Seasonal Dynamics and Legionellosis-Associated Hospitalization in Spain: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Source and Study Population
2.3. Hospitalization and Demographic Data
2.4. Statistical Analysis
2.5. Ethical Statement
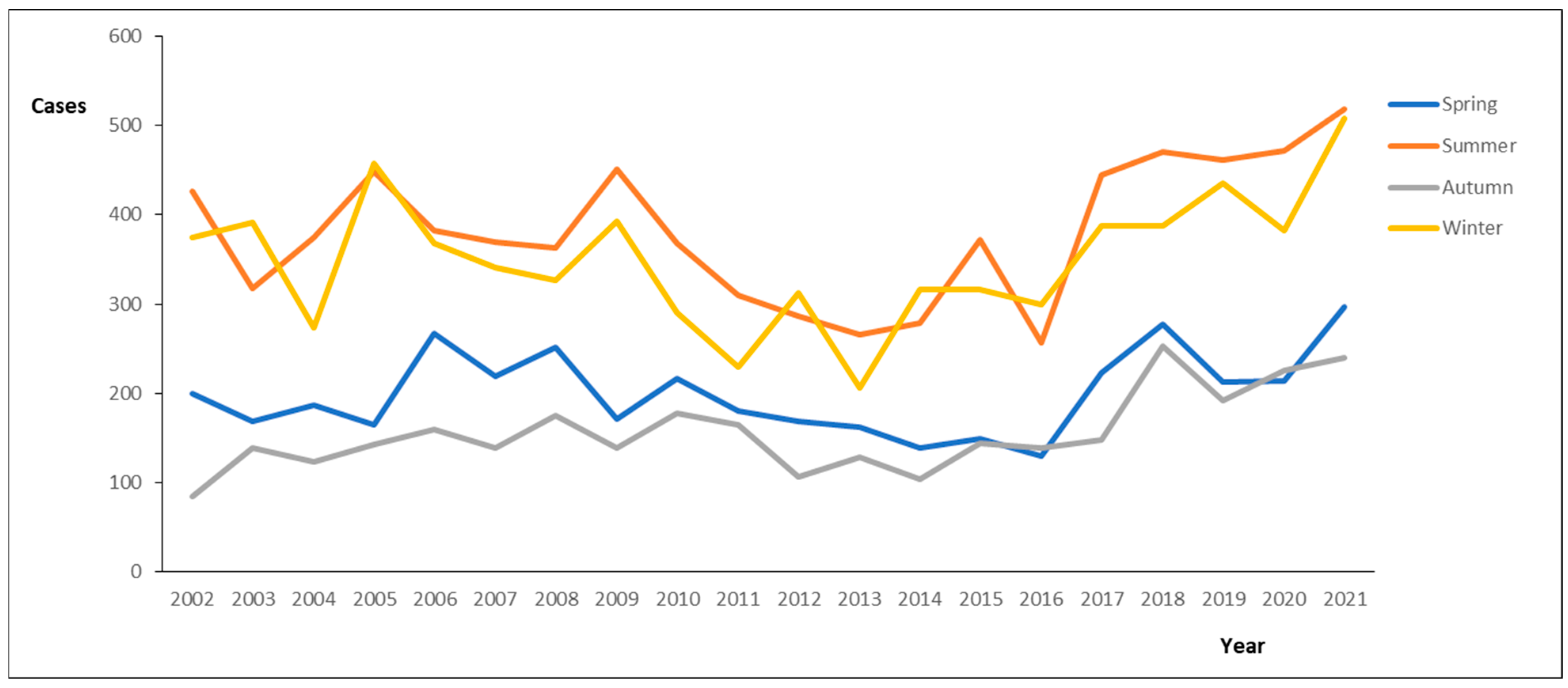
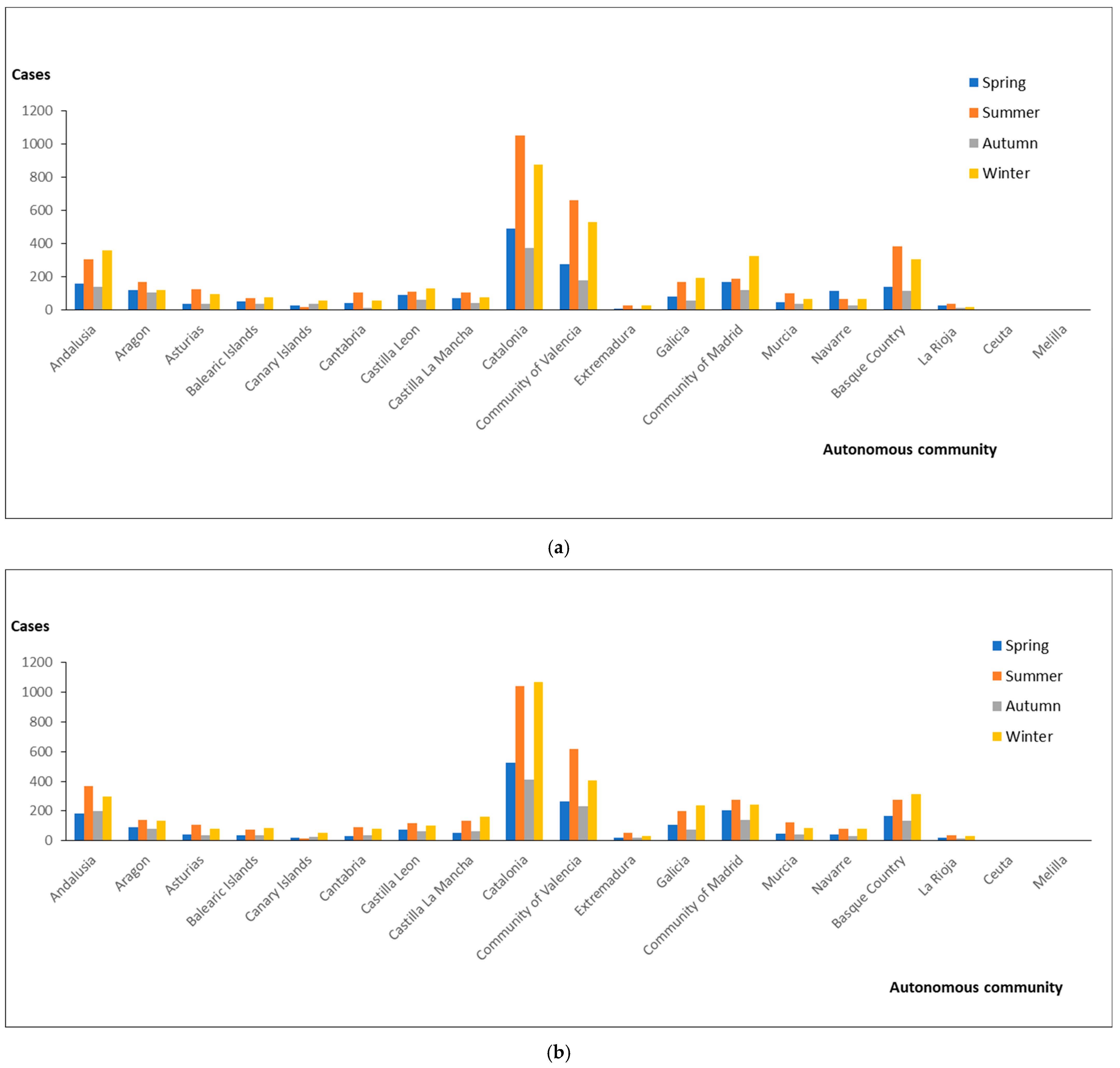
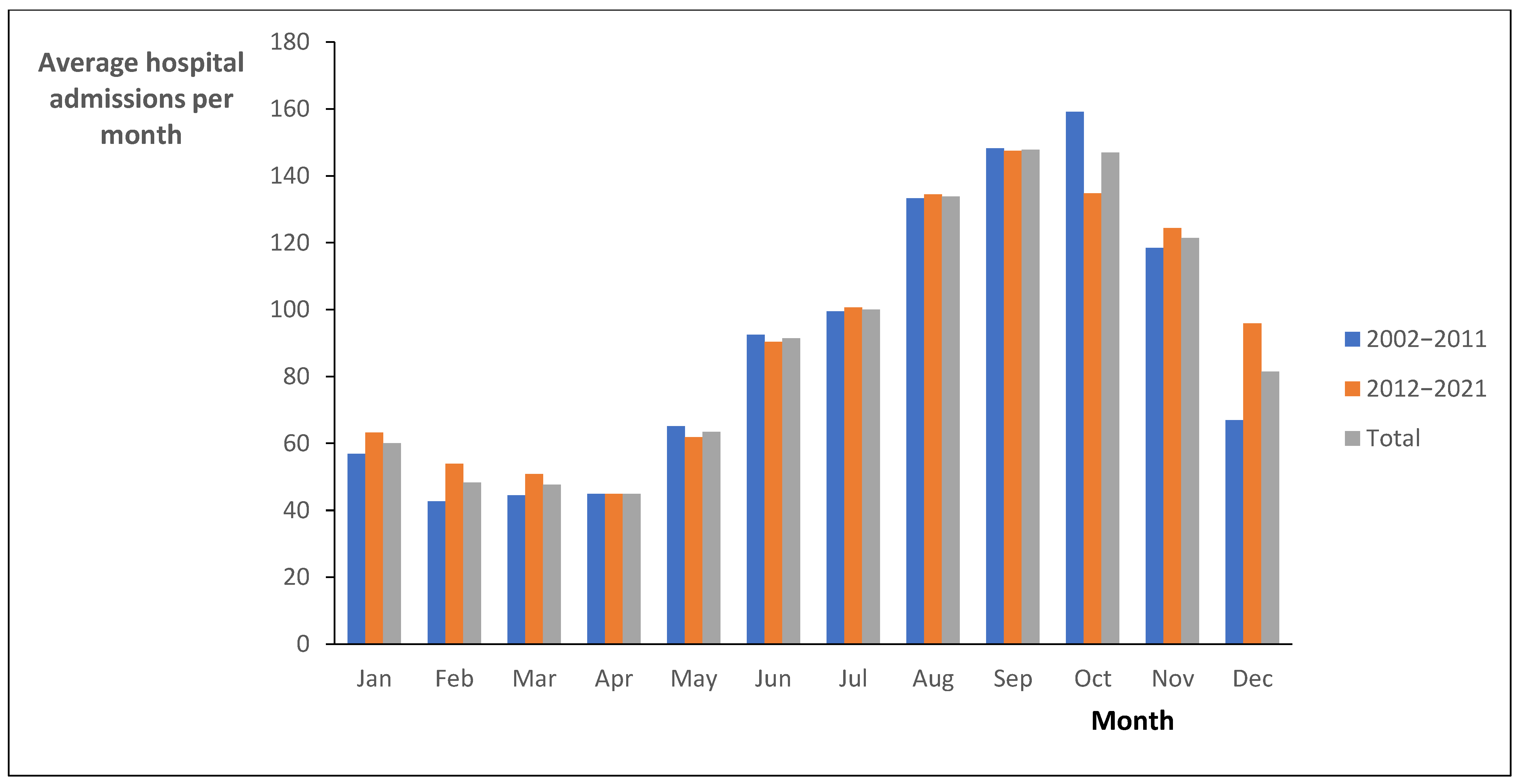
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFR | Case fatality rate |
| HR | Hospitalization rate |
| ICD | International Classification of Diseases |
| ICD-9-CM | International Classification of Diseases 9th Revision |
| ICD-10-ES | International Classification of Diseases 10th Revision |
| MBDS | Minimum Basic Data Set |
References
- Fukushima, S.; Hagiya, H.; Otsuka, Y.; Koyama, T.; Otsuka, F. Trends in the incidence and mortality of legionellosis in Japan: A nationwide observational study, 1999–2017. Sci. Rep. 2021, 11, 7246. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, S.; Ohbe, H.; Kanda, N.; Matsui, H.; Yasunaga, H. Epidemiological analysis of Legionella pneumonia in Japan: A national inpatient database study. J. Epidemiol. 2024, 34, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gorzynski, J.; Wee, B.; Llano, M.; Alves, J.; Cameron, R.; McMenamin, J.; Smith, A.; Lindsay, D.; Fitzgerald, J.R. Epidemiological analysis of Legionnaires’ disease in Scotland: A genomic study. Lancet Microbe 2022, 3, e835–e845. [Google Scholar] [CrossRef] [PubMed]
- Benin, A.L.; Benson, R.F.; Besser, R.E. Trends in legionnaires disease, 1980–1998: Declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 2002, 35, 1039–1046. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. 2023. Available online: http://atlas.ecdc.europa.eu/public/index.aspx (accessed on 21 March 2025).
