Clinical Characteristics of Acute Hepatitis E and Their Correlation with HEV Genotype 3 Subtypes in Italy
Abstract
:1. Introduction
2. Results
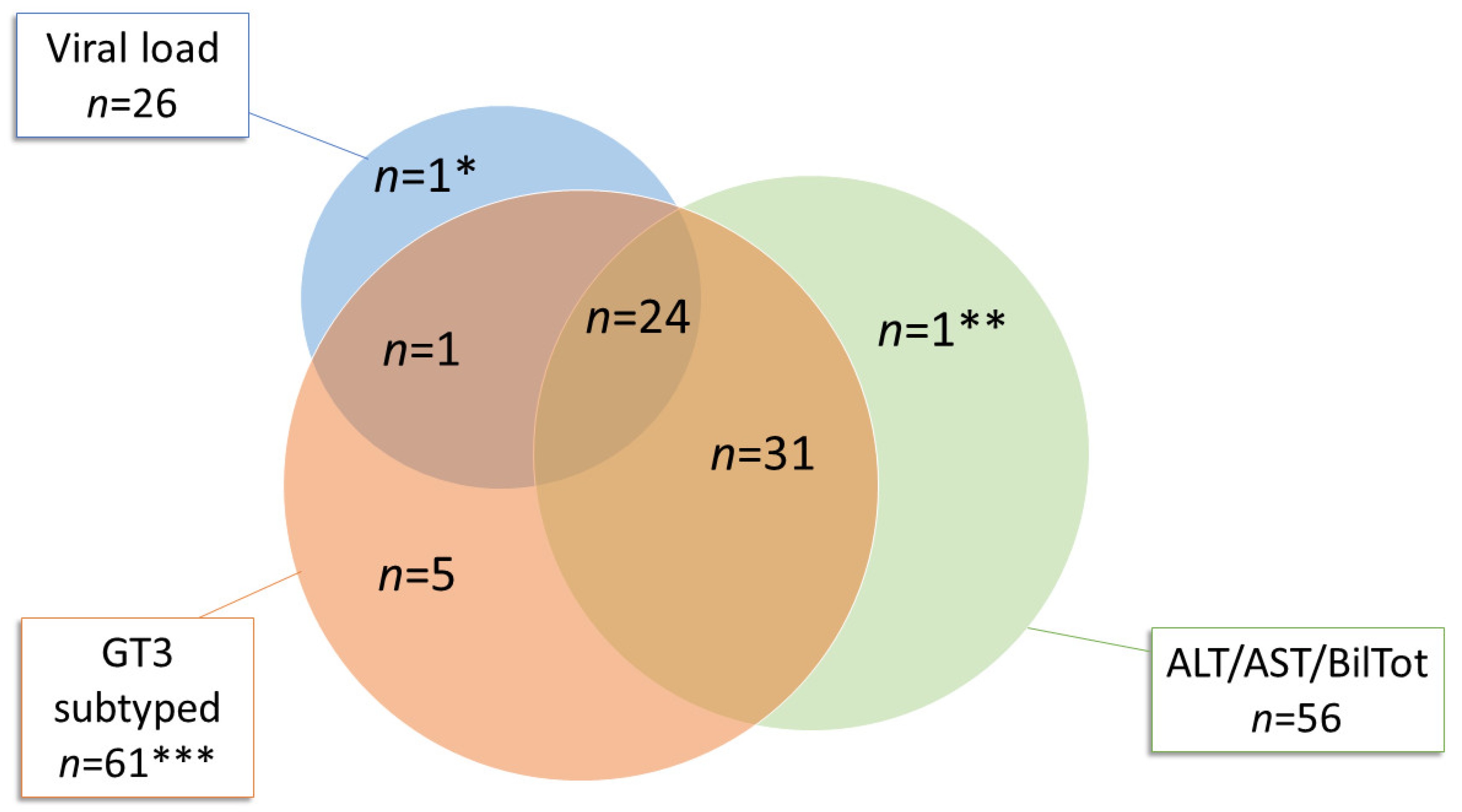
2.1. Acute Hepatitis E Diagnosed between 2011 and 2019
2.2. Genotype and Phylogenetic Analysis
2.3. Clinical Manifestations in HEV GT3 Acute Hepatitis
3. Discussion
4. Methods
4.1. Study Design
4.2. HEV RNA Detection and Sequencing
4.3. Phylogenetic Analysis
4.4. HEV RNA Quantification
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Purdy, M.A.; Members Of The International Committee On The Taxonomy Of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.-J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E virus infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbuglia, A.R.; Scognamiglio, P.; Petrosillo, N.; Mastroianni, C.M.; Sordillo, P.; Gentile, D.; La Scala, P.; Girardi, E.; Capobianchi, M.R. Hepatitis E Virus Genotype 4 Outbreak, Italy, 2011. Emerg. Infect. Dis. 2013, 19, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouamra, Y.; Gerolami, R.; Arzouni, J.-P.; Grimaud, J.-C.; Lafforgue, P.; Nelli, M.; Tivoli, N.; Ferretti, A.; Motte, A.; Colson, P. Emergence of Autochthonous Infections with Hepatitis E Virus of Genotype 4 in Europe. Intervirology 2014, 57, 43–48. [Google Scholar] [CrossRef]
- Rasche, A.; Saqib, M.; Liljander, A.M.; Bornstein, S.; Zohaib, A.; Renneker, S.; Steinhagen, K.; Wernery, R.; Younan, M.; Gluecks, I.; et al. Hepatitis E Virus Infection in Dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983–2015. Emerg. Infect. Dis. 2016, 22, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.; Teng, J.L.; Cao, K.-Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.; Wong, P.-C.; Wong, E.Y.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef]
- Pérez-Gracia, M.T.; Garcia, M.; Suay, B.; Mateos-Lindemann, M.L. Current Knowledge on Hepatitis E. J. Clin. Transl. Hepatol. 2015, 3, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Khuroo, M.S.; Khuroo, M.S. Hepatitis E: An emerging global disease–from discovery towards control and cure. J. Viral. Hepat. 2016, 23, 68–79. [Google Scholar] [CrossRef]
- Adlhoch, C.; Avellón, A.; Baylis, S.A.; Ciccaglione, A.R.; Couturier, E.; De Sousa, R.; Epštein, J.; Ethelberg, S.; Faber, M.; Fehér, Á.; et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J. Clin. Virol. 2016, 82, 9–16. [Google Scholar] [CrossRef]
