Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania hupensis quadrasi
Abstract
:1. Introduction
2. Results
2.1. Description of Collection Sites and Malacological Survey
2.2. eDNA Detection via Conventional PCR and qPCR
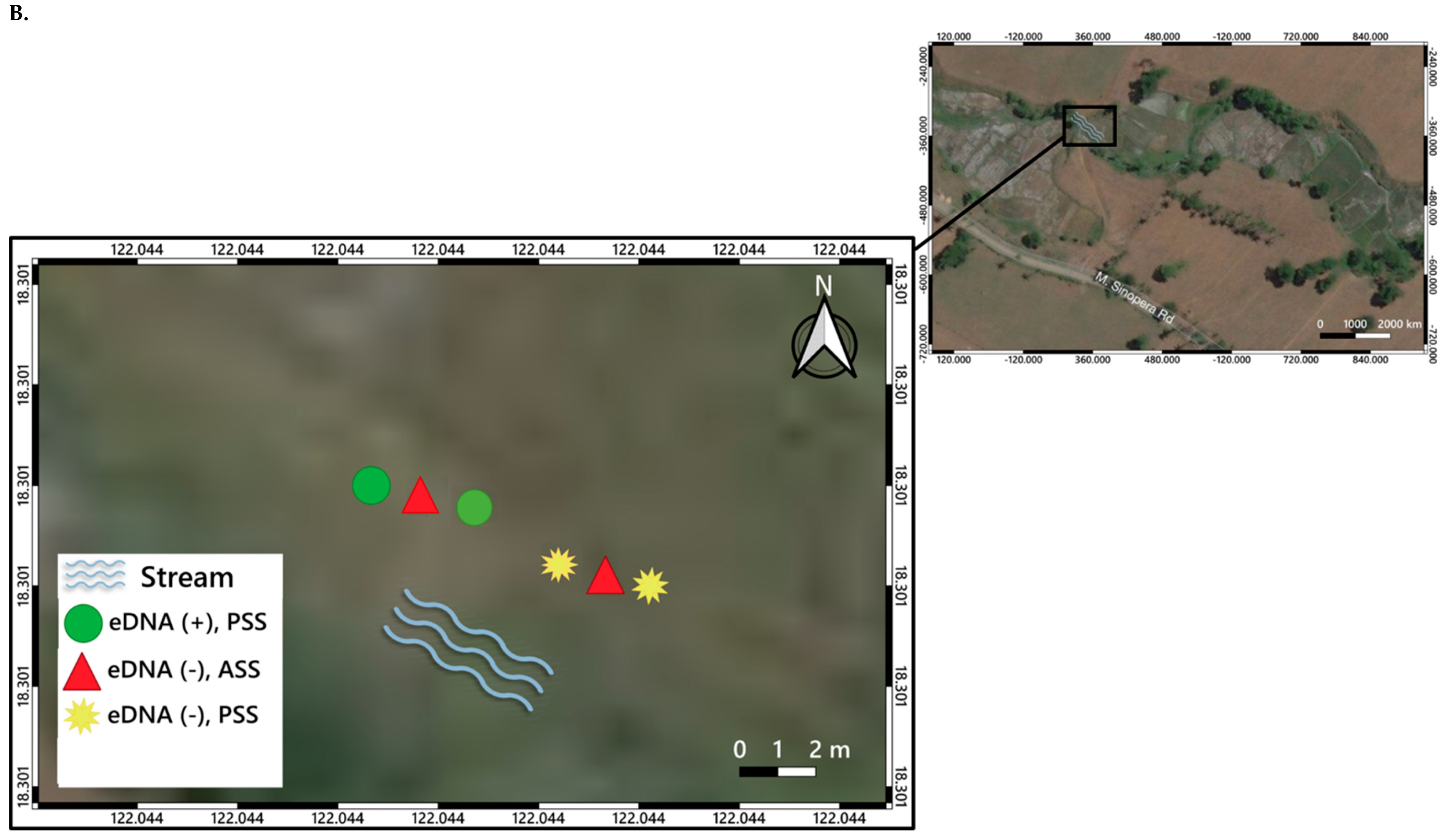
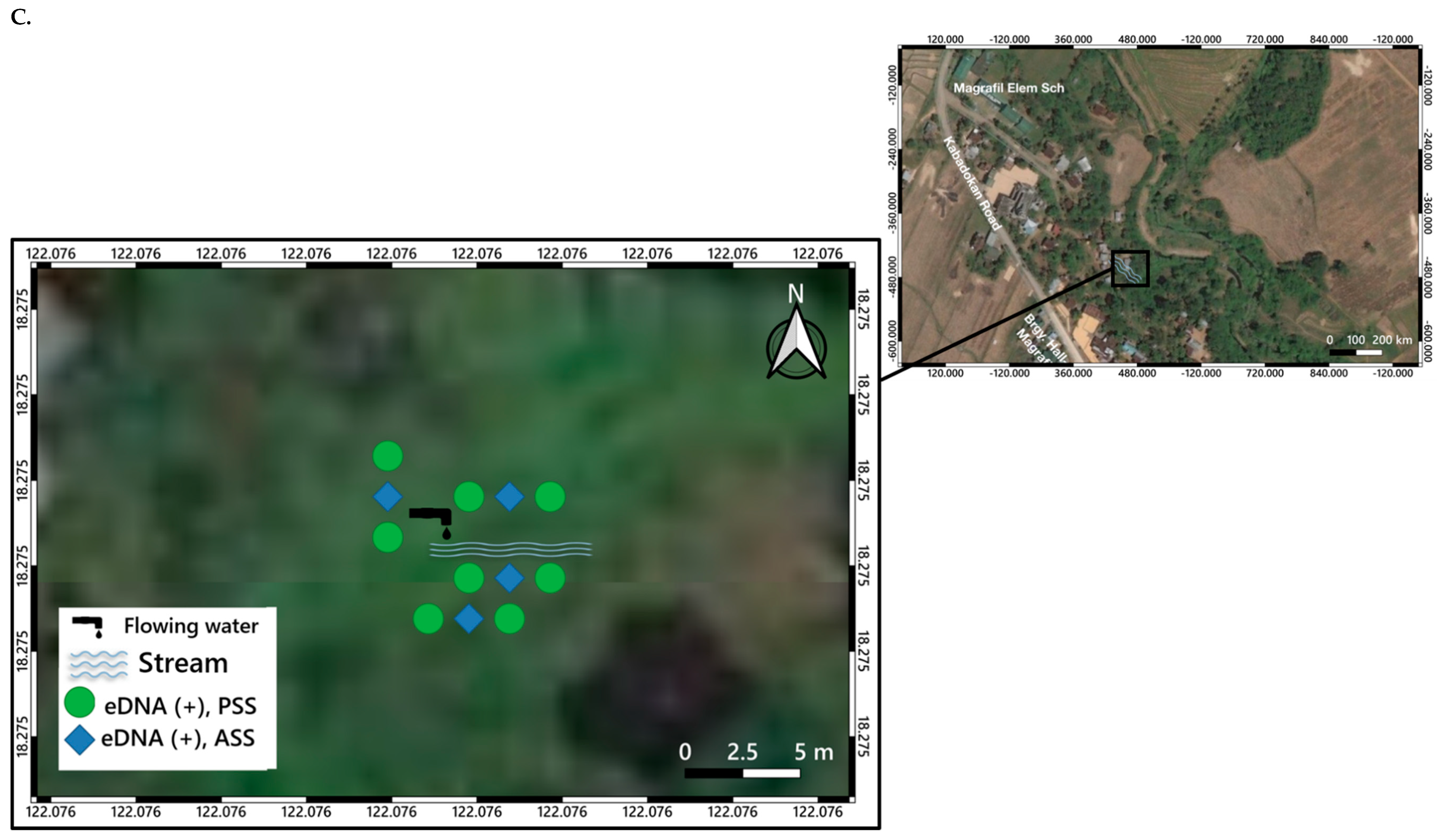
3. Distribution Mapping of eDNA Positive Sites
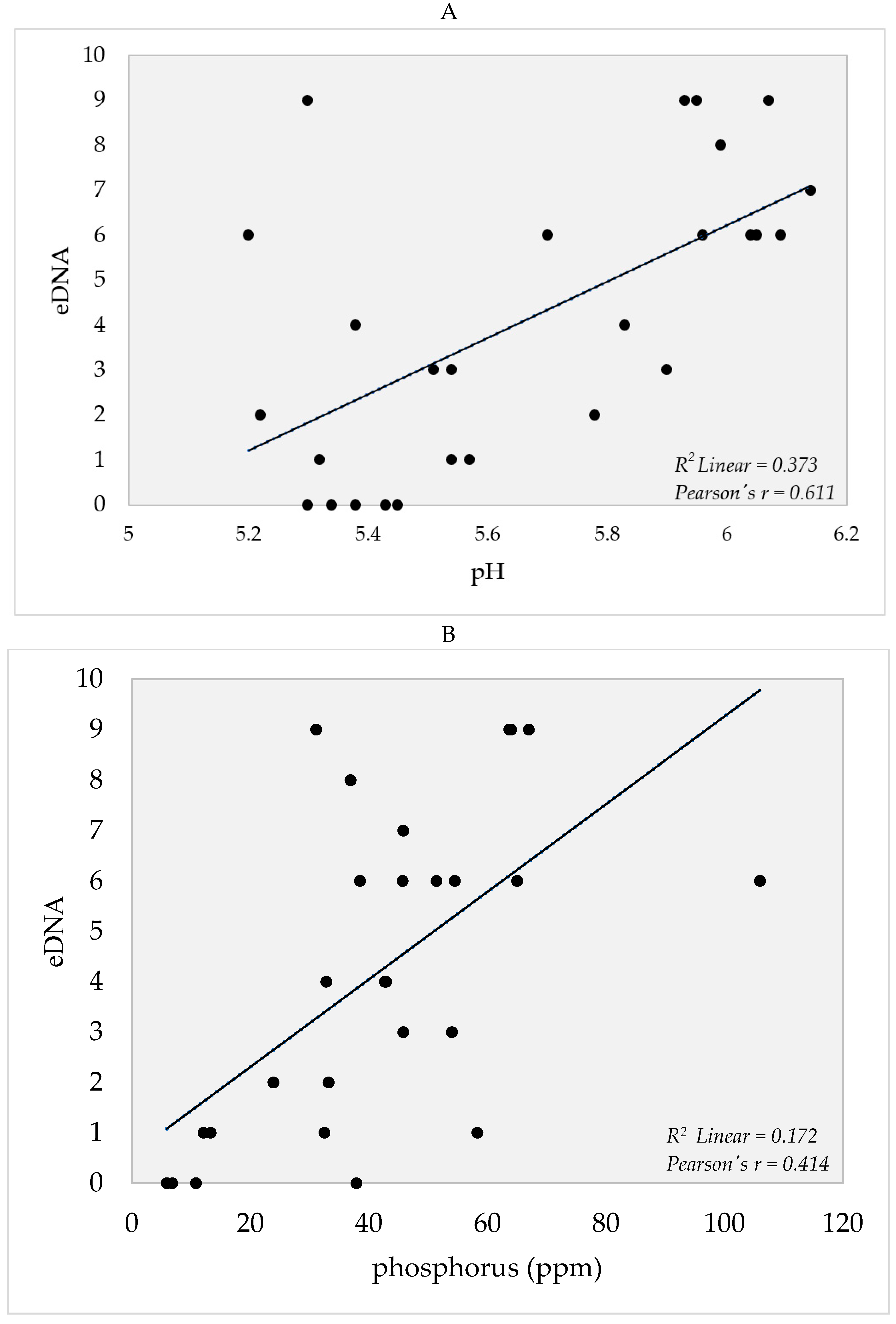
4. Soil Edaphic Factors
5. Discussion
5.1. Detection of eDNA of O. hupensis quadrasi from Soil Samples via Conventional PCR and qPCR
5.2. O. hupensis quadrasi eDNA Potential Distribution
5.3. Edaphic Factors and eDNA Detection
6. Conclusions and Recommendations
7. Materials and Methods
7.1. Soil Sampling, Malacological Survey, and Mapping of Snail Sites
7.2. Storage of Soil Samples and Measurement of Edaphic Factors
7.3. eDNA Extraction from Soil and Nanodrop Spectrophotometry
7.4. Detection via Conventional PCR and qPCR
7.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, C.H.; Dickman, K.; Tisch, D.J. Reassessment of the cost of chronic helmintic infection: A meta-Analysis of disability-Related outcomes in endemic schistosomiasis. Lancet 2005, 365, 1561–1569. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-Analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Schleinitz, M.D.; Carabin, H.; McGarvey, S.T. Decision-Model estimation of the age-Specific disability weight for schistosomiasis japonica: A systematic review of the literature. PLoS Negl. Trop. Dis. 2008, 2, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, H.; Chen, X.; Zhang, L.; Wang, K.; Guo, J.; Huang, Z.; Zhang, B.; Huang, W.; Jin, K.; et al. The Schistosoma japonicum genome reveals features of host-Parasite interplay. Nature 2009, 460, 345. [Google Scholar]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Olveda, D.U.; Li, Y.; Olveda, R.M.; Lam, A.K.; McManus, D.P.; Chau, T.N.P.; Harn, D.A.; Williams, G.M.; Gray, D.J.; Ross, A.G.P. Bilharzia in the Philippines: Past, present, and future. Int. J. Infect. Dis. 2014, 18, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, L.; Chigusa, Y.; Kikuchi, M.; Kato-Hayashi, N.; Kawazu, S.I.; Angeles, J.M.; Fontanilla, I.K.; Tabios, I.K.; Moendeg, K.; Goto, Y.; et al. Schistosomiasis in the Philippines: Challenges and some successes in control. Southeast Asian J. Trop. Med. Public Health 2016, 47, 651–666. [Google Scholar]
