Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Methods
2.2.1. Enzyme-Linked Immunosorbent Spot (ELISpot) Assay for IFN-g T Cell Response Detection
2.2.2. SARS-CoV-2 IgG Antibodies
2.3. Statistical Analysis
3. Results
3.1. Participants’ Demographic Characteristics
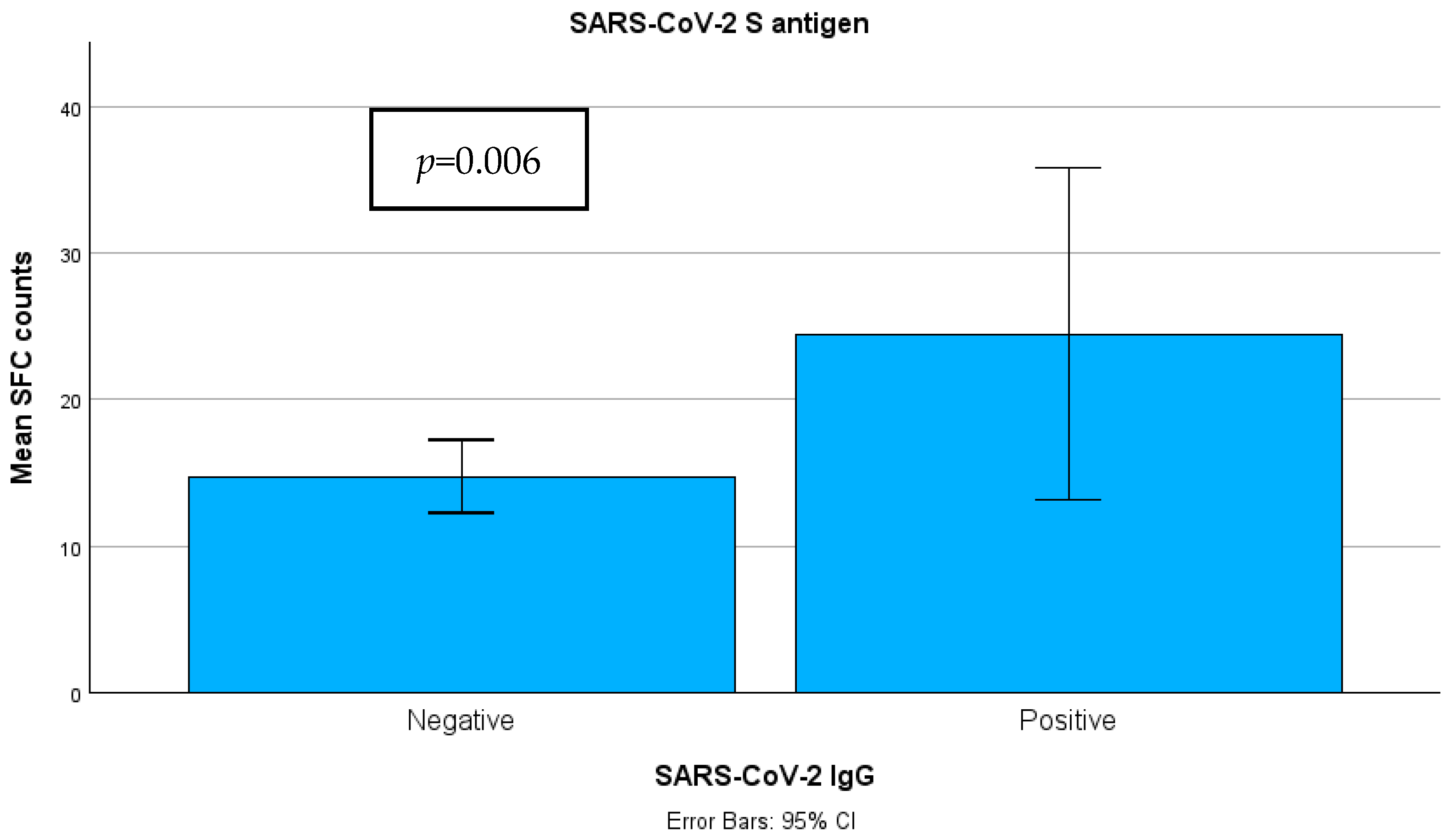
3.2. T Cell Positivity Rate Between Groups
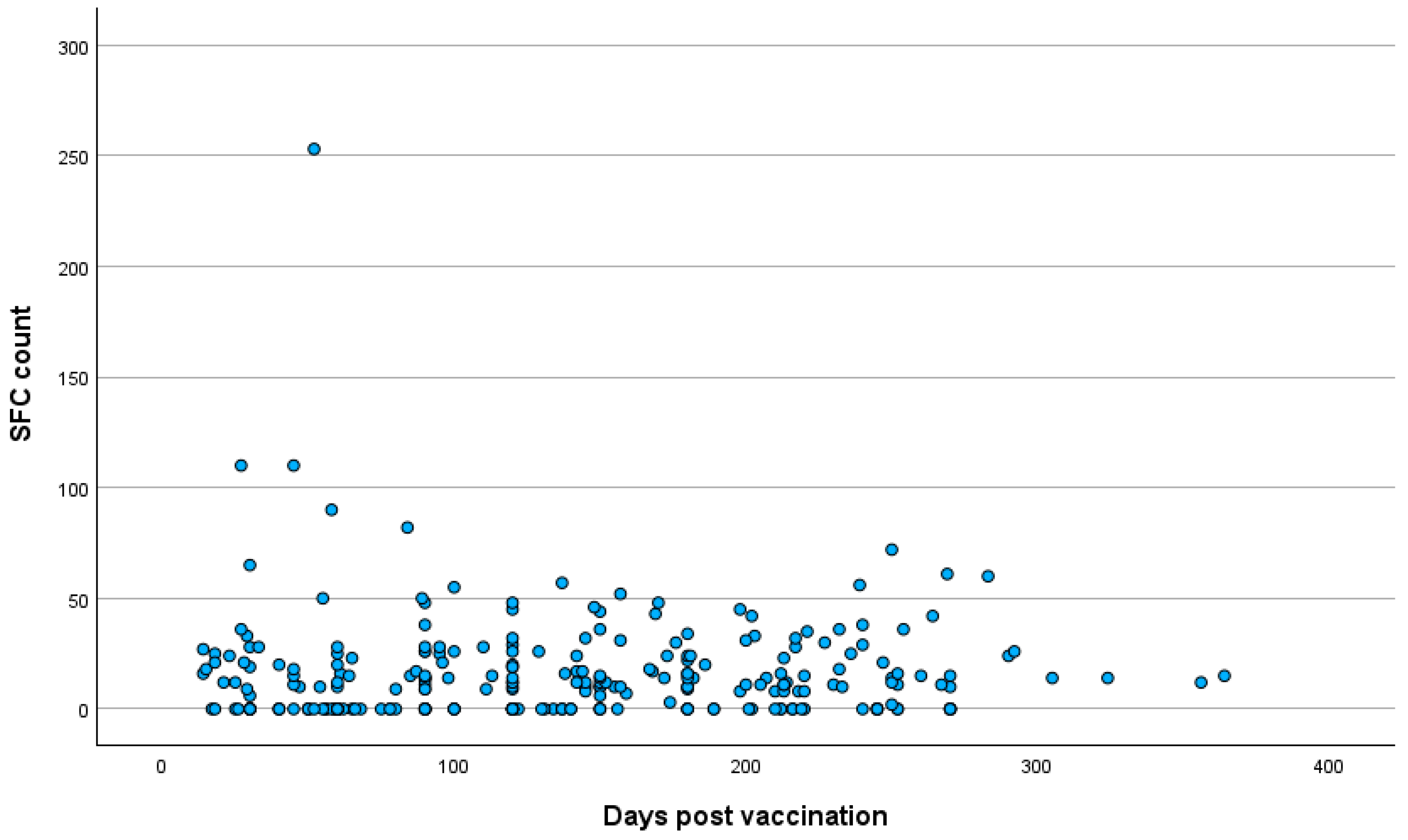
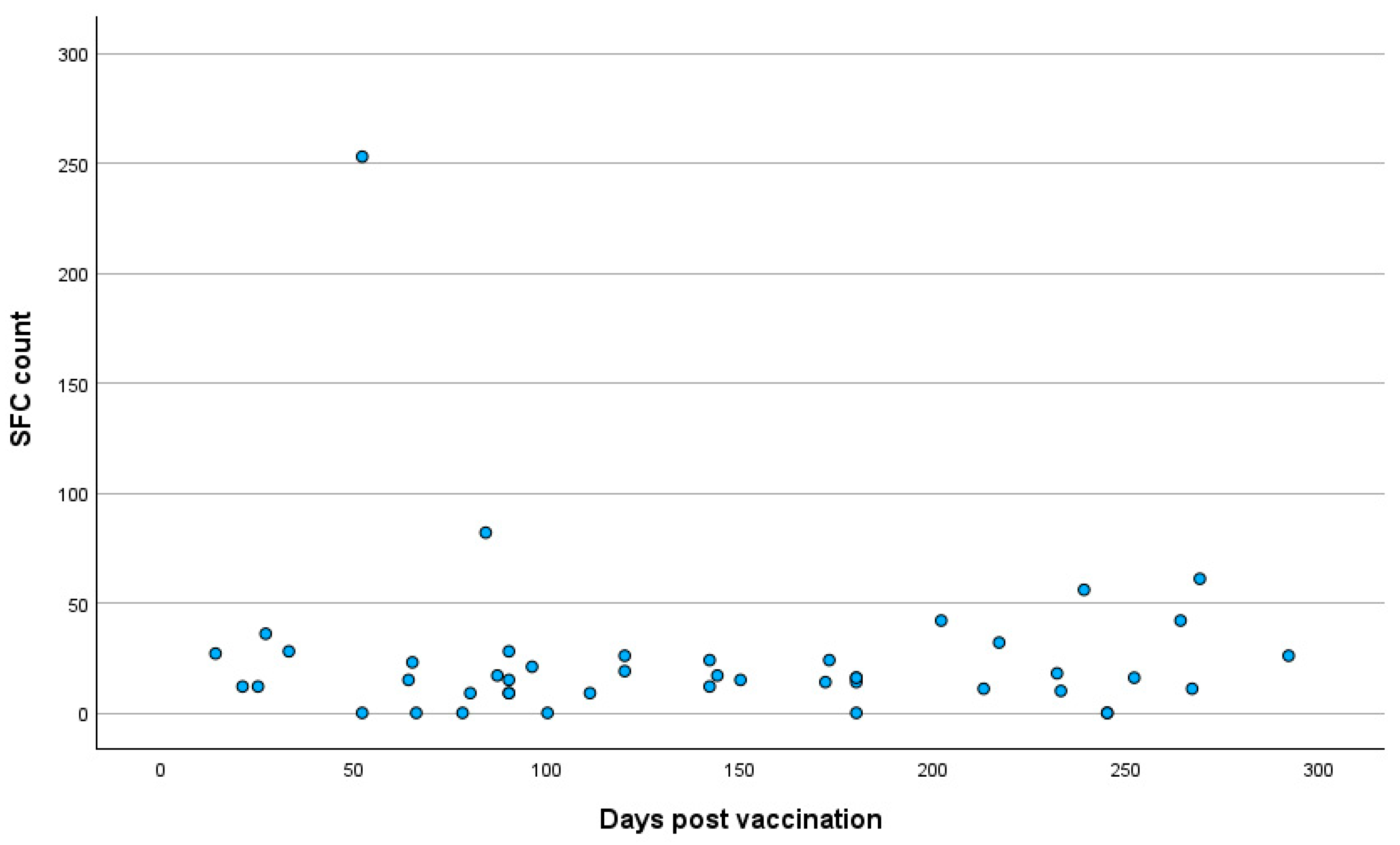
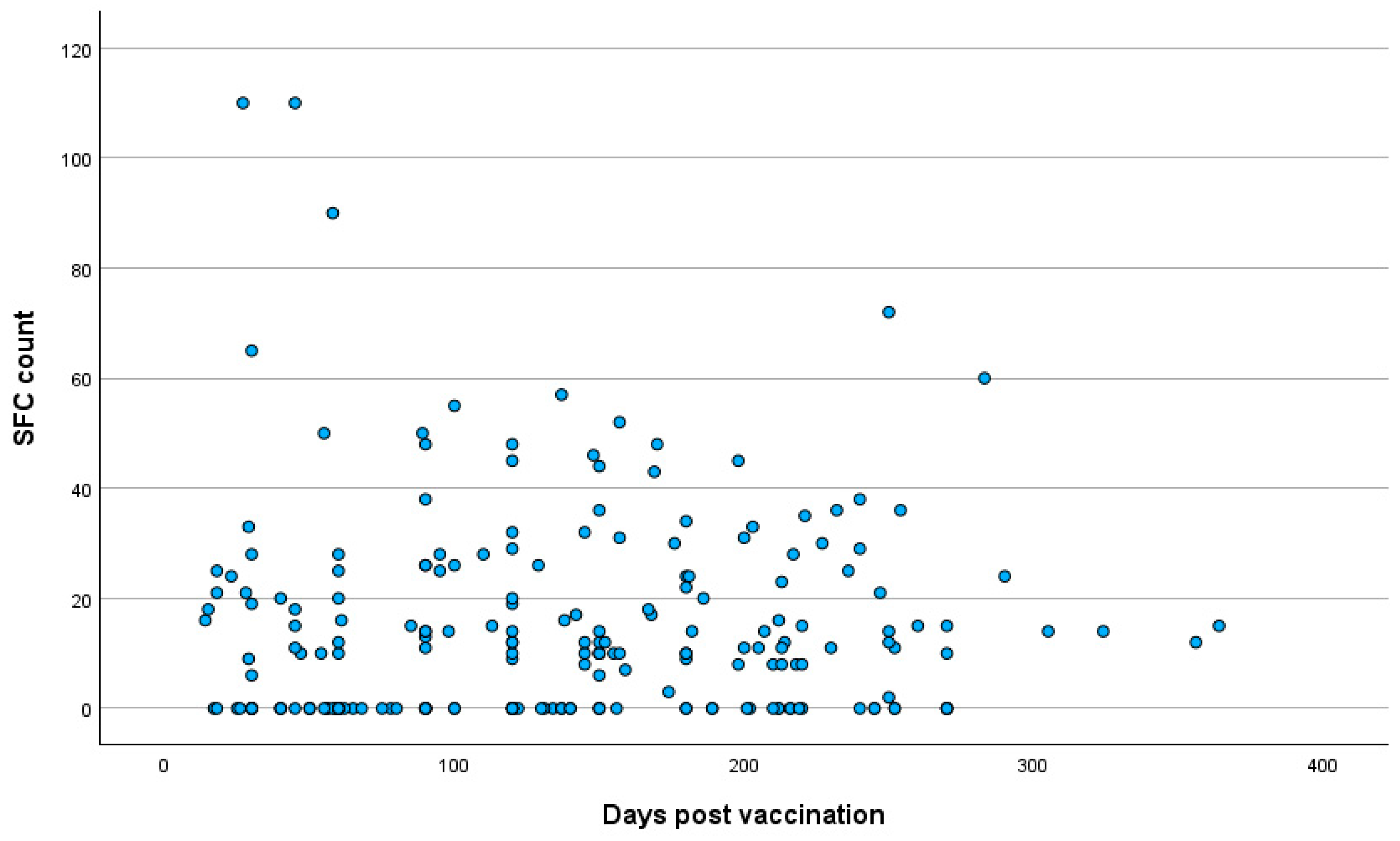
3.3. Quantitative SARS-CoV-2-Specific IFN-g Response
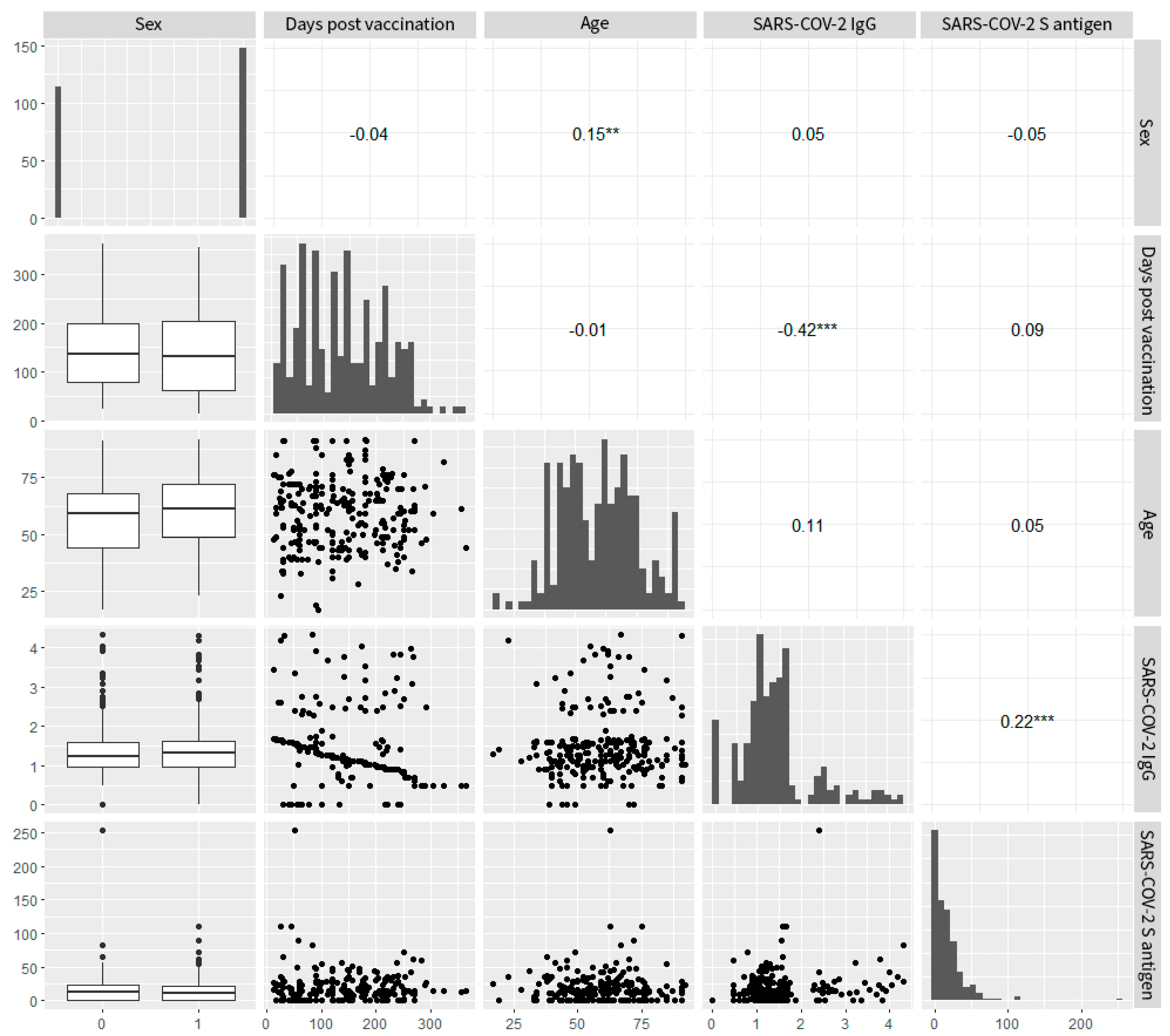
3.4. Correlation Analysis
3.5. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, T.; Xie, H.; Li, Y.; Zhang, J.; Su, X.; Qi, H. The Role of Cellular Immunity in the Protective Efficacy of the SARS-CoV-2 Vaccines. Vaccines 2022, 10, 1103. [Google Scholar] [CrossRef]
- Moga, E.; Lynton-Pons, E.; Domingo, P. The Robustness of Cellular Immunity Determines the Fate of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 904686. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ciriza, L.; González, Á.; del Pozo, J.L.; Fernández-Montero, A.; Carmona-Torre, F.; Carlos, S.; Sarasa, M.d.M.; Reina, G. Humoral and cellular immune response over 9 months of mRNA-1273, BNT162b2 and ChAdOx1 vaccination in a University Hospital in Spain. Sci. Rep. 2022, 12, 15606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef]
