, 139–147. [Google Scholar]
- Franco, D.; Pinelo, M.; Sineiro, J.; Núñez, M.J. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 2007, 98, 3506–3512. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Rosa rubiginosa and Fraxinus ox-ycarpa herbal teas: Characterization of phytochemical profiles by liquid chromatography-mass spectrometry, and evaluation of the antioxidant activity. New J. Chem. 2017, 41, 7681–7688. [Google Scholar] [CrossRef]
- Peña, F.; Valencia, S.; Tereucán, G.; Nahuelcura, J.; Jiménez-Aspee, F.; Cornejo, P.; Ruiz, A. Bioactive compounds and antioxidant activity in the fruit of rosehip (Rosa canina L. and Rosa Rubiginosa L.). Molecules 2023, 28, 3544. [Google Scholar] [CrossRef]
- Üstüner, H.; Barak, T.H.; Servi, H.; Ertekin, S.K.; Sen, A.; Dinç, M.; Özkan, V.K. Anticancer, antidiabetic, and anti-oxidant activities of endemic Stachys buttleri Mill. and Stachys pinardii Boiss. essential oils. J. Essent. Oil Bear. Plants 2023, 26, 1502–1514. [Google Scholar] [CrossRef]
- Servi, H.; Demir, U.; Servi, E.Y.; Gundogdu, B.; Barak, T.H. Antiproliferative and Antibacterial Activities of Four Commer-cial Essential Oil Samples from Boswellia carteri, B. serrata, and two chemotypes of Canarium luzonicum. J. Essent. Oil Bear. Plants 2023, 26, 79–94. [Google Scholar] [CrossRef]
- Alexander, B.; Browse, D.J.; Reading, S.J.; Benjamin, I.S. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods 1999, 41, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, G.; Yörük, E.; Teker, T.; Sefer, Ö. Investigation of antifungal activities of myrcene on Fusarium reference strains. Arch. Microbiol. 2023, 205, 82. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Scussel, V.M. Effects of Ozone Gas Exposure on Toxigenic Fungi Species from Fusarium, Aspergillus, and Penicillium Genera. Ozone Sci. Eng. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Yörük, E. Rutin hydrate induces autophagic cell death and oxidative stress response in phytopathogenic fungus Fusarium graminearum. Zemdirbyste-Agriculture 2023, 110, 375–382. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gargouri-Kammoun, L.; Gargouri, S.; Rezgui, S.; Trifi, M.; Bahri, N.; Hajlaoui, M.R. Pathogenicity and aggressiveness of Fusarium and Microdochium on wheat seedlings under controlled conditions. Tunis. J. Plant Prot. 2009, 4, 135–144. [Google Scholar]
