Enhanced Biofilm Disruption in Methicillin-Resistant Staphylococcus aureus Using Rifampin and Fluoroquinolone Combinations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Biofilm-Forming Ability Assay
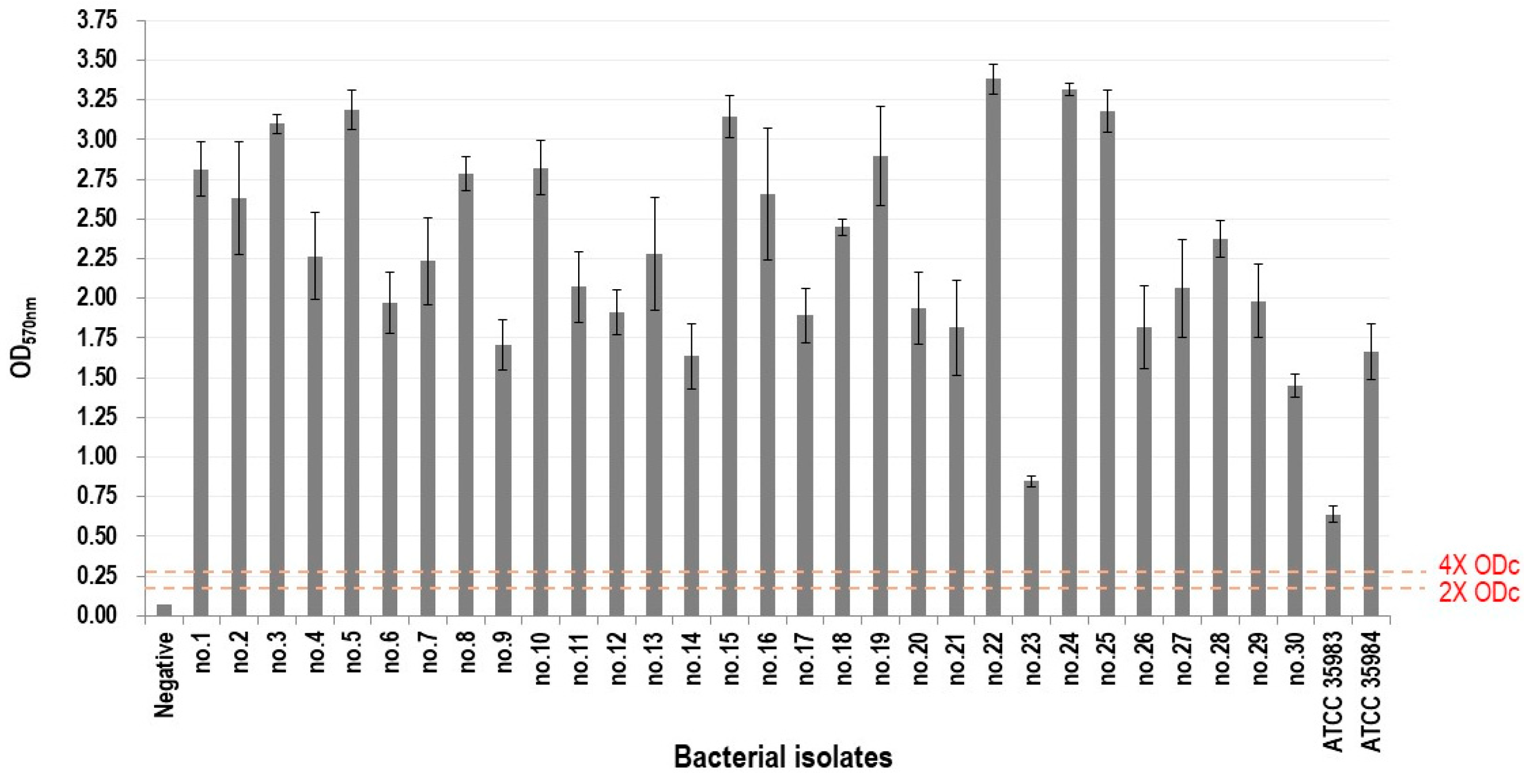
- OD ≤ ODc: no biofilm production;
- ODc < OD ≤ 2 × ODc: weak biofilm production;
- 2 × ODc < OD ≤ 4 × ODc: moderate biofilm production;
- OD > 4 × ODc: strong biofilm production.