- Bura, M.; Lagiedo, M.; Michalak, M.; Sikora, J.; Mozer-Lisewska, I. Hepatitis E virus IgG seroprevalence in HIV patients and blood donors, west-central Poland. Int. J. Infect. Dis. 2017, 61, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Askar, M.; Stark, K. Case-control study on risk factors for acute hepatitis E in Germany, 2012 to 2014. Eurosurveillance 2018, 23, 17-00469. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J. Does chronic hepatitis E virus infection exist in immunocompetent patients? Hepatology 2014, 60, 427. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Epidemiology of Hepatitis E Virus in European Countries. Int. J. Mol. Sci. 2015, 16, 25711–25743. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, S.; Arnold, E.; Banks, M.; Bendall, R.P.; Cramp, M.E.; Cunningham, R.; Dalton, H.R.; Harrison, T.J.; Hill, S.F.; Macfarlane, L.; et al. Non–Travel-Associated Hepatitis E in England and Wales: Demographic, Clinical, and Molecular Epidemiological Characteristics. J. Infect. Dis. 2005, 192, 1166–1172. [Google Scholar] [CrossRef]
- Lanini, S.; Garbuglia, A.R.; Lapa, D.; Puro, V.; Navarra, A.; Pergola, C.; Ippolito, G.; Capobianchi, M.R. Epidemiology of HEV in the Mediterranean basin: 10-year prevalence in Italy. BMJ Open 2015, 5, e007110. [Google Scholar] [CrossRef] [Green Version]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, T.; Diekmann, J.; Eberhardt, M.; Knabbe, C.; Dreier, J. Hepatitis E in blood donors: Investigation of the natural course of asymptomatic infection, Germany, 2011. Eurosurveillance 2016, 21, 30332. [Google Scholar] [CrossRef] [Green Version]
- Wedemeyer, H.; Pischke, S.; Manns, M.P. Pathogenesis and Treatment of Hepatitis E Virus Infection. Gastroenterology 2012, 142, 1388–1397. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; Hepatitis E Incident Investigation Team. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef]
- Guillois, Y.; Abravanel, F.; Miura, T.; Pavio, N.; Vaillant, V.; Lhomme, S.; Le Guyader, F.S.; Rose, N.; Le Saux, J.-C.; King, L.A.; et al. High Proportion of Asymptomatic Infections in an Outbreak of Hepatitis E Associated With a Spit-Roasted Piglet, France, 2013. Clin. Infect. Dis. 2015, 62, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lens, S.; Mensa, L.; Gambato, M.; González, P.; Torras, X.; Soriano, G.; Costa, J.; Capón, A.; Londoño, M.-C.; Mariño, Z.; et al. HEV infection in two referral centers in Spain; epidemiology and clinical outcomes. J. Clin. Virol. 2015, 63, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.C.; Stableforth, W.; Thurairajah, P.; Hazeldine, S.; Remnarace, R.; Usama, W.; Farrington, L.; Hamad, N.; Sieberhagen, C.; Ellis, V.; et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- De Sabato, L.; Di Bartolo, I.; Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Molecular Characterization of HEV Genotype 3 in Italy at Human/Animal Interface. Front. Microbiol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Nakano, T.; Takahashi, M.; Takahashi, K.; Nagashima, S.; Suzuki, Y.; Nishigaki, Y.; Tomita, E.; Okano, H.; Oya, Y.; Shiraki, K.; et al. Hepatitis E virus subtype 3f strains isolated from Japanese hepatitis patients with no history of travel to endemic areas–The origin analyzed by molecular evolution. Virology 2018, 513, 146–152. [Google Scholar] [CrossRef]
