The Main Arboviruses and Virus Detection Methods in Vectors: Current Approaches and Future Perspectives
Abstract
1. Introduction
2. Arboviruses with Veterinary Importance and the “Spillover” of Natural Zoonotic Pathogens into the Human Population
3. The Importance of Viral Detection in Insect Vectors for Predicting Emerging and Re-Emerging Viruses into Populations
4. Different Virus Families Responsible from the Main Arboviruses
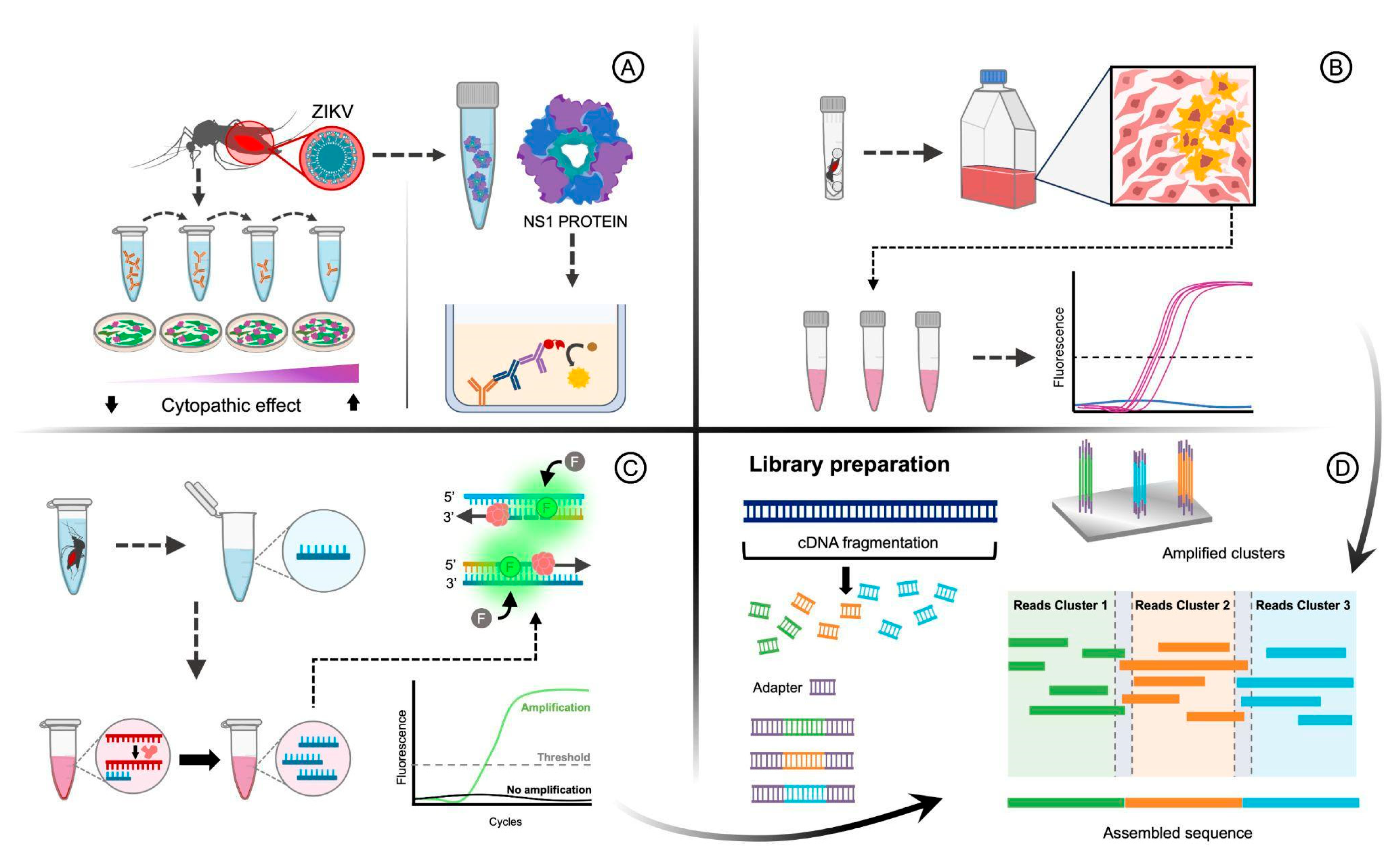
5. Methodologies to Detect Arboviruses in Vector
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Fact Sheet: Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 14 August 2022).