2.3. Determination of Minimum Biofilm Eradication Concentration (MBEC)
2.4. Assessment of Interactions in Antibiotic Combinations Using Fractional Biofilm Eradication Concentration (FBEC) Index
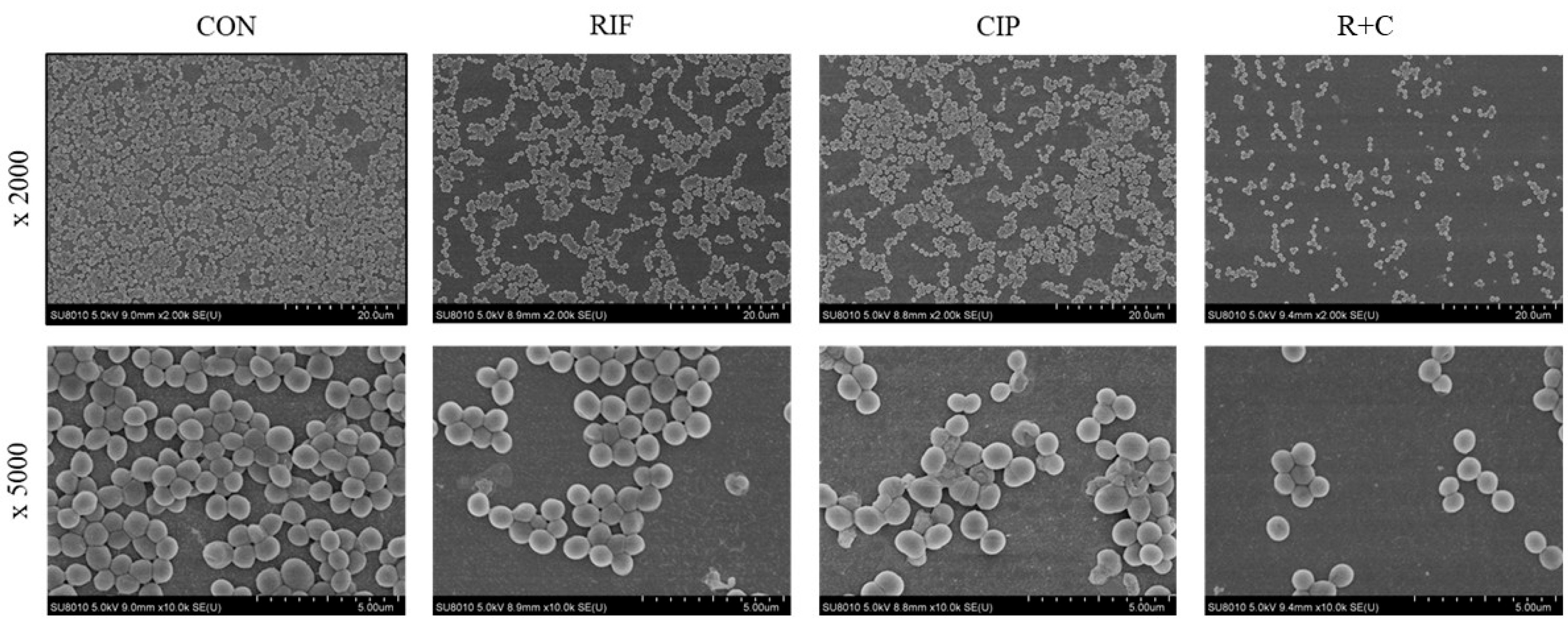
2.5. Visualization of Biofilm Eradication Through Scanning Electron Microscopy (SEM)
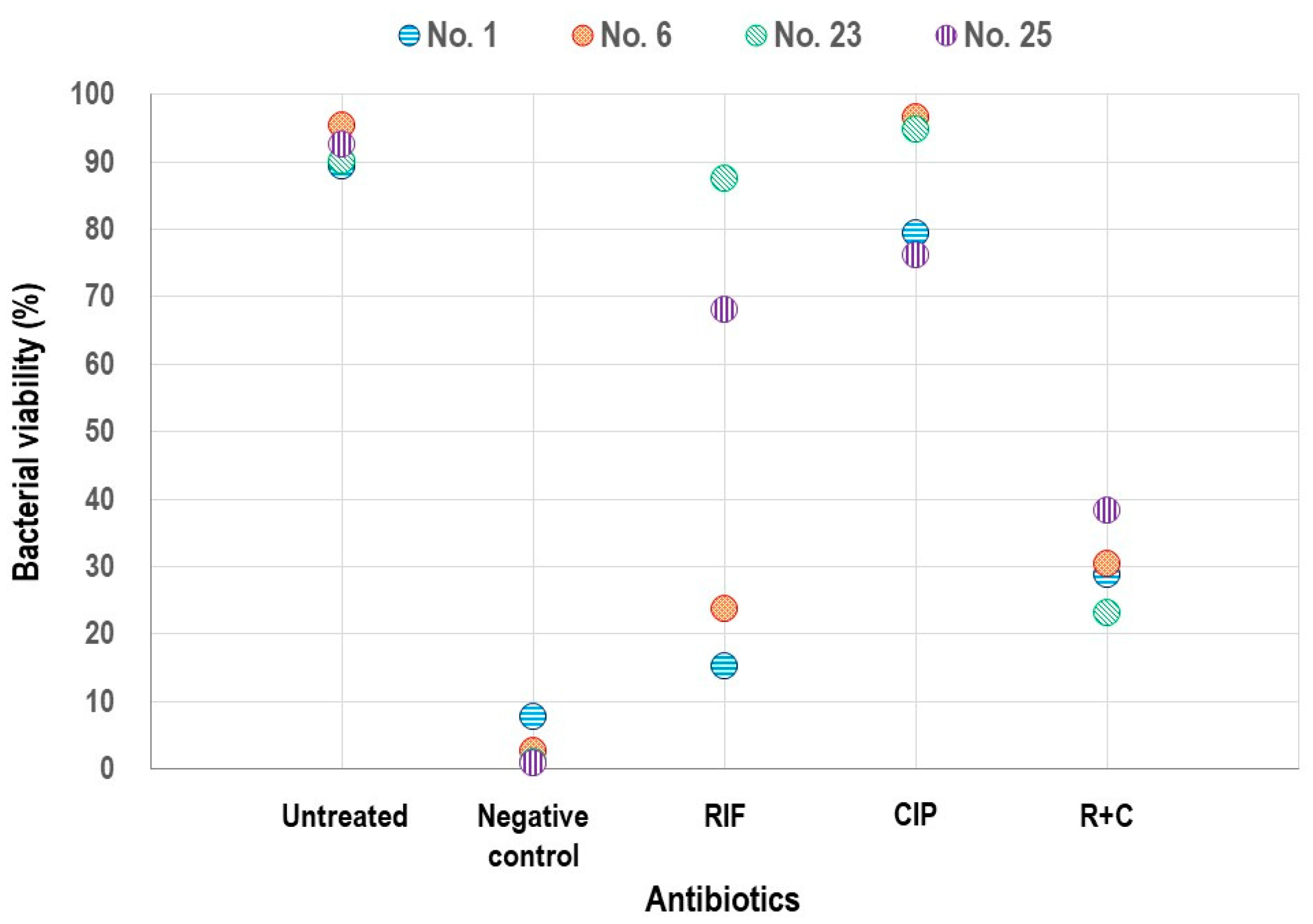
2.6. Analysis of Biofilms Using Confocal Laser Scanning Microscopy (CLSM)
2.7. Statistical Analysis
3. Results
3.1. Determination of Biofilm-Forming Ability
3.2. Determination of Interactions Based on FBEC Index
3.3. Visualization of Biofilm Eradication Through SEM
3.4. Assessment of Biofilm Viability Using CLSM
3.5. Concordance in Interactions of Antibiotic Combinations Between Biofilm and Planktonic Phases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Extracellular polymeric substances |
| RIF | Rifampin |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| VSSA | Vancomycin-susceptible S. aureus |
| VISA | Vancomycin-intermediate S. aureus |
| hVISA | Heterogeneous VISA |
| MIC | Minimum inhibitory concentration |
| CIP | Ciprofloxacin |
| LVX | Levofloxacin |
| OD | Optical density |
| MBEC | Minimum biofilm eradication concentration |
| FBEC | Fractional biofilm eradication concentration |
| SEM | Scanning electron microscopy |
| CLSM | Confocal laser scanning microscopy |
| ANOVA | Analysis of variance |
| R+C | Rifampin + ciprofloxacin |
| R+L | Rifampin + levofloxacin |
References
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A review of biofilm formation of Staphylococcus aureus and its regulation mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- Jørgensen, N.P.; Skovdal, S.M.; Meyer, R.L.; Dagnæs-Hansen, F.; Fuursted, K.; Petersen, E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog. Dis. 2016, 74, ftw019. [Google Scholar] [CrossRef]