- Petrone, L.; Sette, A.; de Vries, R.D.; Goletti, D. The Importance of Measuring SARS-CoV-2-Specific T cell Responses in an Ongoing Pandemic. Pathogens 2023, 12, 862. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, Z. Humoral and cellular immunity against diverse SARS-CoV-2 variants. J. Genet. Genom. 2023, 50, 934–947. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Theodoridou, K.; Vasileiou, I.V.; Tsakris, A. SARS-CoV-2 T Cell Immunity Responses following Natural Infection and Vaccination. Vaccines 2023, 11, 1186. [Google Scholar] [CrossRef]
- Gil-Manso, S.; Carbonell, D.; López-Fernández, L.; Miguens, I.; Alonso, R.; Buño, I.; Muñoz, P.; Ochando, J.; Pion, M.; Correa-Rocha, R. Induction of High Levels of Specific Humoral and Cellular Responses to SARS-CoV-2 After the Administration of COVID-19 mRNA Vaccines Requires Several Days. Front. Immunol. 2021, 12, 726960. [Google Scholar] [CrossRef]
- Graça, D.; Brglez, V.; Allouche, J.; Zorzi, K.; Fernandez, C.; Teisseyre, M.; Cremoni, M.; Benzaken, S.; Pradier, C.; Seitz-Polski, B. Both Humoral and Cellular Immune Responses to SARS-CoV-2 Are Essential to Prevent Infection: A Prospective Study in a Working Vaccinated Population from Southern France. J. Clin. Immunol. 