Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils
2.2. Compounds Identification and Dilution Design
2.3. Bacteria Strains
2.4. Screening for MDR Activity
2.5. Screening for TEO Activity
2.5.1. Disk Diffusion and Broth Microdilution Methods
2.5.2. Determination of Time-Kill Kinetics
2.6. Preliminary Test for Standardization of In Vitro Synthesis of Biofilm Assay
- Nonbiofilm producers (OD ≤ ODc);
- Weak biofilm producers (ODc < OD ≤ 2 × ODc);
- Moderate biofilm producers (2 × ODc < OD ≤ 4 × ODc);
- Strong biofilm producers (4 × ODc < OD).
2.7. Biofilm Production by Isolated Bacteria and TEO Inhibition
2.8. PCR for the Detection of Genes Linked to Biofilm Production
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of the TEO
3.2. Isolated Bacteria
3.3. MDR Activity
3.4. Screening for Antibacterial Activity of TEO
3.4.1. Disk Diffusion Assay
3.4.2. MIC and MBC
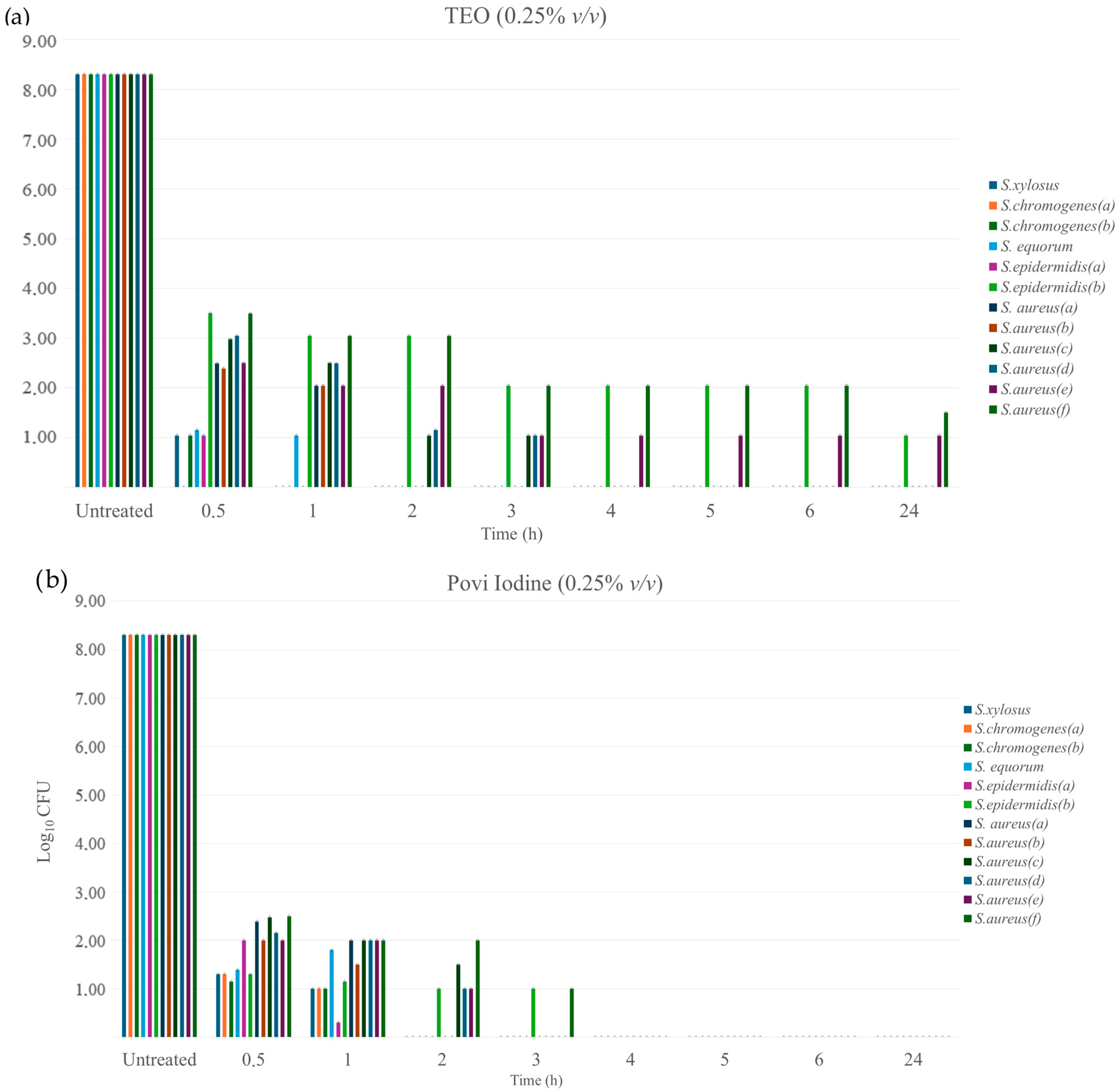
3.4.3. Time-Kill Kinetics
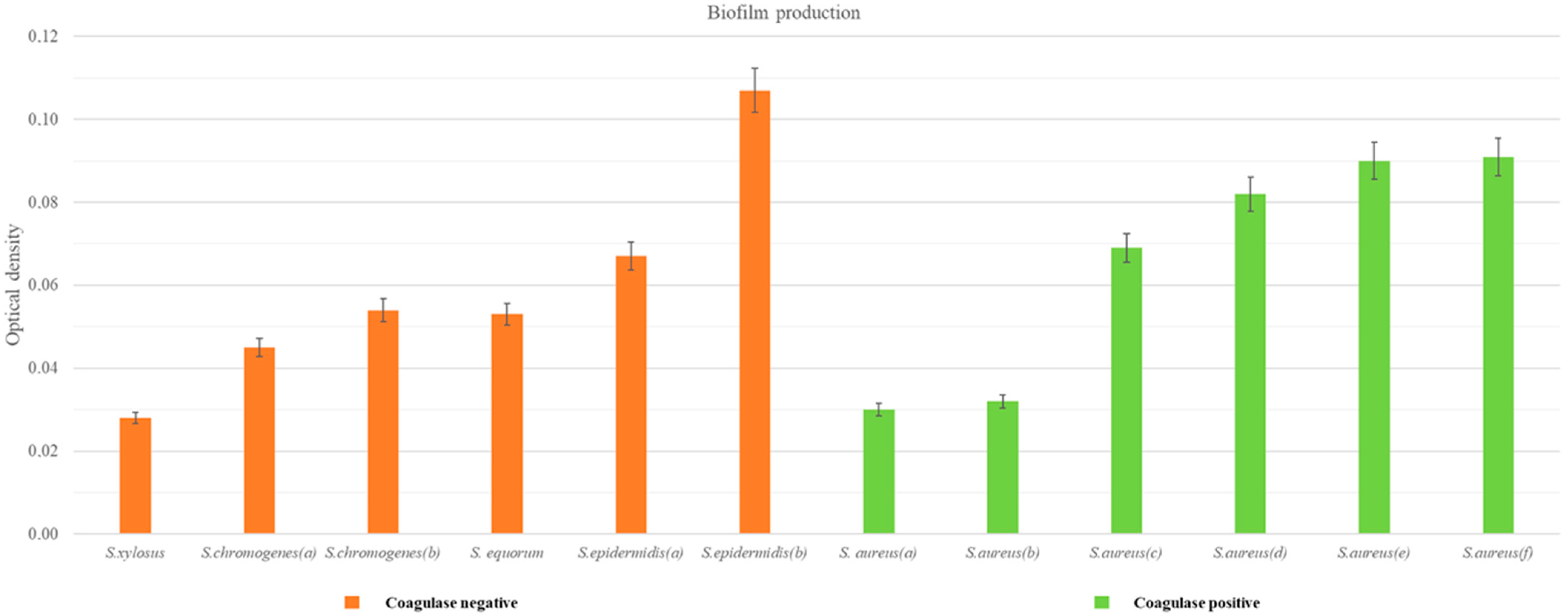
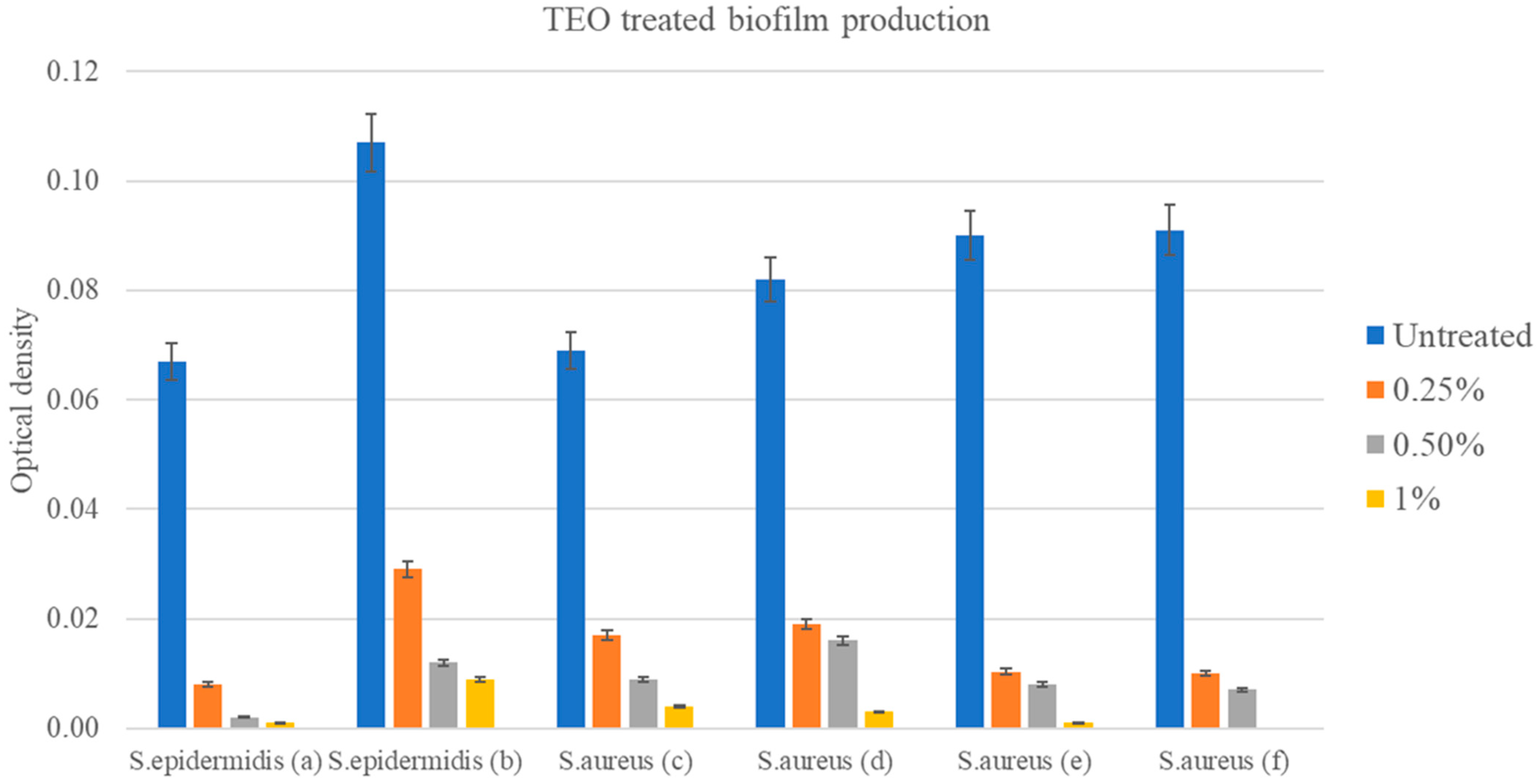
3.4.4. Production of Biofilm
3.4.5. Biofilm-Producing Genes
3.4.6. TEO Activity and Resistance Profile
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.C.; Marco, J.C.; Paape, M.J.; Gonzalo, C. Mastitis in Small Ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Ntuli, V.; Sibanda, T.; Elegbeleye, J.A.; Mugadza, D.T.; Seifu, E.; Buys, E.M. Dairy Production: Microbial Safety of Raw Milk and Processed Milk Products. In Present Knowledge in Food Safety; Elsevier: Amsterdam, The Netherlands, 2023; pp. 439–454. [Google Scholar]
- El-Deeb, W.; Fayez, M.; Alhumam, N.; Elsohaby, I.; Quadri, S.A.; Mkrtchyan, H. The Effect of Staphylococcal Mastitis Including Resistant Strains on Serum Procalcitonin, Neopterin, Acute Phase Response and Stress Biomarkers in Holstein Dairy Cows. PeerJ 2021, 9, e11511. [Google Scholar] [CrossRef]
- Darwish, S.F.; Asfour, H.A.E. Investigation of Biofilm Forming Ability in Staphylococci Causing Bovine Mastitis Using Phenotypic and Genotypic Assays. Sci. World J. 2013, 2013, 378492. [Google Scholar] [CrossRef]
- Vasudevan, P.; Nair, M.K.M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and Genotypic Characterization of Bovine Mastitis Isolates of Staphylococcus aureus for Biofilm Formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef]