- Blas, B. Handbook for the control of Schistosomiasis japonica. Monogr. Schist Jpn. Infect. Philipp. 1991, 72, 745–753. [Google Scholar]
- Ross, A.G.P.; Sleigh, A.C.; Li, Y.; Davis, G.M.; Williams, G.M.; Jiang, Z.; Feng, Z.; Manus, D.P.M.C. Schistosomiasis in the People ’ s Republic of China: Prospects and Challenges for the 21st Century. Clin. Microbiol. Rev. 2001, 14, 270–295. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Y.; Ye, P.; Lin, J.; Cai, Y.; Shen, W.; Bickle, Q.D.; Taylor, M.G. Laboratory and field evaluation of Schistosoma japonicum DNA vaccines in sheep and water buffalo in China. Vaccine 2001, 20, 462–467. [Google Scholar] [CrossRef]
- Blas, B.L.; Rosales, M.I.; Lipayon, I.L.; Yasuraoka, K.; Matsuda, H.; Hayashi, M. The schistosomiasis problem in the Philippines: A review. Parasitol. Int. 2004, 53, 127–134. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, S.T.; Carabin, H.; Balolong, E., Jr.; Bélisle, P.; Fernandez, T.; Joseph, L.; Tallo, V.; Gonzales, R.; Tarafder, M.R.; Alday, P.; et al. Cross-Sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar province, Philippines. Bull. World Health Organ. 2006, 84, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Conlan, J.V.; Sripa, B.; Attwood, S.; Newton, P.N. A review of parasitic zoonoses in a changing Southeast Asia. Vet. Parasitol. 2011, 182, 22–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.P.; Maria, V.J.; Zhang, S.Q.; Wang, F.F.; Wu, W.D.; Zhang, G.H.; Pan, X.P.; Ju, Y.; Niels, Ø. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005, 96, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, H.; Iwanaga, Y.; Nara, T.; Matsuda, H.; Yasuraoka, K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol. Int. 2003, 52, 409–417. [Google Scholar] [CrossRef]