2023, 43, 1724–1739. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Bleotu, C.; Grancea, C.; Chiriac, D.; Botezatu, A.; Iancu, I.V.; Pitica, I.; Necula, L.G.; Neagu, A.; Matei, L.; et al. Kinetics and persistence of cellular and humoral immune responses to SARS-CoV-2 vaccine in healthcare workers with or without prior COVID-19. J. Cell Mol. Med. 2022, 26, 1293–1305. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Morgiel, E.; Szmyrka, M.; Madej, M.; Sebastian, A.; Sokolik, R.; Andrasiak, I.; Chodyra, M.; Walas-Antoszek, M.; Korman, L.; Świerkot, J. Complete (Humoral and Cellular) Response to Vaccination against COVID-19 in a Group of Healthcare Workers-Assessment of Factors Affecting Immunogenicity. Vaccines 2022, 10, 710. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bautista, J.F.; López-Nevot, M.Á.; Gómez-Vicente, E.; Quesada, T.; Marín, E.M.; Rodríguez, A.I.; Rodríguez-Granger, J.; Cobo, F.; Sampedro, A. Study of humoral and cellular immunity in vaccinated with mRNA-1273. Apmis 2022, 130, 261–269. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Godignon, M.; Dutheil, F.; Lambert, C.; Cosme, J.; Mirand, A.; Ollier, A.; et al. Comparative T and B immune responses of four different anti-COVID-19 vaccine strategies 6 months after vaccination. J. Infect. 