- Fischer, F.B.; Saucy, A.; Vienneau, D.; Hattendorf, J.; Fanderl, J.; de Hoogh, K.; Mäusezahl, D. Impacts of weather and air pollution on Legionnaires’ disease in Switzerland: A national case-crossover study. Environ. Res. 2023, 233, 116327. [Google Scholar] [CrossRef]
- Waller, C.; Freeman, K.; Labib, S.; Baird, R. Epidemiological and clinical characteristics of legionellosis in Northern Australia, 2010–2021. Commun. Dis. Intell. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Barskey, A.E.; Derado, G.; Edens, C. Rising incidence of Legionnaires’ disease and associated epidemiologic patterns, United States, 1992–2018. Emerg. Infect. Dis. 2022, 28, 527–538. [Google Scholar] [CrossRef]
- Fisman, D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Gea-Izquierdo, E.; Gil-Prieto, R.; Hernández-Barrera, V.; Rodríguez-Caravaca, G.; Gil-de-Miguel, Á. Legionellosis-associated hospitalization in Spain from 2002 to 2021. Microorganisms 2023, 11, 1693. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Legionnaires’ Disease in Europe, 2014; ECDC: Stockholm, Sweden, 2016. [Google Scholar]
- Report, M.W. Legionellosis USA 2000–2009. MMWR. Morb. Mortal. Wkly. Rep. 2011, 60, 1073–1077. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Legionnaires’ Disease. Annual Epidemiological Report for 2019. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2019 (accessed on 21 March 2025).
- NHS. Health Technical Memorandum (HTM) 04-01: Safe Water in Healthcare Premises. 2016. Available online: https://www.england.nhs.uk/publication/safe-water-in-healthcare-premises-htm-04-01 (accessed on 21 March 2025).
- Li, J.S.; O’Brien, E.D.; Guest, C. A review of national legionellosis surveillance in Australia, 1991 to 2000. Commun. Dis. Intell. Q. Rep. 2002, 26, 461–468. [Google Scholar] [PubMed]
- Karagiannis, I.; Brandsema, P.; Van Der Sande, M. Warm, wet weather associated with increased Legionnaires’ disease incidence in The Netherlands. Epidemiol. Infect. 2009, 137, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, U.; Altmann, D.; Brodhun, B. Differential seasonality of Legionnaires’ disease by exposure category. Int. J. Environ. Res. Public Health 2020, 17, 3049. [Google Scholar] [CrossRef]
- Wade, T.J.; Herbert, C. Weather conditions and legionellosis: A nationwide case-crossover study among Medicare recipients. Epidemiol. Infect. 2024, 152, e125. [Google Scholar] [CrossRef]
- Montagna, M.T.; Brigida, S.; Fasano, F.; Leone, C.M.; D’Ambrosio, M.; Spagnuolo, V.; Lopuzzo, M.; Apollonio, F.; Triggiano, F.; Caringella, M.E.; et al. The role of air temperature in Legionella water contamination and legionellosis incidence rates in southern Italy (2018–2023). Ann. Ig. 2023, 35, 631–640. [Google Scholar]
- NNDSS Annual Report Working Group. Australia’s Notifiable Disease Status, 2014: Annual Report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell. Q. Rep. 2016, 40, E48–E145. [Google Scholar]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, A.; Abubakar, I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- Simmering, J.E.; Polgreen, L.A.; Hornick, D.B.; Sewell, D.K.; Polgreen, P.M. Weather-dependent risk for Legionnaires’ Disease, United States. Emerg. Infect. Dis. 2017, 23, 1843–1851. [Google Scholar] [CrossRef]
- Cunha, B.A.; Connolly, J.; Abruzzo, E. Increase in pre-seasonal community-acquired Legionnaire’s disease due to increased precipitation. Clin. Microbiol. Infect. 2015, 21, e45–e46. [Google Scholar] [CrossRef]
- Gleason, J.A.; Kratz, N.R.; Greeley, R.D.; Fagliano, J.A. Under the weather: Legionellosis and meteorological factors. Ecohealth 2016, 13, 293–302. [Google Scholar] [CrossRef]
- Mitsui, M.; Ito, A.; Ishida, T.; Tachibana, H.; Nakanishi, Y.; Yamazaki, A.; Washio, Y. Increased risk of Legionella pneumonia as community-acquired pneumonia after heavy rainfall in 2018 in west Japan. J. Infect. Chemother. 2021, 27, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.D.; Shaman, J. The effect of seasonal and extreme floods on hospitalizations for Legionnaires’ disease in the United States, 2000–2011. BMC Infect. Dis. 2022, 22, 550. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de España. Real Decreto 487/2022, de 21 de Junio, Por el Que se Establecen los Requisitos Sanitarios Para la Prevención y el Control de la Legionelosis. 2022. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2022-10297 (accessed on 21 March 2025).