- Sauer, K. The Genomics and Proteomics of Biofilm Formation. Genome Biol. 2003, 4, 219. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, F.; Namvar, A.E. Detection of Genes Involved in Biofilm Formation in Staphylococcus aureus Isolates. GMS Hyg. Infect. Control 2016, 11, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Azmi, K.; Qrei, W.; Abdeen, Z. Screening of Genes Encoding Adhesion Factors and Biofilm Production in Methicillin Resistant Strains of Staphylococcus aureus Isolated from Palestinian Patients. BMC Genom. 2019, 20, 578. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, S.; Lou, X.; Cheng, S.; Liu, X.; Zheng, W.; Zheng, Z.; Wang, H. Biofilm Formation and Prevalence of Adhesion Genes among Staphylococcus aureus Isolates from Different Food Sources. Microbiologyopen 2020, 9, e00946. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The Intercellular Adhesion (Ica) Locus Is Present in Staphylococcus aureus and Is Required for Biofilm Formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef]
- Sulaiman, A.; Abdulla, B. Detection of Biofilm Genes (IcaA and IcaD) in Staphylococcus spp. Rafidain J. Sci. 2018, 27, 60–63. [Google Scholar] [CrossRef]
- Elkhashab, T.H.T.; Adel, L.A.; Nour, M.S.; Mahran, M.; Elkaffas, M. Association of Intercellular Adhesion Gene A with Biofilm Formation in Staphylococci Isolates from Patients with Conjunctivitis. J. Lab. Physicians 2018, 10, 309–315. [Google Scholar] [CrossRef]
- Resch, A.; Rosenstein, R.; Nerz, C.; Götz, F. Differential Gene Expression Profiling of Staphylococcus aureus Cultivated under Biofilm and Planktonic Conditions. Appl. Environ. Microbiol. 2005, 71, 2663–2676. [Google Scholar] [CrossRef]
- Gerke, C.; Kraft, A.; Süßmuth, R.; Schweitzer, O.; Götz, F. Characterization of TheN-Acetylglucosaminyltransferase Activity Involved in the Biosynthesis of the Staphylococcus epidermidis Polysaccharide Intercellular Adhesin. J. Biol. Chem. 1998, 273, 18586–18593. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, U.; Ulrich, M.; Steinhuber, A.; Döring, G.; Mack, D.; Landmann, R.; Goerke, C.; Wolz, C. Biofilm Formation, IcaADBC Transcription, and Polysaccharide Intercellular Adhesin Synthesis by Staphylococci in a Device-Related Infection Model. Infect. Immun. 2005, 73, 1811–1819. [Google Scholar] [CrossRef]
- Ózsvári, L.; Ivanyos, D. The Use of Teat Disinfectants and Milking Machine Cleaning Products in Commercial Holstein-Friesian Farms. Front. Vet. Sci. 2022, 9, 956843. [Google Scholar] [CrossRef]
- Cabral, J.F.; Bánkuti, F.I.; Gurgel, A.L.C.; Ítavo, L.C.V.; Sippert, M.R.; Osorio, J.A.C.; Marchi, F.E.d.; Lourenço, J.C.S.; de Almeida, K.V.; Valloto, A.A.; et al. Iodine Concentration in Milk Evaluated by Iodized Agents during Milking. Food Sci. Technol. 2022, 42, e41322. [Google Scholar] [CrossRef]