- Kading, R.C.; Brault, A.C.; Beckham, J.D. Global Perspectives on Arbovirus Outbreaks: A 2020 Snapshot. Trop. Med. Infect. Dis. 2020, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R. Arboviruses: A Family on the Move. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1062, pp. 1–10. [Google Scholar]
- Young, K.I.; Medwid, J.T.; Azar, S.R.; Hu, R.M.; Drumm, H.; Co, L.L.; Pitts, R.J.; Buenemann, M.; Vasilakis, N.; Perera, D.; et al. Identification of Mosquito Bloodmeals Collected in Diverse Habitats in Malaysian Borneo Using COI Barcoding. Trop. Med. Infect. Dis. 2020, 5, 51. [Google Scholar] [CrossRef]
- Lorenz, C.; Freitas Ribeiro, A.; Chiaravalloti-Neto, F. Mayaro Virus Distribution in South America. Acta Trop. 2019, 198, 105093. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-de-Oliveira, R.; Failloux, A.B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes Albopictus and Potential Risk of Urban Epidemics in Brazil, South America. Sci. Rep. 2018, 8, 14337. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, V.A.; Borja, L.S.; Cardoso, C.W.; Weaver, S.C.; Reis, M.G.; Kitron, U.; Ribeiro, G.S. Changes in the Dynamics of Dengue Incidence in South and Central America Are Possibly Due to Cross-Population Immunity after Zika Virus Epidemics. Trop. Med. Int. Health 2021, 26, 272–280. [Google Scholar] [CrossRef]
- PAHO. Report on the Epidemiological Situation of Dengue in the Americas; PAHO: Washington, DC, USA, 2024; Volume 143, pp. 1–3. [Google Scholar]
- Cunha, M.S.; Costa, P.A.G.; Correa, I.A.; de Souza, M.R.M.; Calil, P.T.; da Silva, G.P.D.; Costa, S.M.; Fonseca, V.W.P.; da Costa, L.J. Chikungunya Virus: An Emergent Arbovirus to the South American Continent and a Continuous Threat to the World. Front. Microbiol. 2020, 11, 1297. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef]
- Grossi-Soyster, E.N.; LaBeaud, A.D. Rift Valley Fever: Important Considerations for Risk Mitigation and Future Outbreaks. Trop. Med. Infect. Dis. 2020, 5, 89. [Google Scholar] [CrossRef]
- Chopra, H.; Patel, N.; Sethi, Y.; Emran, T.B. Resurgence of Yellow Fever in Africa in 2022: A Glance on Protective Measures. Int. J. Surg. 2023, 109, 112–114. [Google Scholar] [CrossRef]
- Sorvillo, T.E.; Rodriguez, S.E.; Hudson, P.; Carey, M.; Rodriguez, L.L.; Spiropoulou, C.F.; Bird, B.H.; Spengler, J.R.; Bente, D.A. Towards a Sustainable One Health Approach to Crimean–Congo Hemorrhagic Fever Prevention: Focus Areas and Gaps in Knowledge. Trop. Med. Infect. Dis. 2020, 5, 113. [Google Scholar] [CrossRef]
- Temmam, S.; Monteil-Bouchard, S.; Robert, C.; Baudoin, J.P.; Sambou, M.; Aubadie-Ladrix, M.; Labas, N.; Raoult, D.; Mediannikov, O.; Desnues, C. Characterization of Viral Communities of Biting Midges and Identification of Novel Thogotovirus Species and Rhabdovirus Genus. Viruses 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mclean, R.G. Letter to the Editor: Venezuelan Equine Encephalitis Virus 1B Invasion and Epidemic Control—South Texas, 1971. Trop. Med. Infect. Dis. 2020, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.B.; Schaefer, T.J. West Nile Virus; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Harding, S.; Greig, J.; Mascarenhas, M.; Young, I.; Waddell, L.A. La Crosse Virus: A Scoping Review of the Global Evidence. Zoonoses Public Health 2019, 66, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-Borne Encephalitis in Europe, 2012 to 2016. Euro Surveill. 2018, 23, 1800201. [Google Scholar] [CrossRef]
- Campos, R.K.; Rossi, S.L.; Tesh, R.B.; Weaver, S.C. Zoonotic Mosquito-Borne Arboviruses: Spillover, Spillback, and Realistic Mitigation Strategies. Sci. Transl. Med. 2023, 15, eadj2166. [Google Scholar] [CrossRef]
- Kuno, G.; Mackenzie, J.S.; Junglen, S.; Hubálek, Z.; Plyusnin, A.; Gubler, D.J. Vertebrate Reservoirs of Arboviruses: Myth, Synonym of Amplifier, or Reality? Viruses 2017, 9, 185. [Google Scholar] [CrossRef]
- Escudero-Pérez, B.; Lalande, A.; Mathieu, C.; Lawrence, P. Host–Pathogen Interactions Influencing Zoonotic Spillover Potential and Transmission in Humans. Viruses 2023, 15, 599. [Google Scholar] [CrossRef]
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro Virus: A New Human Disease Agent. II. Isolation from Blood of Patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef]