- Tanaka, H.; Tsuji, M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int. J. Parasitol. 1997, 27, 1465–1480. [Google Scholar] [CrossRef]
- Minggang, C.; Zheng, F. Schistosomiasis control in China. Parasitol. Int. 1999, 48, 11–19. [Google Scholar] [CrossRef]
- Chua, C.J.; Tabios, I.K.; Tamayo, P.G.; Leonardo, L.; Fontanilla, I.K.C.; De Chavez, E.R.C.; Agatsuma, T.; Kikuchi, M.; Kato-Hayashi, N.; Chigusa, Y. Genetic Comparison of Oncomelania hupensis quadrasi Genetic Comparison of Oncomelania hupensis quad rasi (Möllendorf, 1895) (Gastropoda: Pomatiopsidae), the Intermediate Host of Schistosoma japonicum in the Philippines, Based on 16S Ribosomal RNA Sequence. Sci. Diliman 2017, 292, 32–50. [Google Scholar]
- Leonardo, L.; Rivera, P.; Saniel, O.; Solon, J.A.; Chigusa, Y.; Villacorte, E.; Chua, J.C.; Moendeg, K.; Manalo, D.; Crisostomo, B.; et al. New endemic foci of schistosomiasis infections in the Philippines. Acta Trop. 2015, 141, 354–360. [Google Scholar] [CrossRef]
- Pesigan, T.P.; Hairston, N.G.; Jauregui, J.J.; Garcia, E.G.; Santos, A.T.; Santos, B.C.; Besa, A.A. Studies on Schistosoma japonicum infection in the Philippines: 2. The molluscan host. Bull. World Health Organ. 1958, 18, 481. [Google Scholar] [PubMed]
- Nihei, N.; Kanazawa, T.; Blas, B.L.; Saitoh, Y.; Itagaki, H.; Pangilinan, R.; Matsuda, H.; Yasuraoka, K. Soil factors influencing the distribution of Oncomelania quadrasi, the intermediate host of Schistosoma japonicum, on Bohol Island, Philippines. Ann. Trop. Med. Parasitol. 1998, 92, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Mcmullen, D.B. The Control Of Schistosomiasis japonica: I. Observations on the habits, ecology and Life Cycle of Oncomelania quadrasi, The Molluscan Intermediate Host Of Schistosoma japonicum in the Philippine Islands. Am. J. Epidemiol. 1947, 45, 259–273. [Google Scholar] [CrossRef]
- Pm, B.; Ingalls, J.W., Jr. The molluscan intermediate host and schistosomiasis japonica; observations on the production and rate of emergence of cercariae of Schistosoma japonicum from the molluscan intermediate host, Oncomelania quadrasi. Am. J. Trop. Med. Hyg. 1948, 28, 567–575. [Google Scholar]