- Trøan, G.; Dahl, L.; Margrete Meltzer, H.; Hope Abel, M.; Geir Indahl, U.; Haug, A.; Prestløkken, E. A Model to Secure a Stable Iodine Concentration in Milk. Food Nutr. Res. 2015, 59, 29829. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant Essential Oils as a Tool in the Control of Bovine Mastitis: An Update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef]
- Reddy, D.N. Essential Oils Extracted from Medicinal Plants and Their Applications. In Natural Bio-Active Compounds; Springer: Singapore, 2019; pp. 237–283. [Google Scholar]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fussieger, C.; Theodoro, H.; Silveira, S.; Pauletti, G.F.; Ely, M.R.; Lunge, V.R.; Streck, A.F. Antimicrobial Activity of Essential Oils against Staphylococcus aureus and Staphylococcus chromogenes Isolated from Bovine Mastitis. Braz. J. Microbiol. 2023, 54, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Tarabili, R.M.; Bahnass, M.M.; Alshahrani, M.A.; Saif, A.; Alwutayd, K.M.; Safhi, F.A.; Mansour, A.T.; Alblwi, N.A.N.; Ghoneim, M.M.; et al. Partnering Essential Oils with Antibiotics: Proven Therapies against Bovine Staphylococcus aureus Mastitis. Front. Cell Infect. Microbiol. 2023, 13, 1265027. [Google Scholar] [CrossRef]
- Galgano, M.; Mrenoshki, D.; Pellegrini, F.; Capozzi, L.; Cordisco, M.; Del Sambro, L.; Trotta, A.; Camero, M.; Tempesta, M.; Buonavoglia, D.; et al. Antibacterial and Biofilm Production Inhibition Activity of Thymus Vulgaris L. Essential Oil against Salmonella Spp. Isolates from Reptiles. Pathogens 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Budri, P.E.; Silva, N.C.C.; Bonsaglia, E.C.R.; Fernandes, A.; Araújo, J.P.; Doyama, J.T.; Gonçalves, J.L.; Santos, M.V.; Fitzgerald-Hughes, D.; Rall, V.L.M. Effect of Essential Oils of Syzygium aromaticum and Cinnamomum zeylanicum and Their Major Components on Biofilm Production in Staphylococcus aureus Strains Isolated from Milk of Cows with Mastitis. J. Dairy. Sci. 2015, 98, 5899–5904. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics 2024, 13, 625. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Suitability of Goat Milk Protein as a Source of Protein in Infant Formulae and in Follow-on Formulae. EFSA J. 2012, 10, 2603. [Google Scholar] [CrossRef][Green Version]
- Růžička, F.; Holá, V.; Votava, M.; Tejkalová, R.; Horvát, R.; Heroldová, M.; Woznicová, V. Biofilm Detection and the Clinical Significance Of Staphylococcus epidermidis Isolates. Folia Microbiol. 2004, 49, 596–600. [Google Scholar] [CrossRef]