- Renz, N.; Trampuz, A.; Zimmerli, W. Controversy about the role of rifampin in biofilm infections: Is it justified? Antibiotics 2021, 10, 165. [Google Scholar] [CrossRef]
- Kang, Y.R.; Chung, D.R.; Ko, J.H.; Huh, K.; Cho, S.Y.; Kang, C.I.; Peck, K.R. Comparing the synergistic and antagonistic interactions of ciprofloxacin and levofloxacin combined with rifampin against drug-resistant Staphylococcus aureus: A time-kill assay. Antibiotics 2023, 12, 711. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Stefano, V.D. In vitro anti-biofilm activity of Boswellia spp. Oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Ceri, H.; Yerly, J.; Stremick, C.A.; Hu, Y.; Martinuzzi, R.; Turner, R.J. The use of microscopy and three-dimensional visualization to evaluate the structure of microbial biofilms cultivated in the Calgary Biofilm Device. Biol. Proced. Online 2006, 8, 194–215. [Google Scholar] [CrossRef]
- Ghorbani, H.; Memar, M.Y.; Sefidan, F.Y.; Yekani, M.; Ghotaslou, R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS Hyg. Infect. Control 2017, 12, Doc17. [Google Scholar]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef]
- Dall, G.F.; Tsang, S.J.; Gwynne, P.J.; MacKenzie, S.P.; Simpson, A.H.R.W.; Breusch, S.J.; Gallagher, M.P. Unexpected synergistic and antagonistic antibiotic activity against Staphylococcus biofilms. J. Antimicrob. Chemother. 2018, 73, 1830–1840. [Google Scholar] [CrossRef]
- Kong, C.; Chee, C.F.; Richter, K.; Thomas, N.; Rahman, N.A.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef]
- Chin, C.Y.; Hara, Y.; Ghazali, A.K.; Yap, S.J.; Kong, C.; Wong, Y.C.; Rozali, N.; Koh, S.F.; Hoh, C.C.; Puthucheary, S.D.; et al. Global transcriptional analysis of Burkholderia pseudomallei high and low biofilm producers reveals insights into biofilm production and virulence. BMC Genom. 2015, 16, 471. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Cerca, N.; Azeredo, J.; Oliveira, R. Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Curr. Microbiol. 2011, 63, 354–359. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob. Agents Chemother. 2019, 63, e01746-18. [Google Scholar] [CrossRef] [PubMed]