- Belizario, V.Y.; Martinez, R.M.; de Leon, W.U.; Esparar, D.G.; Navarro, J.R.P.; Villar, L.C.; Sunico, L.S.; Velasco, L.R.; Sison, S.M. Cagayan Valley: A newly described endemic focus for schistosomiasis japonicum in the Philippines. Philipp. J. Intern. Med. 2005, 43, 117–122. [Google Scholar]
- Leonardo, L.R.; Acosta, L.P.; Olveda, R.M.; Aligui, G.D.L. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Trop. 2002, 82, 295–299. [Google Scholar] [CrossRef]
- Fornillos, R.J.C.; Fontanilla, I.K.C.; Chigusa, Y.; Kikuchi, M.; Kirinoki, M.; Kato-Hayashi, N.; Kawazu, S.; Angeles, J.M.; Tabios, I.K.; Moendeg, K.; et al. Infection rate of Schistosoma japonicum in the snail Oncomelania hupensis quadrasi in endemic villages in the Philippines: Need for snail surveillance technique o Title. Trop. Biomed. 2019, 36, 402–411. [Google Scholar]
- Driscoll, A.J.; Kyle, J.L.; Remais, J. Development of a novel PCR assay capable of detecting a single Schistosoma japonicum cercaria recovered from Oncomelania hupensis. Parasitology 2005, 131, 497–500. [Google Scholar] [CrossRef]
- Hung, Y.W.; Remais, J. Quantitative detection of Schistosoma japonicum cercariae in water by real-Time PCR. PLoS Negl. Trop. Dis. 2008, 2, 337. [Google Scholar] [CrossRef]
- Sato, M.O.; Rafalimanantsoa, A.; Ramarokoto, C.; Rahetilahy, A.M.; Ravoniarimbinina, P.; Kawai, S.; Minamoto, T.; Sato, M.; Kirinoki, M.; Rasolofo, V.; et al. Usefulness of environmental DNA for detecting Schistosoma mansoni occurrence sites in Madagascar. Int. J. Infect. Dis. 2018, 76, 130–136. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Hellström, M.; Kariuki, H.C.; Olsen, A.; Thomsen, P.F.; Mejer, H.; Willerslev, E.; Mwanje, M.T.; Madsen, H.; Kristensen, T.K.; et al. Environmental DNA for improved detection and environmental surveillance of schistosomiasis. Proc. Natl. Acad. Sci. USA 2019, 116, 8931–8940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Google Earth. 2019. Available online: https://earth.google.com/web/@18.27337328,122.10486135,232.93976988a,88309.32932935d,35y,0h,0t, 0r/data=ChMaEQoJL20vMDZqX2hzGAIgASgC No Title (accessed on 31 July 2019).