- De Jong, M.C.M.; Diekmann, O. A method to calculate–for computer-simulated infections–the threshold value, R0, that predicts whether or not the infection will spread. Prev. Vet. Med. 1992, 12, 269–285. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Frankena, K.; Rutjes, S.A.; Wellenberg, G.J.; Husman, A.M.D.R.; Van Der Poel, W.H.; De Jong, M.C. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Veter. Res. 2008, 39, 40. [Google Scholar] [CrossRef] [Green Version]
- Iaconelli, M.; Ferraro, G.B.; Mancini, P.; Suffredini, E.; Veneri, C.; Ciccaglione, A.R.; Bruni, R.; Della Libera, S.; Bignami, F.; Brambilla, M.; et al. Nine-Year Nationwide Environmental Surveillance of Hepatitis E Virus in Urban Wastewaters in Italy (2011–2019). Int. J. Environ. Res. Public Health 2020, 17, 2059. [Google Scholar] [CrossRef] [Green Version]
- Maunula, L.; Kaupke, A.; Vasickova, P.; Söderberg, K.; Kozyra, I.; Lazic, S.; Van Der Poel, W.H.; Bouwknegt, M.; Rutjes, S.; Willems, K.A.; et al. Tracing enteric viruses in the European berry fruit supply chain. Int. J. Food Microbiol. 2013, 167, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ferrando, E.; Randazzo, W.; Pérez-Cataluña, A.; Sánchez, G. HEV Occurrence in Waste and Drinking Water Treatment Plants. Front. Microbiol. 2020, 10, 2937. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Gallian, P.; DiMeglio, C.; Assal, A.; Abravanel, F.; Tiberghien, P.; Izopet, J. Viral load and clinical manifestations of hepatitis E virus genotype 3 infections. J. Viral Hepat. 2019, 26, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Dimeglio, C.; Castanier, M.; Péron, J.M.; Kamar, N.; Lhomme, S.; Izopet, J. Does HEV-3 subtype play a role in the severity of acute hepatitis E? Liver Int. 2020, 40, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Grierson, S.; Der Honing, R.H.-V.; Martelli, F.; Johne, R.; Reetz, J.; Ulrich, R.G.; Pavio, N.; Van Der Poel, W.H.; Banks, M. Hepatitis E Virus in Pork Liver Sausage, France. Emerg. Infect. Dis. 2012, 19, 264–266. [Google Scholar] [CrossRef]
- Boxman, I.L.; Jansen, C.C.; Hägele, G.; Zwartkruis-Nahuis, A.; Tijsma, A.S.; Vennema, H. Monitoring of pork liver and meat products on the Dutch market for the presence of HEV RNA. Int. J. Food Microbiol. 2019, 296, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal Inactivation of Infectious Hepatitis E Virus in Experimentally Contaminated Food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imagawa, T.; Sugiyama, R.; Shiota, T.; Li, T.-C.; Yoshizaki, S.; Wakita, T.; Ishii, K. Evaluation of Heating Conditions for Inactivation of Hepatitis E Virus Genotypes 3 and 4. J. Food Prot. 2018, 81, 947–952. [Google Scholar] [CrossRef]