- Beldman, M.; Löwik, C.; Soriano, A.; Albiach, L.; Zijlstra, W.P.; Knobben, B.A.S.; Jutte, P.; Sousa, R.; Carvalho, A.; Goswami, K.; et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin. Infect. Dis. 2021, 73, 1634–1641. [Google Scholar] [CrossRef]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic tolerance of Staphylococcus aureus biofilm in periprosthetic joint infections and antibiofilm strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef]
- Zheng, Z.; Stewart, P.S. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2002, 46, 900–903. [Google Scholar] [CrossRef]
- Achermann, Y.; Eigenmann, K.; Ledergerber, B.; Derksen, L.; Rafeiner, P.; Clauss, M.; Nüesch, R.; Zellweger, C.; Vogt, M.; Zimmerli, W. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): A matched case-control study. Infection 2013, 41, 431–437. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Euba, G.; Cobo, J.; Horcajada, J.P.; Soriano, A.; Sandoval, E.; Pigrau, C.; Benito, N.; Falgueras, L.; Palomino, J.; et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: A randomised clinical trial. Int. J. Antimicrob. Agents 2016, 48, 310–316. [Google Scholar]
- Suzuki, H.; Goto, M.; Nair, R.; Livorsi, D.J.; Sekar, P.; Ohl, M.E.; Diekema, D.J.; Perencevich, E.N.; Alexander, B.; Jones, M.P.; et al. Effectiveness and optimal duration of adjunctive rifampin treatment in the management of Staphylococcus aureus prosthetic joint infections after debridement, antibiotics, and implant retention. Open Forum Infect. Dis. 2022, 9, ofac473. [Google Scholar] [CrossRef]
- El Zein, S.; Berbari, E.F.; Passerini, M.; Petri, F.; Maamari, J.; Murad, M.H.; Sendi, P.; Tande, A.J. Rifampin based therapy for patients with Staphylococcus aureus native vertebral osteomyelitis: A systematic review and meta-analysis. Clin. Infect. Dis. 2024, 78, 40–47. [Google Scholar] [CrossRef]