- Wilfinger, W.W.; Mackey, K.; Chomczynski, P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 1997, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. JoVE 2010, 45, 2565. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Valentini, A.; Dejean, T.; Montarsi, F.; Taberlet, P.; Glaizot, O.; Fumagalli, L. Detection of invasive mosquito vectors using environmental DNA (eDNA) from water samples. PLoS ONE 2016, 11, e0162493. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.S.; Groombridge, J.J.; Griffiths, R.A. Seasonal variation in environmental DNA detection in sediment and water samples. PLoS ONE 2018, 13, e0191737. [Google Scholar] [CrossRef] [PubMed]
- Ellison, S.L.R.; English, C.A.; Burns, M.J.; Keer, J.T. Routes to improving the reliability of low level DNA analysis using real-time PCR. BMC Biotechnol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Douglas, W.Y.; De Bruyn, M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef]
- Herder, J.; Valentini, A.; Bellemain, E.; Dejean, T.; Van Delft, J.; Thomsen, P.F.; Taberlet, P. Environmental DNA- A Review of the Possible Applications for the Detection of (Invasive) Species; Technical Report: Report number: 2013-104; RAVON: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.M.; Gough, K.C. The detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Turner, C.R.; Deiner, K.; Klymus, K.E.; Thomsen, P.F.; Murphy, M.A.; Spear, S.F.; McKee, A.; Oyler-McCance, S.J.; Cornman, R.S.; et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods Ecol. Evol. 2016, 7, 1299–1307. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Møller, P.R.; Rasmussen, M.; Willerslev, E. Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLoS ONE 2012, 7, e41732. [Google Scholar] [CrossRef] [PubMed]