- Liberto, M.C.; Matera, G.; Quirino, A.; Lamberti, A.G.; Capicotto, R.; Puccio, R.; Barreca, G.S.; Focà, E.; Cascio, A.; Focà, A. Phenotypic and Genotypic Evaluation of Slime Production by Conventional and Molecular Microbiological Techniques. Microbiol. Res. 2009, 164, 522–528. [Google Scholar] [CrossRef]
- Fratini, F.; Casella, S.; Leonardi, M.; Pisseri, F.; Ebani, V.V.; Pistelli, L.; Pistelli, L. Antibacterial Activity of Essential Oils, Their Blends and Mixtures of Their Main Constituents against Some Strains Supporting Livestock Mastitis. Fitoterapia 2014, 96, 1–7. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing: Carol Stream, IL, USA, 2005; Volume 16. [Google Scholar]
- Koo, I.; Kim, S.; Zhang, X. Comparative Analysis of Mass Spectral Matching-Based Compound Identification in Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2013, 1298, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Molineri, A.I.; Camussone, C.; Zbrun, M.V.; Suárez Archilla, G.; Cristiani, M.; Neder, V.; Calvinho, L.; Signorini, M. Antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis: Systematic review and meta-analysis. Prev. Vet. Med. 2021, 188, 105261. [Google Scholar] [CrossRef] [PubMed]
- Mimica, M.J.; Berezin, E.N.; Carvalho, R.L.B.; Mimica, I.M.; Mimica, L.M.J.; Sáfadi, M.A.P.; Schneider, E.; Caiaffa-Filho, H.H. Detection of Methicillin Resistance in Staphylococcus aureus Isolated from Pediatric Patients: Is the Cefoxitin Disk Diffusion Test Accurate Enough? Braz. J. Infect. Dis. 2007, 11, 415–417. [Google Scholar] [CrossRef]
- Durrafourd, C.; Lapraz, J.C.; Reynier, J. Traité de Phytothérapie Clinique: Endobiogénie et Médecine; Elsevier Masson: Issy-les-Moulineaux, France, 2002. [Google Scholar]
- Sfeir, J.; Lefrançois, C.; Baudoux, D.; Derbré, S.; Licznar, P. In Vitro Antibacterial Activity of Essential Oils against Streptococcus pyogenes. Evid.-Based Complement. Altern. Med. 2013, 2013, 269161. [Google Scholar] [CrossRef] [PubMed]
- Chamdit, S.; Siripermpool, P. Antimicrobial Effect of Clove and Lemongrass Oils against Planktonic Cells and Biofilms of Staphylococcus aureus. Mahidol Univ. J. Pharm. Sci. 2012, 39, 28–36. [Google Scholar]
- Aiemsaard, J.; Borlace, G.N.; Thongkham, E.; Jarassaeng, C. Antibacterial Efficacy of Essential Oil Spray Formulation for Post-Milking Disinfection in Dairy Cows. Vet. World 2023, 16, 1552–1561. [Google Scholar] [CrossRef]