| a | ||||
|---|---|---|---|---|
| Autonomous Community | HR | |||
| 2002–2011 | 2012–2021 | 2002–2021 | p-Value * | |
| Andalusia | 1.21 (1.13–1.29) | 1.24 (1.16–1.32) | 1.23 (1.18–1.28) | 0.000 |
| Aragon * | 3.96 (3.62–4.3) | 3.39 (3.08–3.7) | 3.67 (3.44–3.9) | 0.000 |
| Asturias | 2.81 (2.49–3.13) | 2.55 (2.24–2.86) | 2.68 (2.46–2.9) | 0.000 |
| Balearic Islands | 2.48 (2.17–2.79) | 2.04 (1.78–2.3) | 2.24 (2.04–2.44) | 0.000 |
| Canary Islands | 0.72 (0.6–0.84) | 0.53 (0.43–0.63) | 0.62 (0.54–0.7) | 0.000 |
| Cantabria | 3.89 (3.38–4.4) | 4.14 (3.62–4.66) | 4.02 (3.65–4.39) | 0.000 |
| Castilla Leon | 1.57 (1.41–1.73) | 1.47 (1.32–1.62) | 1.52 (1.41–1.63) | 0.000 |
| Castilla La Mancha * | 1.53 (1.36–1.7) | 2.03 (1.84–2.22) | 1.78 (1.65–1.91) | 0.000 |
| Catalonia * | 3.93 (3.78–4.08) | 4.06 (3.92–4.2) | 3.99 (3.89–4.09) | 0.000 |
| Community of Valencia * | 3.48 (3.31–3.65) | 3.06 (2.91–3.21) | 3.27 (3.16–3.38) | 0.000 |
| Extremadura * | 0.67 (0.52–0.82) | 1.14 (0.94–1.34) | 0.91 (0.78–1.04) | 0.000 |
| Galicia * | 1.84 (1.68–2) | 2.27 (2.09–2.45) | 2.06 (1.94–2.18) | 0.000 |
| Community of Madrid | 1.32 (1.23–1.41) | 1.32 (1.23–1.41) | 1.32 (1.26–1.38) | 0.000 |
| Murcia * | 1.84 (1.61–2.07) | 2.06 (1.83–2.29) | 1.96 (1.8–2.12) | 0.000 |
| Navarre * | 4.57 (4.03–5.11) | 3.57 (3.11–4.03) | 4.06 (3.71–4.41) | 0.000 |
| Basque Country | 4.4 (4.12–4.68) | 4.09 (3.82–4.36) | 4.24 (4.05–4.43) | 0.000 |
| La Rioja | 3.14 (2.51–3.77) | 3.53 (2.87–4.19) | 3.33 (2.88–3.78) | 0.000 |
| Ceuta * | 1.6 (0.7–2.5) | 0.24 (0–0.57) | 0.88 (0.42–1.34) | 0.000 |
| Melilla | 0.28 (0–0.67) | 0.48 (0.01–0.95) | 0.39 (0.08–0.7) | 0.999 |
| Total * | 2.41 (2.36–2.46) | 2.36 (2.32–2.4) | 2.38 (2.35–2.41) | 0.000 |
| b | ||||
| Autonomous Community | CFR | |||
| 2002–2011 | 2012–2021 | 2002–2021 | p-Value * | |
| Andalusia * | 8.83 (7.04–10.62) | 11.57 (9.63–13.51) | 10.25 (8.92–11.58) | 0.452 |
| Aragon | 10.37 (7.73–13.01) | 10.69 (7.83–13.55) | 10.52 (8.58–12.46) | 0.086 |
| Asturias | 7.33 (4.38–10.28) | 4.92 (2.31–7.53) | 6.21 (4.22–8.2) | 0.621 |
| Balearic Islands | 8.91 (5.36–12.46) | 7.63 (4.24–11.02) | 8.28 (5.82–10.74) | 0.837 |
| Canary Islands | 7.91 (3.42–12.4) | 7.83 (2.92–12.74) | 7.87 (4.56–11.18) | 0.821 |
| Cantabria | 5.88 (2.78–8.98) | 2.48 (0.52–4.44) | 4.1 (2.29–5.91) | 0.652 |
| Castilla Leon * | 10.43 (7.41–13.45) | 6.41 (3.88–8.94) | 8.51 (6.52–10.5) | 0.444 |
| Castilla La Mancha | 5.69 (3.06–8.32) | 7.69 (5.13–10.25) | 6.85 (5–8.7) | 0.305 |