- Eichmiller, J.J.; Best, S.E.; Sorensen, P.W. Effects of temperature and trophic state on degradation of environmental DNA in lake water. Environ. Sci. Technol. 2016, 50, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.T.J.; Chase, J.M.; Dosch, K.L.; Hartson, R.B.; Gross, J.A.; Larson, D.J.; Sutherland, D.R.; Carpenter, S.R. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl. Acad. Sci. USA 2007, 104, 15781–15786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R. The potassium paradox: Implications for soil fertility, crop production and human health. Renew. Agric. Food Syst. 2014, 29, 3–27. [Google Scholar] [CrossRef]
- Shuhaimi-Othman, M.; Nur-Amalina, R.; Nadzifah, Y. Toxicity of metals to a freshwater snail, Melanoides tuberculata. Sci. World J. 2012, 2012, 125785. [Google Scholar] [CrossRef]
- Gawad, S.S.A. Acute toxicity of some heavy metals to the fresh water snail, Theodoxus niloticus (Reeve, 1856). Egypt. J. Aquat. Res. 2018, 44, 83–87. [Google Scholar] [CrossRef]
- Moreno, L.I.; McCord, B.R. Understanding metal inhibition: The effect of copper (Cu2+) on DNA containing samples. Forensic Chem. 2017, 4, 89–95. [Google Scholar] [CrossRef]
- Cervantes-Cervantes, M.P.; Calderón-Salinas, J.V.; Albores, A.; Muñoz-Sánchez, J.L. Copper increases the damage to DNA and proteins caused by reactive oxygen species. Biol. Trace Elem. Res. 2005, 103, 229–248. [Google Scholar] [CrossRef]
- Jove, J.A. Use of molluscicides in the control of bilharziasis in Venezuela: Equipment and methods of application. Bull. World Health Organ. 1956, 14, 617. [Google Scholar]
- Chandiwana, S.K.; Ndamba, J.; Makura, O.; Taylor, P. Field evaluation of controlled release copper glass as a molluscicide in snail control. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 952–955. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Abdel-Ghaffar, F.; Bakry, F.A.; Sayed, D.A. Ecotoxicological effect of sublethal exposure to zinc oxide nanoparticles on freshwater snail Biomphalaria alexandrina. Arch. Environ. Contam. Toxicol. 2014, 67, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.Y.; Yang, X.H.; Yang, Y.; Feng, H.H.; Hu, X.Y.; Wang, X.Y. The Facile Preparation and the Performance of Killing Oncomelania Snails of Zinc Oxide Nanoparticles. Adv. Mater. Res. 2014, 1078, 40–44. [Google Scholar] [CrossRef]