- Mezencio, J.M.S.; de Souza, W.; Fonseca, M.E.F.; Rebello, M.A. Ultrastructural Study of Mayaro Virus Replication in BHK-21 Cells. Arch. Virol. 1990, 114, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mezencio, J.M.S.; de Souza, W.; Fonseca, M.E.F.; Rebello, M.A. Replication of Mayaro Virus in Aedes Albopictus Cells: An Electron Microscopic Study. Arch. Virol. 1989, 104, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Celone, M.; Potter, A.M.; Han, B.A.; Beeman, S.P.; Okech, B.; Forshey, B.; Dunford, J.; Rutherford, G.; Mita-Mendoza, N.K.; Estallo, E.L.; et al. A Geopositioned and Evidence-Graded Pan-Species Compendium of Mayaro Virus Occurrence. Sci. Data 2023, 10, 460. [Google Scholar] [CrossRef]
- Hoch, A.L.; Peterson, N.E.; Leduc, J.W.; Pinheiro, F.P. An Outbreak of Mayaro Viru Disease in Belterra, Brazil. Am. J. Trop. Med. Hyg. 1981, 30, 689–698. [Google Scholar] [CrossRef]
- Meegan, J.; Le Guenno, B.; Ksiazek, T.; Jouan, A.; Knauert, F.; Digoutte, J.P.; Peters, C.J. Rapid Diagnosis of Rift Valley Fever: A Comparison of Methods for the Direct Detection of Viral Antigen in Human Sera. Res. Virol. 1989, 140, 59–65. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Davies, F.G.; Kairo, A.; Bailey, C.L. Rift Valley Fever Virus (Family Bunyaviridae, Genus Phlebovirus). Isolations from Diptera Collected during an Inter-Epizootic Period in Kenya. J. Hyg. 1985, 95, 197–209. [Google Scholar] [CrossRef]
- Weaver, S.C.; Ferro, C.; Barrera, R.; Boshell, J.; Navarro, J.C. Venezuelan Equine Encephalitis. Annu. Rev. Entomol. 2004, 49, 141–174. [Google Scholar] [CrossRef]
- Johnson, K.M.; Shelokov, A.; Peralta, P.H.; Dammin, G.J.; Young, N.A. Recovery of Venezuelan Equine Encephalomyelitis in Panamá. Am. J. Trop. Med. Hyg. 1968, 17, 432–440. [Google Scholar] [CrossRef]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats; Springer: Cham, Switzerland, 2010; Volume 85, ISBN 4097472429. [Google Scholar]
- Brès, P. Impact of Arboviruses on Human and Animal Health, 1st ed.; CRC Press: Boca Raton, FL, USA, 1986; ISBN 9780429280221. [Google Scholar]
- Nasci, R.S.; Moore, C.G.; Biggerstaff, B.J.; Panella, N.A.; Liu, H.Q.; Karabatsos, N.; Davis, B.S.; Brannon, E.S. La Crosse Encephalitis Virus Habitat Associations in Nicholas County, West Virginia. J. Med. Entomol. 2000, 37, 559–570. [Google Scholar] [CrossRef]
- Cheng, L.L.; Rodas, J.D.; Schultz, K.T.; Christensen, B.M.; Yuill, T.M.; Israel, B.A. Potential for Evolution of California Serogroup Bunyaviruses by Genome Reassortment in Aedes Albopictus. Am. J. Trop. Med. Hyg. 1999, 60, 430–438. [Google Scholar] [CrossRef]
- Pustijanac, E.; Buršić, M.; Talapko, J.; Škrlec, I.; Meštrović, T.; Lišnjić, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Bhatti, M.F.E.; Shahid, S.; Faiz, A.; Asad, K.; Arshad, M.; Mushtaq, A. Tick-Borne Encephalitis—A Threat to Life. One Health Triad 2023, 3, 8–11. [Google Scholar] [CrossRef]
- Butenko, A.M.; Leshchinskaia, E.V.; Semashko, I.V.; Donets, M.A.; Mart’ianova, L.I. Dhori Virus—A Causative Agent of Human Disease. 5 Cases of Laboratory Infection. Vopr. Virusol. 1987, 32, 724–729. [Google Scholar]
- Ogen-Odoi, A.; Miller, B.R.; Happ, C.M.; Maupin, G.O.; Burkot, T.R. Isolation of Thogoto Virus (Orthomyxoviridae) from the Banded Mongoose, Mongos Mungo (Herpestidae), in Uganda. Am. J. Trop. Med. Hyg. 1999, 60, 439–440. [Google Scholar] [CrossRef]
- Mateo, R.I.; Xiao, S.-Y.; Lei, H.; Da Rosa, A.P.A.T.; Tesh, R.B. Dhori Virus (Orthomyxoviridae: Thogotovirus) Infection in Mice: A Model of the Pathogenesis of Severe Orthomyxovirus Infection. Am. J. Trop. Med. Hyg. 2007, 76, 785–790. [Google Scholar] [CrossRef]
- Al-Khalifa, M.S.; Diab, F.M.; Khalil, G.M. Man-Threatening Viruses Isolated from Ticks in Saudi Arabia. Saudi Med. J. 2007, 28, 1864–1867. [Google Scholar]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; Hewitson, G.R.; Genge, D.; Burtonclay, P.J.; De Jong, A.J.; Pyke, A.T.; Van Den Hurk, A.F. FTA Cards Facilitate Storage, Shipment, and Detection of Arboviruses in Infected Aedes Aegypti Collected in Adult Mosquito Traps. Am. J. Trop. Med. Hyg. 2017, 96, 1241–1243. [Google Scholar] [CrossRef]
- Štefanić, S.; Grimm, F.; Mathis, A.; Winiger, R.; Verhulst, N.O. Xenosurveillance Proof-of-Principle: Detection of Toxoplasma Gondii and SARS-CoV-2 Antibodies in Mosquito Blood Meals by (Pan)-Specific ELISAs. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100076. [Google Scholar] [CrossRef]