2022, 84, e45–e47. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Kocher, K.; Priller, A.; Cheng, C.-C.; Steininger, P.; Liao, B.-H.; Körber, N.; Willmann, A.; Irrgang, P.; Held, J.; et al. Dynamics of humoral and cellular immune responses after homologous and heterologous SARS-CoV-2 vaccination with ChAdOx1 nCoV-19 and BNT162b2. eBioMedicine 2022, 85, 104294. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Navarra, A.; Aiello, A.; Laganà, B.; Cuzzi, G.; Salmi, A.; Vanini, V.; Maggi, F.; Meschi, S.; Matusali, G.; et al. Older Age, a High Titre of Neutralising Antibodies and Therapy with Conventional DMARDs Are Associated with Protection from Breakthrough Infection in Rheumatoid Arthritis Patients after the Booster Dose of Anti-SARS-CoV-2 Vaccine. Vaccines 2023, 11, 1684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aiello, A.; Ruggieri, S.; Navarra, A.; Tortorella, C.; Vanini, V.; Haggiag, S.; Prosperini, L.; Cuzzi, G.; Salmi, A.; Quartuccio, M.E.; et al. Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination. Vaccines 2024, 12, 926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Vasileiou, I.V.; Konstantinidis, A.D.; Spyrou, N.I.; Tsakris, A. Prolonged