- Bolyard, S. Fate of Zinc Oxide in Landfill Leachate. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2012. [Google Scholar]
- Zhou, X.N.; Wang, L.Y.; Chen, M.G.; Wu, X.H.; Jiang, Q.W.; Chen, X.Y.; Zheng, J.; Jürg, U. The public health significance and control of schistosomiasis in China—Then and now. Acta Trop. 2005, 96, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mänd, R.; Tilgar, V.; Leivits, A. Calcium, snails, and birds: A case study. Web Ecol. 2000, 1, 63–69. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calata, F.I.C.; Caranguian, C.Z.; Mendoza, J.E.M.; Fornillos, R.J.C.; Tabios, I.K.B.; Fontanilla, I.K.C.; Leonardo, L.R.; Sunico, L.S.; Kawai, S.; Chigusa, Y.; et al. Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania hupensis quadrasi. Pathogens 2019, 8, 160. https://doi.org/10.3390/pathogens8040160
Calata FIC, Caranguian CZ, Mendoza JEM, Fornillos RJC, Tabios IKB, Fontanilla IKC, Leonardo LR, Sunico LS, Kawai S, Chigusa Y, et al. Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania hupensis quadrasi. Pathogens. 2019; 8(4):160. https://doi.org/10.3390/pathogens8040160
Chicago/Turabian StyleCalata, Fritz Ivy C., Camille Z. Caranguian, Jillian Ela M. Mendoza, Raffy Jay C. Fornillos, Ian Kim B. Tabios, Ian Kendrich C. Fontanilla, Lydia R. Leonardo, Louie S. Sunico, Satoru Kawai, Yuichi Chigusa, and et al. 2019. "Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania hupensis quadrasi" Pathogens 8, no. 4: 160. https://doi.org/10.3390/pathogens8040160
APA StyleCalata, F. I. C., Caranguian, C. Z., Mendoza, J. E. M., Fornillos, R. J. C., Tabios, I. K. B., Fontanilla, I. K. C., Leonardo, L. R., Sunico, L. S., Kawai, S., Chigusa, Y., Kikuchi, M., Sato, M., Minamoto, T., Baoanan, Z. G., & Sato, M. O. (2019). Analysis of Environmental DNA and Edaphic Factors for the Detection of the Snail Intermediate Host Oncomelania hupensis quadrasi. Pathogens, 8(4), 160. https://doi.org/10.3390/pathogens8040160








