Caffeic Acid Phenethyl Ester (CAPE) Inhibits Arginase Activity and Growth of Leishmania amazonensis Promastigotes and Intracellular Amastigotes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
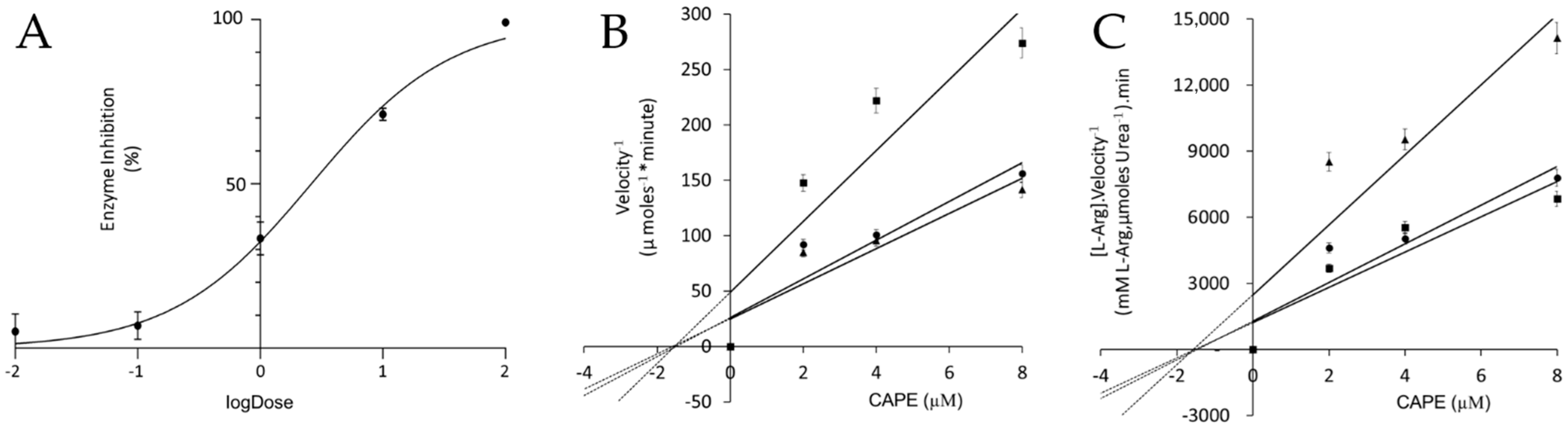
2.2. Kinetics of Arginase Inhibition
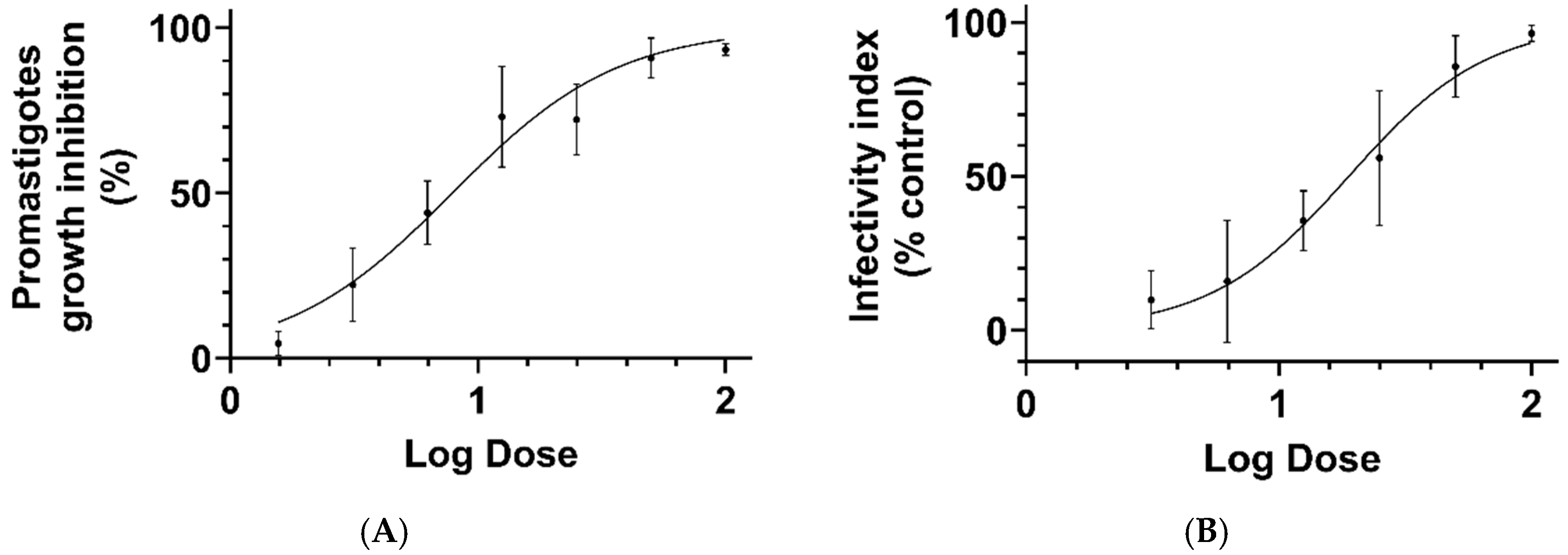
2.3. Promastigote Test Culture
2.4. Amastigote Culture
2.5. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO|Leishmaniasis. 2019. Available online: https://www.who.int/leishmaniasis/en/ (accessed on 27 June 2019).