| Catalonia * | 4.52 (3.75–5.29) | 5.98 (5.14–6.82) | 5.28 (4.71–5.85) | 0.003 |
| Community of Valencia | 6.75 (5.54–7.96) | 6.89 (5.62–8.16) | 6.82 (5.94–7.7) | 0.016 |
| Extremadura | 9.59 (2.84–16.34) | 10.57 (5.14–16) | 10.2 (5.96–14.44) | 0.280 |
| Galicia * | 6.93 (4.71–9.15) | 2.91 (1.58–4.24) | 4.72 (3.48–5.96) | 0.717 |
| Community of Madrid | 5.76 (4.14–7.38) | 6.86 (5.17–8.55) | 6.33 (5.16–7.5) | 0.914 |
| Murcia | 5.56 (2.73–8.39) | 7.21 (4.31–10.11) | 6.46 (4.42–8.5) | 0.706 |
| Navarre * | 2.54 (0.68–4.4) | 7.83 (4.36–11.3) | 4.94 (3.05–6.83) | 0.463 |
| Basque Country | 3.61 (2.42–4.8) | 4.05 (2.75–5.35) | 3.82 (2.94–4.7) | 0.350 |
| La Rioja | 5.21 (0.76–9.66) | 3.6 (0.13–7.07) | 4.35 (1.57–7.13) | 0.854 |
| Ceuta * | 8.33 (0–23.97) | 50 (0–119.3) | 14.29 (0–32.62) | - |
| Melilla | 0 (0–0) | 0 (0–0) | 0 (0–0) | - |
| Total | 6.24 (5.78–6.7) | 6.66 (6.19–7.13) | 6.45 (6.12–6.78) | 0.023 |
| Autonomous Community | 2002–2011 | 2012–2021 | 2002–2021 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |||||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | SD | SD | SD | |||||||||||||
| Andalusia | 6863.55 | 7639.86 | 6950.29 | 10,257.30 | 7884.83 | 11,387.88 | 6512.83 | 6931.17 | 7024.87 | 9717.13 | 5840.44 | 5936.25 | 6591.31 | 8773.90 | 7985.20 | 11,219.31 | 6948.69 | 8785.96 | 6337.63 | 8172.12 | 7117.04 | 9924.15 | 7189.09 | 9175.91 |
| Aragon | 7137.69 | 7365.50 | 6786.12 | 10,742.30 | 6612.23 | 7788.30 | 5995.55 | 6782.63 | 6139.52 | 5976.33 | 5730.94 | 6538.50 | 7971.55 | 11,274.77 | 6339.36 | 9561.66 | 6688.28 | 6778.19 | 6300.14 | 9054.03 | 7178.61 | 9393.96 | 6176.36 | 8345.68 |
| Asturias | 6004.58 | 7934.63 | 5132.42 | 3959.83 | 7318.60 | 9746.68 | 5337.82 | 5566.95 | 4237.12 | 1112.11 | 4760.96 | 3413.01 | 4668.11 | 1724.88 | 4840.15 | 3526.10 | 5083.51 | 5577.38 | 4958.00 | 3709.79 | 6081.71 | 7294.21 | 5111.61 | 4743.23 |
| Balearic Islands | 7403.67 | 8994.24 | 7747.10 | 8281.04 | 8933.13 | 12,741.53 | 8601.52 | 9776.59 | 5115.72 | 2536.53 | 6934.16 | 10,059.61 | 6854.80 | 7736.83 | 7409.57 | 10,628.51 | 6429.59 | 7078.54 | 7345.30 | 9185.46 | 7867.99 | 10,463.83 | 7980.86 | 10,216.24 |
| Canary Islands | 7975.93 | 9179.18 | 10,295.18 | 13,664.18 | 10,677.86 | 13,228.09 | 7508.96 | 15,121.32 | 9356.05 | 14,673.66 | 11,645.10 | 22,034.75 | 6134.60 | 5849.39 | 10,778.36 | 20,296.88 | 8651.91 | 12,074.23 | 10,970.14 | 18,082.73 | 8702.53 | 10,840.82 | 9085.28 | 17,799.24 |