- Ko, K.K.K.; Chng, K.R.; Nagarajan, N. Metagenomics-Enabled Microbial Surveillance. Nat. Microbiol. 2022, 7, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Z.; Bian, C.; Ye, R.-Z.; Cui, X.-M.; Chu, Y.-L.; Yao, N.-N.; Xu, X.-W.; Ye, J.-L.; Chen, L.; Yang, J.-H.; et al. Human Infection with a Novel Tickborne Orthonairovirus Species in China. N. Engl. J. Med. 2025, 392, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Bian, C.; Ye, R.Z.; Cui, X.M.; Yao, N.N.; Yang, J.H.; Chu, Y.L.; Su, X.L.; Wu, Y.F.; Ye, J.L.; et al. A Series of Patients Infected with the Emerging Tick-Borne Yezo Virus in China: An Active Surveillance and Genomic Analysis. Lancet Infect. Dis. 2024, 25, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-A.; Ma, Y.-D.; Zhang, Y.-F.; Hu, Z.-Y.; Zhang, J.-T.; Han, S.; Wang, G.; Li, S.; Wang, X.; Tang, F.; et al. A New Orthonairovirus Associated with Human Febrile Illness. N. Engl. J. Med. 2024, 391, 821–831. [Google Scholar] [CrossRef]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C.; Consortium, I.R. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef]
- Giorgi, C.; Accardi, L.; Nicoletti, L.; Gro, M.C.; Takehara, K.; Hilditch, C.; Morikawa, S.; Bishop, D.H. Sequences and Coding Strategies of the S RNAs of Toscana and Rift Valley Fever Viruses Compared to Those of Punta Toro, Sicilian Sandfly Fever, and Uukuniemi Viruses. Virology 1991, 180, 738–753. [Google Scholar] [CrossRef]
- Martin, M.L.; Lindsey-Regnery, H.; Sasso, D.; Mccormick, J.B.; Palmer, E. Distinction Between Bunyaviridae Genera by Surface Structure and Comparison with Hantaan Virus Using Negative Stain Electron Microscopy. Arch. Virol. 1985, 86, 17–28. [Google Scholar] [CrossRef]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A Global Public Health Threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef]
- Hussain-Alkhateeb, L.; Ramírez, T.R.; Kroeger, A.; Gozzer, E.; Runge-Ranzinger, S. Early Warning Systems (EWSs) for Chikungunya, Dengue, Malaria, Yellow Fever, and Zika Outbreaks: What Is the Evidence? A Scoping Review. PLoS Negl. Trop. Dis. 2021, 15, e0009686. [Google Scholar] [CrossRef]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef]
- Fouet, C.; Kamdem, C. Integrated Mosquito Management: Is Precision Control a Luxury or Necessity? Trends Parasitol. 2019, 35, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.L.; Long, M.T. Perspectives on New Vaccines against Arboviruses Using Insect-Specific Viruses as Platforms. Vaccines 2021, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.J.; Shah, P.S. Mapping Arbovirus-Vector Interactions Using Systems Biology Techniques. Front. Cell. Infect. Microbiol. 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Serodiagnosis of Flaviviral Infections and Vaccinations in Humans. Adv. Virus Res. 2003, 61, 3–65. [Google Scholar] [CrossRef]
- Gyawali, N.; Murphy, A.K.; Hugo, L.E.; Devine, G.J. A Micro-PRNT for the Detection of Ross River Virus Antibodies in Mosquito Blood Meals: A Useful Tool for Inferring Transmission Pathways. PLoS ONE 2020, 15, e0229314. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Chen, C.; Chen, Z.; Zhang, L.; Han, Q.; Xia, X.; Song, Y.; Zhang, J. Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection. Viruses 2018, 10, 634. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, W.; Li, H.; Su, M.P.; Wu, S.; Chen, H.-W.; Pan, C.-Y.; Tsai, J.-J.; Chen, C.-H. Releasing Intracellular NS1 from Mosquito Cells for the Detection of Dengue Virus-Infected Mosquitoes. Viruses 2020, 12, 1105. [Google Scholar] [CrossRef]
- Hundekar, S.L.; Thakare, J.P.; Gokhale, M.D.; Barde, P.V.; Argade, S.V.; Mourya, D.T. Development of Monoclonal Antibody Based Antigen Capture ELISA to Detect Chikungunya Virus Antigen in Mosquitoes. Indian J. Med. Res. 2002, 115, 144–148. [Google Scholar]
- Sithiprasasna, R.; Strickman, D.; Innis, B.L.; Linthicum, K.J. ELISA for Detecting Dengue and Japanese Encephalitis Viral Antigen in Mosquitoes. Ann. Trop. Med. Parasitol. 2017, 88, 397–404. [Google Scholar] [CrossRef]
- Artsob, H.; Spence, L.; Bishai, F.; Kurjanczyk, L.; Sekla, L. Amplified ELISA for the Detection of Western Equine Encephalitis Virus from Mosquitoes in Manitoba, Canada. J. Virol. Methods 1987, 18, 233–242. [Google Scholar] [CrossRef]
- Gregor, K.M.; Michaely, L.M.; Gutjahr, B.; Rissmann, M.; Keller, M.; Dornbusch, S.; Becker, S.; Eiden, M.; Spitzbarth, I.; Baumgärtner, W.; et al. Rift Valley Fever Virus Detection in Susceptible Hosts with Special Emphasis in Insects. Sci. Rep. 2021, 11, 9822. [Google Scholar] [CrossRef] [PubMed]