- Mathur, T.; Singhal, S.; Khan, S.; Upadhyay, D.J.; Fatma, T.; Rattan, A. Detection of Biofilm Formation among the Clinical Isolates of Staphylococci: An Evaluation of Three Different Screening Methods. Indian. J. Med. Microbiol. 2006, 24, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. Presence of IcaA and IcaD Genes and Slime Production in a Collection of Staphylococcal Strains from Catheter-Associated Infections. J. Clin. Microbiol. 2001, 39, 2151–2156. [Google Scholar] [CrossRef]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and Antiviral Effects of Thymus Vulgaris Essential Oil on Feline Coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Cerqueira, C.; Ovesen, L.; Rasmussen, L.B.; Perrild, H.; Andersen, S.; Pedersen, I.B.; Carlé, A. Iodine Intake as a Determinant of Thyroid Disorders in Populations. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Pizauro, L.J.L.; de Almeida, C.C.; Silva, S.R.; MacInnes, J.I.; Kropinski, A.M.; Zafalon, L.F.; de Avila, F.A.; de Mello Varani, A. Genomic Comparisons and Phylogenetic Analysis of Mastitis-Related Staphylococci with a Focus on Adhesion, Biofilm, and Related Regulatory Genes. Sci. Rep. 2021, 11, 17392. [Google Scholar] [CrossRef]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial Activity of Thyme (Tymus vulgaris) and Oregano (Origanum vulgare) Essential Oils against Some Food-Borne Microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A Review on Recent Research Results (2008–2010) on Essential Oils as Antimicrobials and Antifungals. A Review. Flavour. Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef]
- Aouadhi, C.; Jouini, A.; Maaroufi, K.; Maaroufi, A. Antibacterial Effect of Eight Essential Oils against Bacteria Implicated in Bovine Mastitis and Characterization of Primary Action Mode of Thymus Capitatus Essential Oil. Antibiotics 2024, 13, 237. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol Disrupts the Membrane Integrity of Salmonella Ser. Typhimurium in Vitro and Recovers Infected Macrophages from Oxidative Stress in an Ex Vivo Model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural Agents against Bovine Mastitis Pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Liedtke, J.; Plattes, S.; Lipski, A. Bacterial Community Composition of Biofilms in Milking Machines of Two Dairy Farms Assessed by a Combination of Culture-Dependent and –Independent Methods. PLoS ONE 2019, 14, e0222238. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, E.Z.; Ararso, A.B.; Borana, B.M.; Kelbesa, K.A.; Tadese, N.D.; Marami, L.M.; Sarba, E.J. Isolation and Identification of Staphylococcus aureus from Milk and Milk Products, Associated Factors for Contamination, and Their Antibiogram in Holeta, Central Ethiopia. Vet. Med. Int. 2022, 2022, 6544705. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy. Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Götz, F. Staphylococcus and Biofilms. Mol. Microbiol. 2002, 43, 1367–1378. [Google Scholar] [CrossRef]
- Dhanawade, N.B.; Kalorey, D.R.; Srinivasan, R.; Barbuddhe, S.B.; Kurkure, N.V. Detection of Intercellular Adhesion Genes and Biofilm Production in Staphylococcus aureus Isolated from Bovine Subclinical Mastitis. Vet. Res. Commun. 2010, 34, 81–89. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide Intercellular Adhesin in Biofilm: Structural and Regulatory Aspects. Front. Cell Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Omidi, M.; Firoozeh, F.; Saffari, M.; Sedaghat, H.; Zibaei, M.; Khaledi, A. Ability of Biofilm Production and Molecular Analysis of Spa and Ica Genes among Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. BMC Res. Notes 2020, 13, 19. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Borges, A.; Lopez-Romero, J.C.; Oliveira, D.; Giaouris, E.; Simões, M. Prevention, Removal and Inactivation of Escherichia coli and Staphylococcus aureus Biofilms Using Selected Monoterpenes of Essential Oils. J. Appl. Microbiol. 2017, 123, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Chaabouni, Y.; Fedhila, K.; Bakhrouf, A.; Mahdouani, K.; Chaieb, K. Antibacterial and Efflux Pump Inhibitors of Thymol and Carvacrol against Food-Borne Pathogens. Microb. Pathog. 2016, 99, 95–100. [Google Scholar] [CrossRef]
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felińczak, A.; Gołębiewska, J.; Górniak, A.; Junka, A. The Antimicrobial and Antibiofilm In Vitro Activity of Liquid and Vapour Phases of Selected Essential Oils against Staphylococcus aureus. Pathogens 2021, 10, 1207. [Google Scholar] [CrossRef]
- Naccari, V.; Maria Orlandella, B.; Naccari, C.; Ubertini, B. Effectiveness of Thymus Vulgaris Essential Oil on Ovine Mammary Pustular Dermatitis. Sci. Med. Biol. 2019, 107, 3–4. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, S.; Khurana, R. Essential Oils and Mastitis in Dairy Animals: A Review. Haryana Vet. 2020, 59, 1–9. [Google Scholar]