| Cantabria | 10,916.24 | 20,497.84 | 6407.59 | 8676.71 | 7520.81 | 6230.67 | 5364.35 | 3648.98 | 5123.59 | 2997.51 | 6986.77 | 14,676.18 | 5738.44 | 7124.63 | 5037.85 | 2930.43 | 8349.88 | 15,618.26 | 6677.50 | 11,829.14 | 6266.55 | 6862.83 | 5174.09 | 3240.99 |
| Castilla Leon | 8972.97 | 12,154.25 | 6781.55 | 9655.27 | 10,881.70 | 15,002.35 | 6682.21 | 10,567.59 | 8259.99 | 10,468.85 | 7174.45 | 13,284.04 | 7012.31 | 9958.71 | 6060.73 | 7605.74 | 8673.85 | 11,451.10 | 6975.55 | 11,567.26 | 8842.02 | 12,695.49 | 6412.00 | 9381.45 |
| Castilla La Mancha | 7849.45 | 10,782.10 | 5895.38 | 5205.12 | 7983.51 | 10,946.75 | 6072.76 | 3658.39 | 5203.37 | 3319.64 | 6549.02 | 8954.94 | 6606.21 | 5855.10 | 6235.68 | 7968.31 | 6668.89 | 8402.23 | 6262.56 | 7538.25 | 7154.46 | 8243.41 | 6186.19 | 6939.39 |
| Catalonia | 6290.79 | 6633.25 | 5841.48 | 7349.12 | 6918.48 | 8571.28 | 6095.21 | 8219.99 | 7081.54 | 10,594.03 | 6269.66 | 8695.07 | 7523.11 | 10,847.98 | 6647.42 | 9785.86 | 6693.70 | 8879.70 | 6054.57 | 8048.12 | 7234.83 | 9827.08 | 6397.76 | 9113.22 |
| Community of Valencia | 6558.39 | 11,366.23 | 5533.92 | 7108.07 | 6389.87 | 9786.04 | 5928.45 | 8884.88 | 7668.51 | 12,720.18 | 6567.80 | 10,875.39 | 6923.09 | 9845.09 | 6039.69 | 8900.37 | 7082.56 | 12,026.39 | 6013.09 | 9062.25 | 6675.75 | 9809.89 | 5975.53 | 8886.96 |
| Extremadura | 4754.15 | 1449.05 | 4231.98 | 1561.15 | 7849.51 | 10,652.66 | 11,654.14 | 23,320.37 | 6095.52 | 4808.75 | 5180.81 | 2640.92 | 10,435.71 | 18,528.66 | 4870.77 | 1748.59 | 5706.09 | 4138.83 | 4860.43 | 2365.20 | 9522.94 | 16,058.36 | 8011.22 | 16,110.20 |
| Galicia | 5270.73 | 3250.48 | 7187.84 | 10,622.49 | 6079.52 | 3514.93 | 5746.85 | 5353.33 | 6404.91 | 8876.73 | 6207.69 | 8706.00 | 6869.04 | 10,067.71 | 5500.07 | 4749.77 | 5919.70 | 7050.50 | 6666.97 | 9650.98 | 6531.54 | 7942.12 | 5608.43 | 5019.26 |
| Community of Madrid | 7117.93 | 9690.96 | 6713.52 | 8607.71 | 7217.97 | 8450.72 | 7275.33 | 9713.59 | 8702.07 | 14,545.18 | 7235.56 | 10,446.57 | 8842.34 | 12,356.84 | 8502.46 | 13,359.02 | 7968.67 | 12,543.04 | 7027.45 | 9748.22 | 8108.55 | 10,780.20 | 7802.17 | 11,427.25 |
| Murcia | 4477.66 | 1440.11 | 6170.58 | 7890.75 | 6716.33 | 9004.18 | 4501.92 | 1523.15 | 5326.59 | 3437.08 | 5333.36 | 5227.01 | 6490.12 | 6971.41 | 5926.46 | 5411.40 | 4942.34 | 2741.63 | 5714.24 | 6572.13 | 6595.14 | 7930.69 | 5301.50 | 4226.73 |