- Perez-Alba, E.; Flores-Treviño, S.; Villarreal-Salazar, V.; Bocanegra-Ibarias, P.; Vilchez-Cavazos, F.; Camacho-Ortiz, A. Planktonic and biofilm states of Staphylococcus aureus isolated from bone and joint infections and the in vitro effect of orally available antibiotics. J. Appl. Microbiol. 2023, 134, lxad258. [Google Scholar] [CrossRef]
- Bahl, D.; Miller, D.A.; Leviton, I.; Gialanella, P.; Wolin, M.J.; Liu, W.; Perkins, R.; Miller, M.H. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1997, 41, 1293–1297. [Google Scholar] [CrossRef] [PubMed]


| Strain | Phenotype | Planktonic MIC (mg/L) | Biofilm MBEC (mg/L) | R+C Combination (Biofilm) | Planktonic MIC (mg/L) | Biofilm MBEC (mg/L) | R+L Combination (Biofilm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIF | CIP | RIF | CIP | FBEC Index (Median) | FBEC Index (Range) | Interaction | RIF | LVX | RIF | LVX | FBEC Index (Median) | FBEC Index (Range) | Interaction | ||
| 1 | VSSA | 0.015 | 32 | 32 | ≥1024 | 0.204 | 0.039–1.031 | S | 0.015 | 8 | 32 | 4 | 0.438 | 0.281–0.75 | S |
| 2 | VSSA | 0.015 | 0.5 | 64 | 0.25 | 0.750 | 0.500–1.125 | I | 0.015 | 0.25 | 64 | 0.5 | 0.266 | 0.156–0.625 | S |
| 3 | VSSA | 0.015 | >64 | 2 | 1024 | 0.523 | 0.501–1.0 | I | 0.015 | 32 | 2 | 16 | 0.688 | 0.563–1.0 | I |
| 4 | VSSA | 0.015 | 0.5 | 64 | 0.5 | 0.258 | 0.141–0.625 | S | 0.015 | 0.25 | 64 | 0.5 | 3.063 | 0.375–128.063 | I |
| 5 | VSSA | 0.015 | 64 | 32 | 1024 | 0.094 | 0.033–0.531 | S | 0.015 | 16 | 32 | 16 | 0.234 | 0.093–0.563 | S |
| 6 | VSSA | 0.015 | >64 | 64 | 1024 | 0.071 | 0.018–0.516 | S | 0.015 | >32 | 64 | ≥1024 | 0.071 | 0.017–1.016 | S |
| 7 | VSSA | 0.015 | >64 | 64 | ≥1024 | 0.127 | 0.035–1.016 | S | 0.015 | >32 | 64 | ≥1024 | 0.281 | 0.063–1.031 | S |