- Moradi, M.; Dehne, H.-W.; Steiner, U.; Oerke, E.-C. Improved procedure for mass inoculum production of Fusarium species in a short period of time. Appl. Entomol. Phytopathol. 2017, 84, 21–31. [Google Scholar]
- Moya-Elizondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Covarelli, L.; Gardiner, D.; Beccari, G.; Nicholson, P. Fusarium virulence assay on wheat and barley seedlings. Bio-Protocol 2013, 3, e446. [Google Scholar] [CrossRef]
- Hermann, D.; Stenzel, K. FRAC Mode-of-action Classification and Resistance Risk of Fungicides. In Modern Crop Protection Compounds, 1st ed.; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 589–608. [Google Scholar] [CrossRef]
- Russell, P.E. Fungicide resistance action committee (FRAC): A resistance activity update. Outlooks Pest Man-Agement 2009, 20, 122–125. [Google Scholar] [CrossRef]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental risks of fungicides used in hor-ticultural production systems. Fungicides 2010, 1, 273–304. [Google Scholar]
- Wiederhold, N. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar]
- Umaru, I.J.; Badruddin, F.A.; Umaru, H.A.; Umaru, K.I. Antifungal potential of some medicinal plants on selected pathogenic fungi. MOJ Proteom. Bioinform. 2018, 7, 271–276. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Bertoli, A.; Giamperi, L.; Bucchini, A.; Ricci, D.; Menichini, F.; Trinciarelli, E.; Pistelli, L. Antifungal Evaluation of Hypericum triquetrifolium Polar Extracts against Fusarium spp. Nat. Prod. Commun. 2006, 1, 1934578X0600101209. [Google Scholar] [CrossRef]
- Harcarova, M.; Conkova, E.; Proskovcova, M.; Váczi, P.; Marcincakova, D.; Bujnak, L. Comparison of antifungal activity of selected essential oils against Fusarium graminearum in vitro. Ann. Agric. Environ. Med. 2021, 28, 414–418. [Google Scholar] [CrossRef]
- Kong, W.; Huo, H.; Gu, Y.; Cao, Y.; Wang, J.; Liang, J.; Niu, S. Antifungal activity of camphor against four phyto-pathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Barak, T.H.; Dogenci, İ.; Bardakcı, H. Evaluation of the essential oil samples that are sold as “Eucalyptus oil” on the market in Türkiye in terms of European Pharmacopoeia 10.0 criteria. J. Res. Pharm. 2023, 27, 1463–1473. [Google Scholar]
- Yaghoobi, M.; Farimani, M.M.; Sadeghi, Z.; Asghari, S.; Rezadoost, H. Chemical analysis of Iranian Rosa damascena essential oil, concrete, and absolute oil under different bio-climatic conditions. Ind. Crops Prod. 2022, 187, 115266. [Google Scholar] [CrossRef]