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef]
- Colotti, G.; Ilari, A. Polyamine metabolism in Leishmania: From arginine to trypanothione. Amino Acids 2011, 40, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Balã Na-Fouce, R.; Calvo-Álvarez, E.; Álvarez-Velilla, R.; Prada, C.F.; Pérez-Pertejo, Y.; Reguera, R.M. Role of trypanosomatid’s arginase in polyamine biosynthesis and pathogenesis. Mol. Biochem. Parasitol. 2011, 181, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Krauth-Siegel, R.L.; Meiering, S.K.; Schmidt, H. The parasite-specific trypanothione metabolism of trypanosoma and leishmania. Biol. Chem. 2003, 384, 539–549. [Google Scholar] [CrossRef]
- da Silva, M.F.L.; Zampieri, R.A.; Muxel, S.M.; Beverley, S.M.; Floeter-Winter, L.M. Leishmania amazonensis Arginase Compartmentalization in the Glycosome Is Important for Parasite Infectivity. PLoS ONE 2012, 7, e34022. [Google Scholar] [CrossRef]
- Aoki, J.I.; Laranjeira-Silva, M.F.; Muxel, S.M.; Floeter-Winter, L.M. The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr. Opin. Microbiol. 2019, 52, 110–115. [Google Scholar] [CrossRef]
- Pham, T.N.; Bordage, S.; Pudlo, M.; Demougeot, C.; Thai, K.M.; Girard-Thernier, C. Cinnamide derivatives as mammalian arginase inhibitors: Synthesis, biological evaluation and molecular docking. Int. J. Mol. Sci. 2016, 17, 1656. [Google Scholar] [CrossRef]
- da Silva, E.R.; Come, J.A.A.d.S.S.; Brogi, S.; Calderone, V.; Chemi, G.; Campiani, G.; Oliveira, T.M.F.d.S.; Pham, T.-N.; Pudlo, M.; Girard, C.; et al. Cinnamides Target Leishmania amazonensis Arginase Selectively. Molecules 2020, 25, 5271. [Google Scholar] [CrossRef]
- Riley, E.; Roberts, S.C.; Ullman, B. Inhibition profile of Leishmania mexicana arginase reveals differences with human arginase I. Int. J. Parasitol. 2011, 41, 545–552. [Google Scholar] [CrossRef]
- Ogeturk, M.; Kus, I.; Colakoglu, N.; Zararsiz, I.; Ilhan, N.; Sarsilmaz, M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J. Ethnopharmacol. 2005, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Shiao, M.-S.; Hsu, M.-L.; Tsai, T.-H.; Wang, S.-Y. Effect of Caffeic Acid Phenethyl Ester, an Antioxidant from Propolis, on Inducing Apoptosis in Human Leukemic HL-60 Cells. J. Agric. Food Chem. 2001, 49, 5615–5619. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Hassham, M.; Bin Asad, H. Caffeic Acid Phenethyl Ester and Therapeutic Potentials. BioMed Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.C.; Marcucci, M.C.; Giorgio, S. Effects of Brazilian propolis on Leishmania amazonensis. Mem. Inst. Oswaldo Cruz 2007, 102, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, O.; Grishanin, R.N.; Calder, P. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Wang, L.-C.; Lin, Y.-L.; Liang, Y.-C.; Yang, Y.-H.; Lee, J.-H.; Yu, H.-H.; Wu, W.-M.; Chiang, B.-L. The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells. BMC Immunol. 2009, 10, 39. [Google Scholar] [CrossRef]
- da Silva, E.R.; da Silva, M.F.L.; Fischer, H.; Mortara, R.A.; Mayer, M.G.; Framesqui, K.; Silber, A.M.; Floeter-Winter, L.M. Biochemical and biophysical properties of a highly active recombinant arginase from Leishmania (Leishmania) amazonensis and subcellular localization of native enzyme. Mol. Biochem. Parasitol. 2008, 159, 104–111. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef]
- Maquiaveli, C.C.; Lucon-Júnior, J.F.; Brogi, S.; Campiani, G.; Gemma, S.; Vieira, P.C.; Silva, E.R. Verbascoside Inhibits Promastigote Growth and Arginase Activity of Leishmania amazonensis. J. Nat. Prod. 2016, 79, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Maquiaveli, C.D.C.; Vieira, P.C.; da Silva, E.R. Antileishmanial activity of verbascoside: Selective arginase inhibition of intracellular amastigotes of Leishmania (Leishmania) amazonensis with resistance induced by LPS plus IFN-γ. Biochem. Pharmacol. 2017, 127, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Gómez-Nieto, L.C.; Corraliza, I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 2001, 193, 777–784. [Google Scholar] [CrossRef]