SARS-CoV-2 T Cell Responses in a Vaccinated COVID-19-Naive Population. Vaccines 2024, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Molodtsov, I.A.; Kegeles, E.; Mitin, A.N.; Mityaeva, O.; Musatova, O.E.; Panova, A.E.; Pashenkov, M.V.; Peshkova, I.O.; Alsalloum, A.; Asaad, W.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific T Cells and Antibodies in Coronavirus Disease 2019 (COVID-19) Protection: A Prospective Study. Clin. Infect. Dis. 2022, 75, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Kaplonek, P.; Fischinger, S.; Cizmeci, D.; Bartsch, Y.C.; Kang, J.; Burke, J.S.; Shin, S.A.; Dayal, D.; Martin, P.; Mann, C.; et al. mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern. Immunity 2022, 55, 355–365.e4. [Google Scholar] [CrossRef]
- Scurr, M.J.; Lippiatt, G.; Capitani, L.; Bentley, K.; Lauder, S.N.; Smart, K.; Somerville, M.S.; Rees, T.; Stanton, R.J.; Gallimore, A.; et al. Magnitude of venous or capillary blood-derived SARS-CoV-2-specific T cell response determines COVID-19 immunity. Nat. Commun. 2022, 13, 5422. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Jo, D.-H.; Minn, D.; Lim, J.; Lee, K.-D.; Kang, Y.-M.; Choe, K.-W.; Kim, K.-N. Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines 2021, 9, 1145. [Google Scholar] [CrossRef]