- Sousa, M.; Machado, I.; Simões, L.C.; Simões, M. Biocides as Drivers of Antibiotic Resistance: A Critical Review of Environmental Implications and Public Health Risks. Environ. Sci. Ecotechnol. 2025, 25, 100557. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of Plant Extracts and Essential Oils in the Control of Bovine Mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Talbaoui, A.; Khouchlaa, A.; Charfi, S.; Abrini, J.; Dakka, N. Résistance Aux Antibiotiques et Mécanismes d’action Des Huiles Essentielles Contre Les Bactéries. Phytothérapie 2017, 16 (Suppl. S1), 173–183. [Google Scholar] [CrossRef]
| (a) | ||||||
|---|---|---|---|---|---|---|
| Antibiotics | Staphylococcus Coagulase-Negative | |||||
| S. xylosus | S. chromogenes (a) | S. chromogenes (b) | S. equorum | S. epidermidis (a) | S. epidermidis (b) | |
| AML | R | S | S | S | R | R |
| AMP | I | I | I | I | R | R |
| P | I | I | R | I | R | R |
| CL | I | S | I | S | I | I |
| FOX | I | S | S | S | I | I |
| CRO | S | S | S | S | S | S |
| CD | R | S | S | S | S | R |
| TE | R | S | S | S | S | R |
| OX | I | S | S | S | R | R |
| CN | S | S | S | S | I | I |
| E | R | S | I | S | S | R |
| RD | S | S | S | S | I | R |
| ENR | S | S | S | S | S | S |
| (b) | ||||||
| Antibiotics | Staphylococcus Coagulase-Positive | |||||
| S. aureus | S. aureus | S. aureus | S. aureus | S. aureus | S. aureus | |
| AML | R | R | R | I | I | R |
| AMP | R | R | R | I | S | R |
| P | R | R | R | I | S | R |
| CL | I | S | R | S | S | R |
| FOX | I | S | R | S | I | R |
| CRO | S | S | I | S | S | I |
| CD | S | S | I | S | S | S |
| TE | S | S | R | S | S | R |
| OX | S | S | R | S | S | R |
| CN | S | S | S | S | S | S |
| E | R | R | R | S | S | R |
| RD | S | S | I | S | S | R |
| ENR | S | S | S | S | S | S |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Staphylococcus Coagulase-Negative | ||||||
| %TEO (v/v) | S. xylosus | S. chromogenes (a) | S. chromogenes (b) | S. equorum | S. epidermidis (a) | S. epidermidis (b) |
| 1% | +++ | +++ | +++ | +++ | +++ | +++ |
| 0.5% | +++ | ++ | +++ | +++ | +++ | ++ |
| 0.25% | + | ++ | ++ | ++ | ++ | + |
| (b) | ||||||
| Staphylococcus Coagulase-Positive | ||||||
| %TEO (v/v) | S. aureus (a) | S. aureus (b) | S. aureus (c) | S. aureus (d) | S. aureus (e) | S. aureus (f) |
| 1% | +++ | +++ | +++ | +++ | +++ | +++ |
| 0.5% | +++ | +++ | ++ | ++ | +++ | ++ |
| 0.25% | ++ | ++ | ++ | + | ++ | - |
| Coagulase-Negative Strains | MIC | MBC |
|---|---|---|
| S. xylosus | 0.25% | 0.25% |
| S. chromogenes (a) | 0.25% | 0.25% |
| S. chromogenes (b) | 0.25% | 0.25% |
| S. equorum | 0.25% | 0.25% |
| S. epidermidis (a) | 0.25% | 0.25% |
| S. epidermidis (b) | 0.25% | 0.50% |
| Coagulase-Positive Strains | MIC | MBC |