- Brien, C.A.O.; Hobson-Peters, J.; Yam, A.W.Y.; Colmant, A.M.G.; McLean, B.J.; Prow, N.A.; Watterson, D.; Hall-Mendelin, S.; Warrilow, D.; Ng, M.-L.; et al. Viral RNA Intermediates as Targets for Detection and Discovery of Novel and Emerging Mosquito-Borne Viruses. PLoS Negl. Trop. Dis. 2015, 9, e0003629. [Google Scholar] [CrossRef]
- Nasrin, F.; Tsuruga, K.; Utomo, D.I.S.; Chowdhury, A.D.; Park, E.Y. Design and Analysis of a Single System of Impedimetric Biosensors for the Detection of Mosquito-Borne Viruses. Biosensors 2021, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Burkhalter, K.L.; Savage, H.M. Laboratory Evaluation of the Rapid Analyte Measurement Platform Assay to Detect Dengue Virus in Mosquito Pools. J. Am. Mosq. Control Assoc. 2021, 37, 152–156. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Van Den Hurk, A.F.; Meyer, D.B.; Ritchie, S.A. Searching for the Proverbial Needle in a Haystack: Advances in Mosquito-Borne Arbovirus Surveillance. Parasites Vectors 2018, 11, 320. [Google Scholar] [CrossRef]
- Kuwata, R.; Torii, S.; Shimoda, H.; Ishijima, K.; Yonemitsu, K.; Minami, S.; Kuroda, Y.; Tatemoto, K.; Tran, N.T.B.; Takano, A.; et al. Mosquito-Borne Viruses, Insect-Specific Flaviviruses (Family Flaviviridae, Genus Flavivirus), Banna Virus (Family Reoviridae, Genus Seadornavirus), Bogor Virus (Unassigned Member of Family Permutotetraviridae), and Alphamesoniviruses 2 and 3 (Family Mesoniviridae, Genus Alphamesonivirus) Isolated from Indonesian Mosquitoes. J. Vet. Med. Sci. 2020, 82, 1030–1041. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, S.; Song, S.; Cai, L.; Zhang, H.; Gao, L.; Cao, L.; Li, M.; Gao, X.; He, Y.; et al. Isolation and Genome Phylogenetic Analysis of Arthropod-Borne Viruses, Including Akabane Virus, from Mosquitoes Collected in Hunan Province, China. Vector-Borne Zoonotic Dis. 2019, 19, 62–72. [Google Scholar] [CrossRef]
- Moi, M.L.; Kobayashi, D.; Isawa, H.; Sasaki, T.; Saijo, M.; Kurane, I.; Sawabe, K.; Takasaki, T. Dengue Virus Isolation in Mosquito Aedes Albopictus Captured During an Outbreak in Tokyo, 2014, by a Method Relying on Antibody-Dependent Enhancement Mechanism Using FcγR-Expressing BHK Cells. Vector-Borne Zoonotic Dis. 2016, 16, 810–812. [Google Scholar] [CrossRef]
- Xia, H.; Liu, H.; Zhao, L.; Atoni, E.; Wang, Y.; Yuan, Z. First Isolation and Characterization of a Group C Banna Virus (BAV) from Anopheles Sinensis Mosquitoes in Hubei, China. Viruses 2018, 10, 555. [Google Scholar] [CrossRef]
- Lei, W.; Guo, X.; Fu, S.; Feng, Y.; Song, J.; Zhou, H.; Liang, G. Isolation and Identification of the Nam Dinh Virus from Mosquitoes on the China-Laos-Myanmar Border. Bing Du Xue Bao Chin. J. Virol. 2016, 32, 782–789. [Google Scholar]
- Sutherland, G.L.; Nasci, R.S. Detection of West Nile Virus in Large Pools of Mosquitoes. J. Am. Mosq. Control Assoc. 2007, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Andreadis, T.G.; Finan, S.L.; Shepard, J.J.; Thomas, M.C. Detection of Infectious Virus from Field-Collected Mosquitoes by Vero Cell Culture Assay. J. Vis. Exp. 2011, 52, e2889. [Google Scholar] [CrossRef]
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reyn, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles (Anopheles) Coustani, Anopheles (Anopheles) Squamosus, and Culex (Culex) Antennatus of the Haute Matsiatra Region, Madagascar. Vector-Borne Zoonotic Dis. 2011, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Mwaengo, D.; Lorenzo, G.; Iglesias, J.; Warigia, M.; Sang, R.; Bishop, R.P.; Brun, A. Detection and Identification of Rift Valley Fever Virus in Mosquito Vectors by Quantitative Real-Time PCR. Virus Res. 2012, 169, 137–143. [Google Scholar] [CrossRef]
- de Curcio, J.S.; Salem-Izacc, S.M.; Neto, L.M.P.; Nunes, E.B.; Anunciação, C.E.; de Paula Silveira-Lacerda, E. Detection of Mayaro Virus in Aedes Aegypti Mosquitoes Circulating in Goiânia-Goiás-Brazil. Microbes Infect. 2022, 24, 104948. [Google Scholar] [CrossRef]
- da Silva Pessoa Vieira, C.J.; de Andrade, C.D.; Kubiszeski, J.R.; da Silva, D.J.F.; Barreto, E.S.; Massey, A.L.; Canale, G.R.; Bernardo, C.S.S.; Levi, T.; Peres, C.A.; et al. Detection of Ilheus Virus in Mosquitoes from Southeast Amazon, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 424–427. [Google Scholar] [CrossRef]
- Burkhalter, K.L.; Savage, H.M. Detection of Zika Virus in Desiccated Mosquitoes by Real-Time Reverse Transcription PCR and Plaque Assay. Emerg. Infect. Dis. 2017, 23, 680–681. [Google Scholar] [CrossRef]