| 8 | VSSA | 0.015 | 64 | 64 | ≥1024 | 0.078 | 0.032–1.016 | S | 0.015 | 32 | 64 | 64 | 0.148 | 0.078–0.516 | S |
| 9 | VSSA | 0.015 | >64 | 4 | ≥1024 | 0.375 | 0.254–1.25 | S | 0.015 | 32 | 4 | 32 | 0.438 | 0.281–0.750 | S |
| 10 | VSSA | 0.015 | 0.5 | 32 | 0.5 | 0.516 | 0.281–0.625 | I | 0.015 | 0.25 | 32 | 0.25 | 1.250 | 0.750–2.125 | I |
| 11 | VSSA | 0.015 | 32 | 8 | ≥1024 | 0.188 | 0.126–1.125 | S | 0.015 | 8 | 8 | 256 | 0.219 | 0.129–0.625 | S |
| 12 | VSSA | 0.015 | 0.25 | 64 | 0.5 | 6.266 | 1.125–16.016 | A | 0.015 | 0.25 | 32 | 0.25 | 0.438 | 0.281–0.75 | S |
| 13 | VSSA | 0.015 | 64 | 8 | ≥1024 | 0.188 | 0.126–1.125 | S | 0.015 | 32 | 8 | 128 | 0.250 | 0.133–0.625 | S |
| 14 | VSSA | 0.015 | 0.25 | 64 | 1 | 0.258 | 0.094–0.563 | S | 0.015 | 0.25 | 64 | 0.25 | 0.563 | 0.282–1.25 | I |
| 15 | VSSA | 1 | 4 | 8 | 8 | 0.750 | 0.5–1.125 | I | 1 | 4 | 8 | 4 | 1.500 | 1.0–2.250 | I |
| 16 | VISA | 16 | 16 | 512 | ≥1024 | 0.531 | 0.502–1.125 | I | 16 | 8 | 512 | 32 | 0.563 | 0.266–0.750 | I |
| 17 | VISA | 16 | 64 | 512 | ≥1024 | 1.188 | 1.016–1.5 | I | 16 | 16 | 512 | 32 | 1.125 | 1.016–4.500 | I |
| 18 | VISA | 16 | 1 | ≥512 | 0.5 | 1.125 | 0.516–1.5 | I | 16 | 0.25 | ≥512 | 1 | 0.750 | 0.375–1.500 | I |
| 19 | hVISA | 0.015 | 0.25 | 64 | 0.25 | 0.750 | 0.516–1.25 | I | 0.015 | 0.5 | 64 | 0.25 | 0.625 | 0.516–1.250 | I |
| 20 | hVISA | 0.015 | 16 | 64 | ≥1024 | 0.102 | 0.023–1.016 | S | 0.015 | 8 | 64 | 16 | 0.266 | 0.156–0.563 | S |
| 21 | hVISA | 0.015 | 16 | 64 | ≥1024 | 0.071 | 0.017–1.016 | S | 0.015 | 8 | 64 | 16 | 0.227 | 0.141–0.563 | S |
| 22 | hVISA | 0.015 | 16 | 64 | ≥1024 | 0.071 | 0.017–1.016 | S | 0.015 | 8 | 64 | 64 | 0.281 | 0.063–1.016 | S |
| 23 | hVISA | 16 | >64 | 512 | ≥1024 | 0.594 | 0.375–1.125 | I | 16 | 16 | 512 | 16 | 1.750 | 1.016–4.500 | I |
| 24 | hVISA | 0.015 | 32 | 64 | ≥1024 | 0.281 | 0.125–1.016 | S | 0.015 | 8 | 64 | ≥1024 | 0.141 | 0.047–1.016 | S |