- Rathore, S.; Kundlas, K.; Kumar, R. Variability in essential oil content and constituent profile of damask rose (Rosa damascena Mill.) at altered intervals of harvest in the Indian Western Himalaya. J. Appl. Res. Med. Aromat. Plants 2024, 39, 100537. [Google Scholar] [CrossRef]
- Izgi, M.N. Effect of different harvest dates to essential oil components of oil-bearing rose (Rosa damascena Mill.) in Mardin. J. Essent. Oil Bear. Plants 2022, 25, 250–261. [Google Scholar] [CrossRef]
- Aslan, E.G.; Ulusoy, S.; Kınaytürk, N.K.; Sarıçam, T.; Periz, Ç.D. Bioinsecticidal potential of rose essential oil against the cowpea beetle, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae). J. Appl. Entomol. 2024, 148, 1144–1156. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A. Evaluation of the effect of treated wastewater and well water irrigation on the quality, quantity and antibacterial/antifungal activities of Rosa× damascena Herrm. essential oil. Agric. Water Manag. 2022, 269, 107644. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Baser, K.H.C.; Akterian, S.G.; Fidan, H.N.; Stoyanova, A.S. Chemical composition, sensory evaluation and antimicrobial activity of Taif rose (Rosa damascena Mill.) essential oils. Bulg. Chem. Commun 2020, 52, 460–466. [Google Scholar]
- Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.K.; Chander, R.; Kumar, R.; Agnihotri, V.K. In-vitro antidiabetic and antimicrobial studies of volatile organic compounds (VOCs) from the roots of Rosa damascena Mill. of North-western Himalaya. S. Afr. J. Bot. 2024, 168, 518–528. [Google Scholar] [CrossRef]
- Barakat, H.; Ghazal, G.A. Physicochemical properties of Moringa oleifera seeds and their edible oil cultivated at different regions in Egypt. Food Nutr. Sci. 2016, 7, 472–484. [Google Scholar]
- Shahina, Z.; Al Homsi, R.; Price, J.D.; Whiteway, M.; Sultana, T.; Dahms, T.E. Rosemary essential oil and its com-ponents 1, 8-cineole and α-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans. PLoS ONE 2022, 17, e0277097. [Google Scholar] [CrossRef] [PubMed]

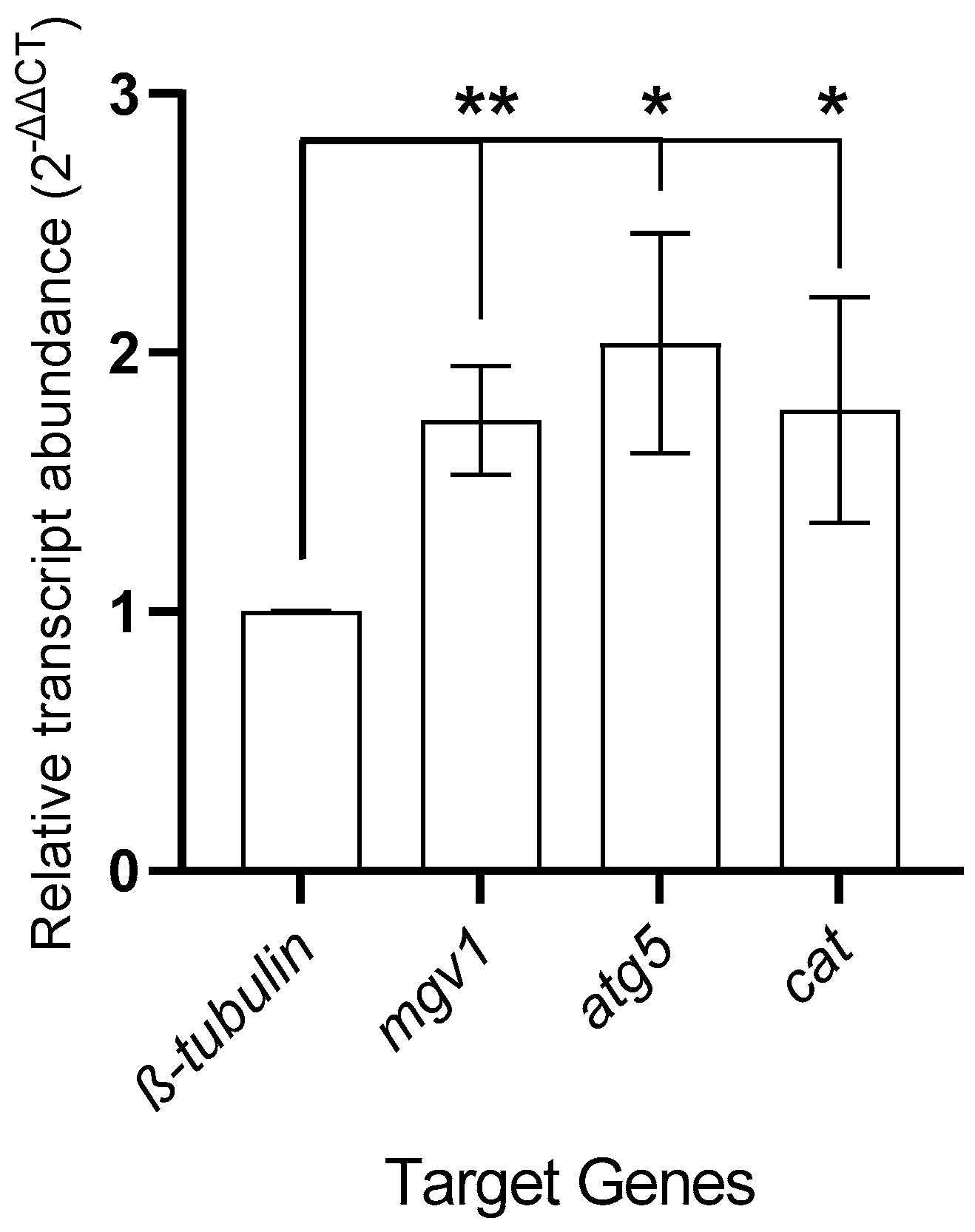

| Gene | Primer Set | Forward Sequence/Reverse Sequence (5′–3′) | Band Size (bp) |
|---|---|---|---|
| β-tubulin | betaF/betaR | AGGGTCATTACACCGAGGGT/GTACCACCACCAAGAGAGTGG | 121 |