- Abamor, E.S. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes in vitro. Asian Pac. J. Trop. Med. 2017, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Froelich, S.; Gupta, M.P.; Siems, K.; Jenett-Siems, K. Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant. Rev. Bras. Farmacogn. 2008, 18, 517–520. [Google Scholar] [CrossRef]
- Montrieux, E.; Perera, W.H.; García, M.; Maes, L.; Cos, P.; Monzote, L. In vitro and in vivo activity of major constituents from Pluchea carolinensis against Leishmania amazonensis. Parasitol. Res. 2014, 113, 2925–2932. [Google Scholar] [CrossRef]
- da Silva, E.R.; Brogi, S.; Grillo, A.; Campiani, G.; Gemma, S.; Vieira, P.C.; Maquiaveli, C.D.C. Cinnamic acids derived compounds with antileishmanial activity target Leishmania amazonensis arginase. Chem. Biol. Drug Des. 2019, 93, 139–146. [Google Scholar] [CrossRef]
- Bocedi, A.; Dawood, K.F.; Fabrini, R.; Federici, G.; Gradoni, L.; Pedersen, J.Z.; Ricci, G. Trypanothione efficiently intercepts nitric oxide as a harmless iron complex in trypanosomatid parasites. FASEB J. 2010, 24, 1035–1042. [Google Scholar] [CrossRef]
- Castilho-Martins, E.A.; Laranjeira da Silva, M.F.; dos Santos, M.G.; Muxel, S.M.; Floeter-Winter, L.M. Axenic Leishmania amazonensis Promastigotes Sense both the External and Internal Arginine Pool Distinctly Regulating the Two Transporter-Coding Genes. PLoS ONE 2011, 6, e27818. [Google Scholar] [CrossRef]
- Wanasen, N.; Soong, L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 2008, 41, 15–25. [Google Scholar] [CrossRef]
- Pessenda, G.; da Silva, J.S. Arginase and its mechanisms in Leishmania persistence. Parasite Immunol. 2020, 42, e12722. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, A. Th1/Th2-regulated arginase availability modulates Leishmania infection. Trends Parasitol. 2001, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.; Escobar, P.; Croft, S.L. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 2010, 65, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Terreros, M.J.S.; de Luna, L.A.V.; Giorgio, S. Evaluation of antileishmanial drugs activities in an ex vivo model of leishmaniasis. Parasitol. Int. 2019, 71, 163–166. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, E.R.; Mesquita, A.; do Carmo Maquiaveli, C. Caffeic Acid Phenethyl Ester (CAPE) Inhibits Arginase Activity and Growth of Leishmania amazonensis Promastigotes and Intracellular Amastigotes. Pathogens 2025, 14, 384. https://doi.org/10.3390/pathogens14040384
da Silva ER, Mesquita A, do Carmo Maquiaveli C. Caffeic Acid Phenethyl Ester (CAPE) Inhibits Arginase Activity and Growth of Leishmania amazonensis Promastigotes and Intracellular Amastigotes. Pathogens. 2025; 14(4):384. https://doi.org/10.3390/pathogens14040384
Chicago/Turabian Styleda Silva, Edson Roberto, André Mesquita, and Claudia do Carmo Maquiaveli. 2025. "Caffeic Acid Phenethyl Ester (CAPE) Inhibits Arginase Activity and Growth of Leishmania amazonensis Promastigotes and Intracellular Amastigotes" Pathogens 14, no. 4: 384. https://doi.org/10.3390/pathogens14040384
APA Styleda Silva, E. R., Mesquita, A., & do Carmo Maquiaveli, C. (2025). Caffeic Acid Phenethyl Ester (CAPE) Inhibits Arginase Activity and Growth of Leishmania amazonensis Promastigotes and Intracellular Amastigotes. Pathogens, 14(4), 384. https://doi.org/10.3390/pathogens14040384