| 25 | hVISA | 16 | 8 | ≥512 | ≥1024 | 1.023 | 0.750–1.250 | S | 16 | 8 | ≥512 | 8 | 1.188 | 1.016–2.5 | I |
| 26 | hVISA | 0.015 | >64 | 32 | ≥1024 | 0.110 | 0.039–1.031 | S | 0.015 | 32 | 32 | 64 | 0.156 | 0.047–1.016 | S |
| 27 | hVISA | 0.015 | 0.5 | 8 | 0.25 | 0.625 | 0.375–1.25 | I | 0.015 | 0.5 | 8 | 0.5 | 0.375 | 0.25–0.625 | S |
| 28 | hVISA | 0.015 | 0.25 | 64 | 0.5 | 1.813 | 0.5–128.031 | I | 0.015 | 0.25 | 64 | 0.125 | 0.813 | 0.531–1.25 | I |
| 29 | hVISA | 0.015 | 32 | 64 | ≥1024 | 0.031 | 0.016–0.5 | S | 0.015 | 8 | 64 | 16 | 0.375 | 0.141–0.563 | S |
| 30 | hVISA | 16 | >64 | ≥512 | ≥1024 | 1.094 | 0.75–1.25 | I | 16 | 32 | ≥512 | 32 | 1.125 | 1.016–5.0 | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.R.; Park, J.-Y.; Chung, D.R.; Kang, M.; Ko, J.-H.; Huh, K.; Cho, S.Y.; Kang, C.-I.; Peck, K.R. Enhanced Biofilm Disruption in Methicillin-Resistant Staphylococcus aureus Using Rifampin and Fluoroquinolone Combinations. Pathogens 2025, 14, 404. https://doi.org/10.3390/pathogens14050404
Kang YR, Park J-Y, Chung DR, Kang M, Ko J-H, Huh K, Cho SY, Kang C-I, Peck KR. Enhanced Biofilm Disruption in Methicillin-Resistant Staphylococcus aureus Using Rifampin and Fluoroquinolone Combinations. Pathogens. 2025; 14(5):404. https://doi.org/10.3390/pathogens14050404
Chicago/Turabian StyleKang, Yu Ri, Joo-Young Park, Doo Ryeon Chung, Minhee Kang, Jae-Hoon Ko, Kyungmin Huh, Sun Young Cho, Cheol-In Kang, and Kyong Ran Peck. 2025. "Enhanced Biofilm Disruption in Methicillin-Resistant Staphylococcus aureus Using Rifampin and Fluoroquinolone Combinations" Pathogens 14, no. 5: 404. https://doi.org/10.3390/pathogens14050404
APA StyleKang, Y. R., Park, J.-Y., Chung, D. R., Kang, M., Ko, J.-H., Huh, K., Cho, S. Y., Kang, C.-I., & Peck, K. R. (2025). Enhanced Biofilm Disruption in Methicillin-Resistant Staphylococcus aureus Using Rifampin and Fluoroquinolone Combinations. Pathogens, 14(5), 404. https://doi.org/10.3390/pathogens14050404