| FgMgv1 | MgvRTF/MgvRTR | AGGTTCAACGATTCCGACAG/ GACCATTACCCTGAGGCAGA | 100 |
| catalase_3 | CatrtF/CatrtR | AATTCCACGTTCGTTTCGTC/ CCATACTAGGCTCGCTTTGC | 130 |
| atg5 | Atg5rtF/Atg5rtR | ATGTCTTCTCCCATCCCGC/ GCTGAAGCGTGGAATACTGG | 108 |
| RT 1 | RRI Exp. 2 | RRI Lit. 3 | Compound | (%) |
|---|---|---|---|---|
| 5.438 | 903 | 899 | Heptanal | 0.1 |
| 6.351 | 934 | 939 | α-Pinene | 0.4 |
| 7.374 | 969 | 970 | 1-Heptanol | 0.1 |
| 7.647 | 978 | 980 | β-Pinene | 0.1 |
| 8.070 | 992 | 991 | Myrcene | 0.2 |
| 12.223 | 1103 | 1098 | Linalool | 0.5 |
| 12.351 | 1106 | 1102 | Nonanal | 0.3 |
| 12.636 | 1113 | 1111 | Cis-Rose oxide | 0.4 |
| 12.810 | 1117 | 1117 | Phenylethyl alcohol | 1.1 |
| 13.318 | 1129 | 1126 | Trans-Rose oxide | 0.2 |
| 15.227 | 1174 | 1172 | Nonanol | 0.2 |
| 15.483 | 1180 | 1177 | Terpinen 4-ol | 0.8 |
| 16.090 | 1195 | 1189 | α-Terpineol | 0.1 |
| 18.149 | 1243 | 1228 | Citronellol | 37.1 |
| 18.212 | 1244 | 1237 | Isogeraniol | 0.1 |
| 18.332 | 1247 | 1240 | β-Citral | 0.3 |
| 19.118 | 1265 | 1255 | Geraniol | 10.6 |
| 19.585 | 1276 | 1270 | α-Citral | 0.5 |
| 21.801 | 1328 | 1323 | (E)-Methylgeranate | 0.1 |
| 23.036 | 1357 | 1354 | Citronellol acetate | 0.5 |
| 23.268 | 1363 | 1356 | Eugenol | 0.6 |
| 24.328 | 1388 | 1365 | Neryl acetate | 1.1 |
| 25.273 | 1411 | 1401 | Methyl eugenol | 2.7 |
| 25.761 | 1423 | 1418 | (E)-Caryophyllene | 0.8 |
| 26.544 | 1442 | 1443 | α-Guaiene | 1.2 |
| 26.672 | 1445 | 1449 | β-Phenylethyl butyrate | 0.1 |
| 27.157 | 1457 | 1455 | α-Caryophyllene | 0.8 |
| 28.285 | 1485 | 1480 | Germacrene D | 1.6 |
| 28.482 | 1490 | 1485 | β-Selinene | 0.2 |
| 28.995 | 1502 | 1500 | Pentadecane | 0.5 |
| 29.264 | 1509 | 1495 | δ-Guaiene | 1.0 |
| 36.590 | 1703 | 1700 | Heptadecane | 1.8 |
| 37.429 | 1727 | 1717 | Trans-Farnesol | 1.2 |
| 40.084 | 1803 | 1800 | Octadecane | 0.2 |
| 42.615 | 1878 | 9-Nonadecene | 2.9 | |
| 43.635 | 1909 | 1900 | Nonadecane | 13.8 |
| 45.801 | 1977 | 1-Eicosene | 0.1 | |
| 46.685 | 2005 | 2000 | Eicosane | 2.1 |
| 49.398 | 2093 | Henicos-1-ene | 0.2 | |
| 49.852 | 2108 | 2100 | Heneicosane | 8.9 |
| 52.662 | 2204 | 2200 | Docosane | 0.3 |
| 55.222 | 2295 | 1-Tricosene | 0.1 | |
| 55.520 | 2306 | 2300 | Tricosane | 2.6 |
| 60.807 | 2505 | 2500 | Pentacosane | 0.7 |
| 66.127 | 2705 | 2700 | Heptacosane | 0.4 |
| Monoterpenes | 0.7 | |||
| Monoterpenoids | 52.3 | |||