- Barnarda, T.R.; Wanga, A.B.; Sagan, S.M. A Highly Sensitive Strand-Specific Multiplex RT-QPCR Assay for Quantitation of Zika Virus Replication. J. Virol. Methods 2022, 307, 114556. [Google Scholar] [CrossRef]
- Pantawane, P.B.; Dhanze, H.; Ravi Kumar, G.V.P.P.S.; Grace, M.R.; Dudhe, N.C.; Bhilegaonkar, K.N. TaqMan Real-Time RT-PCR Assay for Detecting Japanese Encephalitis Virus in Swine Blood Samples and Mosquitoes. Anim. Biotechnol. 2019, 30, 267–272. [Google Scholar] [CrossRef]
- Hadfield, T.L.; Turell, M.; Dempsey, M.P.; David, J.; Park, E.J. Detection of West Nile Virus in Mosquitoes by RT-PCR. Mol. Cell. Probes 2001, 15, 147–150. [Google Scholar] [CrossRef]
- Tang, Z.; Yamada, H.; Kraupa, C.; Canic, S.; Busquets, N.; Talavera, S.; Jiolle, D.; Vreysen, M.J.B.; Bouyer, J.; Abd-Alla, A.M.M. High Sensitivity of One-Step Real-Time Reverse Transcription Quantitative PCR to Detect Low Virus Titers in Large Mosquito Pools. Parasites Vectors 2020, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Davis, B.S.; Chang, G.J. Development of Multiplex Real-Time Reverse Transcriptase PCR Assays for Detecting Eight Medically Important Flaviviruses in Mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Balingit, J.C.; Carvajal, T.M.; Obata, M.S.; Gamboa, M.; Nicolasora, A.D.; Sy, A.K.; Oshitani, H.; Watanabe, K. Surveillance of Dengue Virus in Individual Aedes Aegypti Mosquitoes Collected Concurrently with Suspected Human Cases in Tarlac City, Philippines. Parasites Vectors 2020, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Rademan, R.; Markotter, W.; Paweska, J.T.; van Vuren, P.J. Multiplex Real-Time RT-PCR for Detection and Distinction of Spondweni and Zika Virus. J. Virol. Methods 2019, 266, 72–76. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Su, C.; Teng, H.; Lu, L.; Lin, C.; Wang, C.; Shu, P.; Huang, J.; Wu, H. Screening of Mosquitoes Using SYBR Green I-Based Real-Time RT-PCR with Group-Specific Primers for Detection of Flaviviruses and Alphaviruses in Taiwan. J. Virol. Methods 2010, 168, 147–151. [Google Scholar] [CrossRef]
- Villinger, J.; Mbaya, M.K.; Ouso, D.; Kipanga, P.N. Arbovirus and Insect-Specific Virus Discovery in Kenya by Novel Six Genera Multiplex High-Resolution Melting Analysis. Mol. Ecol. Resour. 2017, 17, 466–480. [Google Scholar] [CrossRef]
- Guarido, M.M.; Govender, K.; Riddin, M.A.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Paulo, A.; Almeida, G.; Venter, M. Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses 2021, 13, 2148. [Google Scholar] [CrossRef]
- Hoyos-López, R.; Suaza-Vasco, J.; Rúa-Uribe, G.; Uribe, S.; Gallego-Gómez, J.C. Molecular Detection of Flaviviruses and Alphaviruses in Mosquitoes (Diptera: Culicidae) from Coastal Ecosystems in the Colombian Caribbean. Mem. Inst. Oswaldo Cruz 2016, 111, 625–634. [Google Scholar] [CrossRef]
- Fang, Y.; Tambo, E.; Xue, J.; Zhang, Y.; Zhou, X.-N.; Khater, E.I.M. Detection of DENV-2 and Insect-Specific Flaviviruses in Mosquitoes Collected from Jeddah, Saudi Arabia. Front. Cell. Infect. Microbiol. 2021, 11, 626368. [Google Scholar] [CrossRef]
- Lura, T.; Su, T.; Thieme, J.; Brown, M.Q. A Validated Triplex RT-QPCR Protocol to Simultaneously Detect Chikungunya, Dengue and Zika Viruses in Mosquitoes. J. Vector Borne Dis. 2022, 59, 198–205. [Google Scholar] [CrossRef]
- Siriyasatien, P.; Wacharapluesadee, S.; Kraivichian, K.; Suwanbamrung, C.; Sutthanont, N.; Cantos-Barreda, A.; Phumee, A. Development and Evaluation of a Visible Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP) for the Detection of Asian Lineage ZIKV in Field-Caught Mosquitoes. Acta Trop. 2022, 236, 106691. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimena, B.; Bakheit, M.; Bekaert, M.; Harold, G.; Frischmann, S.; Fall, C.; Diagne, C.T.; Faye, O.; Faye, O.; Sall, A.A.; et al. Development and Validation of Real-Time RT-LAMP Assays for the Specific Detection of Zika Virus. In Zika Virus; Humana: New York, NY, USA, 2020; pp. 147–164. [Google Scholar]
- Silva, S.J.R.; Paiva, M.H.S.; Guedes, D.R.D.; Krokovsky, L.; De Melo, F.L.; Silva, M.A.L.; Silva, A.; Ayres, C.F.J.; Pena, L.J. Development and Validation of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) for Rapid Detection of ZIKV in Mosquito Samples from Brazil. Sci. Rep. 2019, 9, 4494. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhao, N.; Zhao, L.; Wang, Y.; Zhao, W.; Yuan, Z. Rapid Detection of Banna Virus by Reverse Transcription-Loop-Mediated Isothermal Ampli Fi Cation (RT-LAMP). Int. J. Infect. Dis. 2019, 78, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Aonuma, H.; Iizuka, I.; Li, J.; Ote, M.; Tajima, S.; Saijo, M.; Chen, C.-H.; Kanuka, H. LAMP Detection of Virus-Derived DNA of Zika Virus in Vector Mosquito. Front. Trop. Dis. 2022, 3, 759375. [Google Scholar] [CrossRef]