| Navarre | 4451.36 | 2593.88 | 5824.42 | 9186.62 | 4226.43 | 1091.62 | 4748.76 | 2917.44 | 6964.02 | 10,378.17 | 7314.94 | 14,836.94 | 8214.14 | 14,330.44 | 5632.46 | 7169.13 | 5110.16 | 5825.35 | 6633.01 | 12,554.78 | 6449.09 | 10,837.60 | 5230.78 | 5649.34 |
| Basque Country | 6311.50 | 7963.24 | 6155.51 | 7724.76 | 6315.65 | 8596.43 | 5719.06 | 5658.23 | 6046.02 | 10,315.16 | 5814.94 | 8862.04 | 5828.48 | 7194.05 | 5909.36 | 7555.49 | 6166.62 | 9307.00 | 6010.51 | 8223.43 | 6048.37 | 7845.94 | 5816.04 | 6687.97 |
| La Rioja | 4582.55 | 1419.94 | 4401.33 | 1193.68 | 6457.55 | 4993.25 | 5784.14 | 4809.92 | 4477.97 | 1918.80 | 5221.72 | 5116.69 | 8740.28 | 17,198.15 | 4527.23 | 989.08 | 4532.44 | 1660.04 | 4822.32 | 3757.54 | 7856.65 | 13,707.13 | 5010.66 | 3097.57 |
| Ceuta | 3959.35 | 587.26 | 6349.93 | 1156.85 | 5721.18 | 1965.59 | 6578.31 | 4828.28 | 5054.09 | 5497.51 | 1284.32 | 3959.35 | 587.26 | 5917.98 | 1108.55 | 5721.18 | 1965.59 | 6218.04 | 3824.75 | |||||
| Melilla | 13,255.49 | 4174.19 | 12,502.50 | 4874.01 | 3919.85 | 4524.88 | 748.42 | 5490.88 | 1791.97 | 9064.75 | 5926.60 | 3919.85 | 4407.98 | 566.62 | 7243.78 | 3798.88 | ||||||||
| Total | 6620.07 | 8782.21 | 6126.22 | 8089.84 | 7176.14 | 9525.28 | 6221.14 | 8162.09 | 6945.08 | 10,430.73 | 6303.21 | 9339.47 | 7127.43 | 10,049.91 | 6559.21 | 9415.94 | 6780.38 | 9630.82 | 6214.89 | 8738.13 | 7149.92 | 9809.56 | 6392.76 | 8821.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gea-Izquierdo, E.; Ruiz-Urbaez, R.; Hernández-Barrera, V.; Gil-de-Miguel, Á. Seasonal Dynamics and Legionellosis-Associated Hospitalization in Spain: A Retrospective Study. Pathogens 2025, 14, 411. https://doi.org/10.3390/pathogens14050411
Gea-Izquierdo E, Ruiz-Urbaez R, Hernández-Barrera V, Gil-de-Miguel Á. Seasonal Dynamics and Legionellosis-Associated Hospitalization in Spain: A Retrospective Study. Pathogens. 2025; 14(5):411. https://doi.org/10.3390/pathogens14050411
Chicago/Turabian StyleGea-Izquierdo, Enrique, Rossana Ruiz-Urbaez, Valentín Hernández-Barrera, and Ángel Gil-de-Miguel. 2025. "Seasonal Dynamics and Legionellosis-Associated Hospitalization in Spain: A Retrospective Study" Pathogens 14, no. 5: 411. https://doi.org/10.3390/pathogens14050411
APA StyleGea-Izquierdo, E., Ruiz-Urbaez, R., Hernández-Barrera, V., & Gil-de-Miguel, Á. (2025). Seasonal Dynamics and Legionellosis-Associated Hospitalization in Spain: A Retrospective Study. Pathogens, 14(5), 411. https://doi.org/10.3390/pathogens14050411