- Agrati, C.; Castilletti, C.; Goletti, D.; Sacchi, A.; Bordoni, V.; Mariotti, D.; Notari, S.; Matusali, G.; Meschi, S.; Petrone, L.; et al. Persistent Spike-specific T cell immunity despite antibody reduction after 3 months from SARS-CoV-2 BNT162b2-mRNA vaccine. Sci. Rep. 2022, 12, 6687. [Google Scholar] [CrossRef]
- Forgacs, D.; Silva-Moraes, V.; Sautto, G.A.; Hanley, H.B.; Gattiker, J.L.; Jefferson, A.M.; Kolhe, R.; Ross, T.M. The Effect of Waning on Antibody Levels and Memory B Cell Recall following SARS-CoV-2 Infection or Vaccination. Vaccines 2022, 10, 696. [Google Scholar] [CrossRef]
- Agallou, M.; Koutsoni, O.S.; Michail, M.; Zisimopoulou, P.; Tsitsilonis, O.E.; Karagouni, E. Antibody and T cell Subsets Analysis Unveils an Immune Profile Heterogeneity Mediating Long-term Responses in Individuals Vaccinated Against SARS-CoV-2. J. Infect. Dis. 2023, 227, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Kronsteiner, B.; Longet, S.; Adele, S.; Deeks, A.S.; Liu, C.; Dejnirattisai, W.; Reyes, L.S.; Meardon, N.; Faustini, S.; et al. Evolution of long-term vaccine-induced and hybrid immunity in healthcare workers after different COVID-19 vaccine regimens. Med 2023, 4, 191–215.e9. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Johnston, T.S.; Lundgreen, K.A.; Santos, J.J.S.; Qin, J.S.; Goel, R.R.; Apostolidis, S.A.; Mathew, D.; Fulmer, B.; Williams, J.C.; et al. Prior vaccination enhances immune responses during SARS-CoV-2 breakthrough infection with early activation of memory T cells followed by production of potent neutralizing antibodies. Nat Immunol. 2023, 24, 1711–1724. [Google Scholar] [CrossRef]
- Tan, C.C.S.; Owen, C.J.; Tham, C.Y.L.; Bertoletti, A.; van Dorp, L.; Balloux, F. Pre-existing T cell-mediated cross-reactivity to SARS-CoV-2 cannot solely be explained by prior exposure to endemic human coronaviruses. Infect. Genet. Evol. 2021, 95, 105075. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Garcia, D.; Keilich, S.R.; Haynes, L. Age-related factors that affect B cell responses to vaccination in mice and humans. Immunol. Rev. 2020, 296, 142–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| VARIABLES | Group A (N = 216) | Group B (N = 46) | p-Value |
|---|---|---|---|
| Demographic characteristics | |||
| Sex (F/M) | 118/98 | 26/20 | NS |
| Age (years ± SD) | 58.8 ± 15.6 | 62.4 ± 14.6 | NS |
| Comorbidities | |||
| Respiratory disorders | 32 (14.8) | 3 (6.5) | NS |
| Cardiovascular diseases | 30 (13.8) | 3 (6.5) | NS |
| Central nervous system disorders | 1 (0.4) | 0 | NS |