- Burkhalter, K.L.; Keefe, M.O.; Holbert-Watson, Z.; Green, T.; Savage, H.M.; Markowski, D.M. Laboratory and Field Evaluations of a Commercially Available Commercially Real-Time Loop-Mediated Isothermal Amplification Assay for the Detection of West Nile Virus in Mosquito Pools. J. Am. Mosq. Control Assoc. 2021, 37, 256–262. [Google Scholar] [CrossRef]
- Diagne, C.T.; Faye, M.; Lopez-Jimena, B.; El Wahed, A.A.; Loucoubar, C.; Fall, C.; Mencatelli, G.; Faye, O.; Faye, O.; Weidmann, M.; et al. Comparative Analysis of Zika Virus Detection by RT-QPCR, RT-LAMP, and RT-RPA. In Zika Virus; Humana: New York, NY, USA, 2020; pp. 165–179. ISBN 9781071605806. [Google Scholar]
- Huang, D.; Ma, H.; Zhao, L.; Wang, X.; Huang, Y.; Wang, F.; Yuan, Z.; Xia, H. Mosquito-Associated Virus Isolation from Field-Collected Mosquitoes. J. Vis. Exp. 2022, 186, e63852. [Google Scholar] [CrossRef]
- Thomas, K.M.; Pelletier, N.J.; França, C.M.B. Using Metagenomics to Detect West Nile Virus in Mosquitoes Collected in Oklahoma. Bios 2022, 93, 139–148. [Google Scholar] [CrossRef]
- Hameed, M.; Khan, S.; Xu, J.; Zhang, J.; Wnag, X.; Di, D.; Chen, Z.; Anwar, M.N.; Wahaab, A.; Ma, X.; et al. Detection of Japanese Encephalitis Virus in Mosquitoes from Xinjiang during Next Generation Sequencing Arboviral Surveillance. Transbound. Emerg. Dis. 2021, 68, 467–476. [Google Scholar] [CrossRef]
- Ribeiro, G.D.O.; Julio, F.; Monteiro, C.; Octavio, M.; Rego, S.; Soares, E.; Ribeiro, D.A.; De Castro, D.F.; Caseiro, M.M.; Souza, S.; et al. Detection of RNA-Dependent RNA Polymerase Sequencing in Aedes Aegypti and Culex Quinquefasciatus Mosquitoes from Brazil. Viruses 2019, 11, 147. [Google Scholar] [CrossRef]
- Duarte, M.A.; Campos, F.S.; Neto, O.F.A.; Silva, L.A.; Silva, A.B.; Aguiar, T.C.; Santos, R.N.; Souza, U.J.B.; Alves, G.B.; Melo, F.L.; et al. Identification of Potential New Mosquito-Associated Viruses of Adult Aedes Aegypti Mosquitoes from Tocantins State, Brazil. Braz. J. Microbiol. 2022, 53, 51–62. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Klein, T.A.; Kim, H.; Fung, C.K.; Figueroa, K.L.; Yang, Y.; Asafo-Adjei, E.A.; Jarman, R.G.; Hang, J. Metagenomic Analysis Reveals Three Novel and Prevalent Mosquito Viruses from a Single Pool of Aedes Vexans Nipponii Collected in the Republic of Korea. Viruses 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Batson, J.; Dudas, G.; Haas-Stapleton, E.; Kistler, A.L.; Li, L.M.; Logan, P.; Ratnasiri, K.; Retallack, H. Single Mosquito Metatranscriptomics Identifies Vectors, Emerging Pathogens and Reservoirs in One Assay. eLife 2021, 10, e64943. [Google Scholar] [CrossRef] [PubMed]
- Thannesberger, J.; Rascovan, N.; Eisenmann, A.; Klymiuk, I.; Zittra, C.; Fuehrer, H.P.; Manning, T.S.; Gittens-St. Hilaire, M.; Austin, S.; Landis, R.C.; et al. Viral Metagenomics Reveals the Presence of Novel Zika Virus Variants in Aedes Mosquitoes from Barbados. Parasites Vectors 2021, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Chaintoutis, S.C.; Papadopoulou, E.; Melidou, A.; Papa, A.; Dovas, C.I. A PCR-Based NGS Protocol for Whole Genome Sequencing of West Nile Virus Lineage 2 Directly from Biological Specimens. Mol. Cell. Probes 2019, 46, 101412. [Google Scholar] [CrossRef]
- Martin, E.; Borucki, M.K.; Thissen, J.; Garcia-Luna, S.; Hwang, M.; De Valdez, W.; Jaing, C.J.; Hamer, G.L.; Frank, M. Mosquito-Borne Viruses and Insect-Specific Viruses Revealed in Field-Collected Mosquitoes by a Monitoring Tool Adapted from a Microbial Detection Array. Appl. Environ. Microbiol. 2019, 85, e01202-19. [Google Scholar] [CrossRef]
- Bakhshi, H.; Mousson, L.; Moutailler, S.; Vazeille, M.; Piorkowski, G.; Zakeri, S.; Raz, A.; De Lamballerie, X.; Dinparast-Djadid, N.; Failloux, A.-B. Detection of Arboviruses in Mosquitoes: Evidence of Circulation of Chikungunya Virus in Iran. PLoS Negl. Trop. Dis. 2021, 14, e0008135. [Google Scholar] [CrossRef]
- Glushakova, L.G.; Bradley, A.; Bradley, K.M.; Alto, B.W.; Hoshika, S.; Hutter, D.; Sharma, N.; Yang, Z.; Kim, M.; Benner, S.A. High-Throughput Multiplexed XMAP Luminex Array Panel for Detection of Twenty Two Medically Important Mosquito-Borne Arboviruses Based on Innovations in Synthetic Biology. J. Virol. Methods 2015, 214, 60–74. [Google Scholar] [CrossRef]