| Sesquiterpenes | 5.6 | |||
| Sesquiterpenoids | 1.2 | |||
| n-Alkane derivatives | 35.3 | |||
| Others | 4.5 | |||
| Total | 99.6 |
| r correlation values obtained via Pearson’s correlation matrix | |||||||
| wst1 | severity | dcfda | md | atg5 | cat | mgv1 | |
| wst1 | 1.00 | 0.22 | −0.54 | −0.36 | 0.77 | 0.19 | 0.86 |
| severity | 0.22 | 1.00 | −0.06 | 0.31 | 0.27 | 0.72 | 0.43 |
| dcfda | −0.54 | −0.06 | 1.00 | 0.71 | −0.65 | −0.28 | −0.25 |
| md | −0.36 | 0.31 | 0.71 | 1.00 | −0.31 | −0.06 | −0.01 |
| atg5 | 0.77 | 0.27 | −0.65 | −0.31 | 1.00 | 0.59 | 0.83 |
| cat | 0.19 | 0.72 | −0.28 | −0.06 | 0.59 | 1.00 | 0.48 |
| mgv1 | 0.86 | 0.43 | −0.25 | −0.01 | 0.83 | 0.48 | 1.00 |
| p values obtained via Pearson’s correlation matrix | |||||||
| wst1 | severity | dcfda | md | atg5 | cat | mgv1 | |
| wst1 | 0.66 | 0.26 | 0.47 | 0.07 | 0.71 | 0.02 | |
| severity | 0.66 | 0.90 | 0.55 | 0.60 | 0.10 | 0.39 | |
| dcfda | 0.26 | 0.90 | 0.11 | 0.15 | 0.59 | 0.63 | |
| md | 0.47 | 0.55 | 0.11 | 0.54 | 0.91 | 0.98 | |
| atg5 | 0.07 | 0.60 | 0.15 | 0.54 | 0.22 | 0.04 | |
| cat | 0.71 | 0.10 | 0.59 | 0.91 | 0.22 | 0.33 | |
| mgv1 | 0.02 | 0.39 | 0.63 | 0.98 | 0.04 | 0.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özsoy, E.; Barak, T.H.; Yörük, E.; Servi, H.; Yli-Mattila, T. Chemical Composition, Antifungal Activity, and Plant-Protective Potential of Rosa damascena Mill. Essential Oil Against Fusarium graminearum. Pathogens 2025, 14, 383. https://doi.org/10.3390/pathogens14040383
Özsoy E, Barak TH, Yörük E, Servi H, Yli-Mattila T. Chemical Composition, Antifungal Activity, and Plant-Protective Potential of Rosa damascena Mill. Essential Oil Against Fusarium graminearum. Pathogens. 2025; 14(4):383. https://doi.org/10.3390/pathogens14040383
Chicago/Turabian StyleÖzsoy, Esma, Timur Hakan Barak, Emre Yörük, Hüseyin Servi, and Tapani Yli-Mattila. 2025. "Chemical Composition, Antifungal Activity, and Plant-Protective Potential of Rosa damascena Mill. Essential Oil Against Fusarium graminearum" Pathogens 14, no. 4: 383. https://doi.org/10.3390/pathogens14040383
APA StyleÖzsoy, E., Barak, T. H., Yörük, E., Servi, H., & Yli-Mattila, T. (2025). Chemical Composition, Antifungal Activity, and Plant-Protective Potential of Rosa damascena Mill. Essential Oil Against Fusarium graminearum. Pathogens, 14(4), 383. https://doi.org/10.3390/pathogens14040383