| Diabetes mellitus | 12 (5.5) | 3 (6) | NS |
| Hypertension | 28 (12.9) | 7 (15.2) | NS |
| Lipidemia | 52 (24) | 9 (19.5) | NS |
| Obesity | 27 (12.5) | 8 (17.4) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitiriga, V.C.; Papamentzelopoulou, M.; Nikoloudis, D.; Saldari, C.; Konstantinakou, K.E.; Vasileiou, I.V.; Tsakris, A. Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies. Pathogens 2025, 14, 415. https://doi.org/10.3390/pathogens14050415
Pitiriga VC, Papamentzelopoulou M, Nikoloudis D, Saldari C, Konstantinakou KE, Vasileiou IV, Tsakris A. Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies. Pathogens. 2025; 14(5):415. https://doi.org/10.3390/pathogens14050415
Chicago/Turabian StylePitiriga, Vassiliki C., Myrto Papamentzelopoulou, Dimitris Nikoloudis, Chrysa Saldari, Kanella E. Konstantinakou, Irene V. Vasileiou, and Athanasios Tsakris. 2025. "Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies" Pathogens 14, no. 5: 415. https://doi.org/10.3390/pathogens14050415
APA StylePitiriga, V. C., Papamentzelopoulou, M., Nikoloudis, D., Saldari, C., Konstantinakou, K. E., Vasileiou, I. V., & Tsakris, A. (2025). Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies. Pathogens, 14(5), 415. https://doi.org/10.3390/pathogens14050415