- Glushakova, L.G.; Alto, B.W.; Kim, M.; Hutter, D.; Bradley, A.; Bradley, K.M.; Burkett-Cadena, N.D.; Benner, S.A. Multiplexed Kit Based on Luminex Technology and Achievements in Synthetic Biology Discriminates Zika, Chikungunya, and Dengue Viruses in Mosquitoes. BMC Infect. Dis. 2019, 19, 418. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Santos, L.M.B.; Chouin-Carneiro, T.; Pavan, M.G.; Garcia, G.A.; David, M.R.; Beier, J.C.; Dowell, F.E.; Maciel-de-Freitas, R.; Sikulu-Lord, M.T. Rapid, Noninvasive Detection of Zika Virus in Aedes Aegypti Mosquitoes by Near-Infrared Spectroscopy. Sci. Adv. 2018, 4, eaat0496. [Google Scholar] [CrossRef]
- Sikulu-Lord, M.; Maciel-de-Freitas, R. Application of Infrared Techniques for Characterisation of Vector-Borne Disease Vectors. In Infrared Spectroscopy—Perspectives and Applications; IntechOpen: London, UK, 2022; pp. 1–18. [Google Scholar]

| Viral Family | Shape | Diameter | Genome | Genome Size |
|---|---|---|---|---|
| Togaviridae | Spherical | 70 nM | +ssRNA | 9.7–11.8 kb |
| Flaviviridae | Spherical | 50 nM | +ssRNA | 9.2–11 kb |
| Naioroviridae | Spherical | 80–120 nM | 3 segments −ssRNA | Small 2 kb Medium 5 kb Large 12 kb |
| Phenuiviridae | Spherical | 80–120 nM | 3 segments −ssRNA | Small 1.7 kb Medium 3.2 kb Large 6.4 kb |
| Peribunyaviridae | Spherical | 80–120 nM | 3 segments −ssRNA | Small 1 kb Medium 4 kb Large 6.8 kb |
| Orthomyxoviridae | Spherical | 80–120 nM | 6 segments −ssRNA | 10 kb |
| Method | Time | Equipment | Advantages | Disadvantages | Main Applications |
|---|---|---|---|---|---|
| ELISA | 2–4 h | Microplate reader | High-throughput, detects proteins | Lower sensitivity | Antibody detection, surveillance studies |
| PRNT | 5–7 days | Cell culture, biosafety laboratory | Gold standard for neutralizing antibodies | Labor-intensive, requires live virus | Differentiates viable viruses, serological surveys |
| Electrochemical Impedimetric | 30 min | Portable sensor | Label-free, real-time, portable | Requires sensor optimization | Field biosensors, smart traps |
| RAMP | 15–30 min | Water bath/heat block (37–42 °C) | Faster than LAMP, room temp possible | Less validated, limited commercial kits | Rapid field diagnostic |
| Virus isolation | 3–14 days | Cell culture, biosafety laboratory | Confirms infectious virus, gold standard for viability | Slow, laborious, biosafety risks | Research, vaccine development |
| RT-qPCR | 1–3 h | Real-time PCR machine | Quantitative, high sensitivity, multiplex capable | Expensive reagents | Outbreak monitoring, diagnostic |
| RT-LAMP | 30–60 min | Heat block | Isothermal, Field-deployable, visual readout | Primer design complex, false positives | Point-of-care testing, field surveillance |
| NGS | 1–3 days | Sequencer, bioinformatics | Comprehensive genomic data, novel pathogen | High cost, complex data analysis | Virus discovery |
| LLMDA | 6–24 h | Microarray scanner | Detects thousands of pathogens simultaneously | Slow, expensive | Biodefense, unknown pathogens |
| NIRS | 1–5 min | Portable NIR spectrometer | No sample preparation, rapid, reagent-free | Lower specificity, needs calibration | Mass mosquito screening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cintra, A.M.; Noda-Nicolau, N.M.; Soman, M.L.d.O.; Affonso, P.H.d.A.; Valente, G.T.; Grotto, R.M.T. The Main Arboviruses and Virus Detection Methods in Vectors: Current Approaches and Future Perspectives. Pathogens 2025, 14, 416. https://doi.org/10.3390/pathogens14050416
Cintra AM, Noda-Nicolau NM, Soman MLdO, Affonso PHdA, Valente GT, Grotto RMT. The Main Arboviruses and Virus Detection Methods in Vectors: Current Approaches and Future Perspectives. Pathogens. 2025; 14(5):416. https://doi.org/10.3390/pathogens14050416
Chicago/Turabian StyleCintra, Amanda Montezano, Nathália Mayumi Noda-Nicolau, Milena Leite de Oliveira Soman, Pedro Henrique de Andrade Affonso, Guilherme Targino Valente, and Rejane Maria Tommasini Grotto. 2025. "The Main Arboviruses and Virus Detection Methods in Vectors: Current Approaches and Future Perspectives" Pathogens 14, no. 5: 416. https://doi.org/10.3390/pathogens14050416
APA StyleCintra, A. M., Noda-Nicolau, N. M., Soman, M. L. d. O., Affonso, P. H. d. A., Valente, G. T., & Grotto, R. M. T. (2025). The Main Arboviruses and Virus Detection Methods in Vectors: Current Approaches and Future Perspectives. Pathogens, 14(5), 416. https://doi.org/10.3390/pathogens14050416