| S. aureus (a) | 0.25% | 0.25% |
| S. aureus (b) | 0.25% | 0.25% |
| S. aureus (c) | 0.25% | 0.25% |
| S. aureus (d) | 0.25% | 0.25% |
| S. aureus (e) | 0.25% | 0.50% |
| S. aureus (f) | 0.50% | 0.50% |
| Coagulase-Negative Strains | ODs * | OD (ODs-ODc) ** |
|---|---|---|
| S. xylosus | 0.087 | 0.028 |
| S. chromogenes (a) | 0.104 | 0.045 |
| S. chromogenes (b) | 0.113 | 0.054 |
| S. equorum | 0.115 | 0.053 |
| S. epidermidis (a) | 0.130 | 0.067 |
| S. epidermidis (b) | 0.166 | 0.107 |
| Coagulase-Positive Strains | ODs * | OD (ODs-ODCc *) ** |
| S. aureus (a) | 0.093 | 0.030 |
| S. aureus (b) | 0.095 | 0.032 |
| S. aureus (c) | 0.132 | 0.069 |
| S. aureus (d) | 0.145 | 0.082 |
| S. aureus (e) | 0.153 | 0.090 |
| S. aureus (f) | 0.154 | 0.091 |
| Coagulase-Negative Strains | icaA | icaB |
|---|---|---|
| S. xylosus | - | - |
| S. chromogenes (a) | - | - |
| S. chromogenes (b) | - | - |
| S. equorum | - | - |
| S. epidermidis (a) | - | - |
| S. epidermidis (b) | - | - |
| Coagulase-Positive Strains | icaA | icaB |
| S. aureus (a) | + | + |
| S. aureus (b) | + | + |
| S. aureus (c) | - | + |
| S. aureus (d) | + | + |
| S. aureus (e) | + | + |
| S. aureus (f) | + | + |
| Control Strains | icaA | icaB |
| ATCC 25923 | + | + |
| ATCC 11632 | + | + |
| ATCC 12228 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, M.; Pellegrini, F.; Mrenoshki, D.; Addante, L.; Sposato, A.; Del Sambro, L.; Capozzi, L.; Catalano, E.; Solito, M.; D’Amico, F.; et al. Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants. Pathogens 2025, 14, 412. https://doi.org/10.3390/pathogens14050412
Galgano M, Pellegrini F, Mrenoshki D, Addante L, Sposato A, Del Sambro L, Capozzi L, Catalano E, Solito M, D’Amico F, et al. Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants. Pathogens. 2025; 14(5):412. https://doi.org/10.3390/pathogens14050412
Chicago/Turabian StyleGalgano, Michela, Francesco Pellegrini, Daniela Mrenoshki, Luciana Addante, Alessio Sposato, Laura Del Sambro, Loredana Capozzi, Elisabetta Catalano, Marianna Solito, Francesco D’Amico, and et al. 2025. "Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants" Pathogens 14, no. 5: 412. https://doi.org/10.3390/pathogens14050412
APA StyleGalgano, M., Pellegrini, F., Mrenoshki, D., Addante, L., Sposato, A., Del Sambro, L., Capozzi, L., Catalano, E., Solito, M., D’Amico, F., Messina, D., Parisi, A., Pratelli, A., & Capozza, P. (2025). Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants. Pathogens, 14(5), 412. https://doi.org/10.3390/pathogens14050412