- Subissi, L.; Peeters, M.; Lamoral, S.; Klamer, S.; Suin, V.; Van Gucht, S. Subtype-specific differences in the risk of hospitalisation among patients infected with hepatitis E virus genotype 3 in Belgium, 2010-2018. Epidemiol. Infect. 2019, 147, e224. [Google Scholar] [CrossRef]
- Abravanel, F.; Mansuy, J.-M.; Dubois, M.; Kamar, N.; Péron, J.-M.; Rostaing, L.; Izopet, J. Hepatitis E Virus Genotype 3 Diversity, France. Emerg. Infect. Dis. 2009, 15, 110–114. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Peng, J.; Li, X.; Zhang, D.; Yan, J.; Zhang, Y.; Lu, C.; Xun, J.; Li, W.; et al. Investigation of underlying comorbidities as risk factors for symptomatic human hepatitis E virus infection. Aliment. Pharmacol. Ther. 2017, 45, 701–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rosa, G.; Muscillo, M.; Vennarucci, V.S.; Garbuglia, A.R.; La Scala, P.; Capobianchi, M.R. Hepatitis E virus in Italy: Molecular analysis of travel-related and autochthonous cases. J. Gen. Virol. 2011, 92, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- BLASTX. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 8 April 2020).
- Hillis, D.M.; Bull, J.J. An Empirical Test of Bootstrapping as a Method for Assessing Confidence in Phylogenetic Analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]








| Characteristics | HEV GT3 | HEV GT3c | HEV GT3e | HEV GT3f | p-Value |
|---|---|---|---|---|---|
| (n = 37) | (n = 6) | (n = 8) | (n = 21) | ||
| Age, years median (IQR) | 59 (52–68) | 59.5 (51.5–63) | 59 (49–66) | 65 (53–71) | 0.2 |
| Male sex (n, %) | 32 (86.5%) | 6 (100%) | 7 (87.5%) | 17 (80.9%) | 0.2 |
| BMI, kg/m2 | 24.66 ± 3.13 | 27.9 ± 4.9 | 24.6 ± 2.9 | 24.0 ± 2.9 | 0.6 |
| Alcohol user, (n, %) | 11/27 (40.7%) | 1/2 (50%) | 1/7 (14.3%) | 7/17 (41.2%) | 0.4 |
| Smoker, (n, %) | 8/26 (30.8%) | 1/2 (50%) | 1/6 (16.7%) | 5/16 (31.2%) | 0.6 |
| PWID, (n, %) | 2/37 (5.4%) | 0 | 0 | 2/21 (9.5%) | 0.9 |
| Chronic liver disease, (n, %) | 3/37 (8.1%) | 1 (16.7%) | 0 | 2 (9.5%) | 0.9 |
| Number of comorbidities | |||||
| 0 | 10 (27%) | 1 (16.7%) | 4 (50%) | 3 (14.2%) | |
| 1 | 15 (40.5%) | 2 (33.3%) | 2 (25%) | 11 (52.4%) | |
| 2 | 2 (5.4%) | 1 (16.7%) | 0 | 1 (4.7%) | |
| 3 | 7 (18.9%) | 2 (33.3%) | 2 (25%) | 3 (14.3%) | |
| 4 | 1 (2.7%) | 0 | 0 | 1 (4.7%) | |
| 5 | 2 (5.4%) | 0 | 0 | 2 (9.5%) | |
| Diabetes, (n, %) | 13 (35.1%) | 3 (50%) | 2 (25%) | 8 (38.1%) | 0.6 |
| Cardiovascular diseases, (n, %) | 8 (21.6%) | 1 (16.7%) | 1 (12.5%) | 6 (28.6%) | 0.6 |
| Lipid disorders, (n, %) | 7 (18.9%) | 1 (16.7%) | 1 (12.5%) | 5 (23.8%) | 0.7 |
| Renal diseases, (n, %) | 1 (2.7%) | 0 | 0 | 1 (4.8%) | |
| Arterial hypertension, (n, %) | 17 (45.9%) | 4 (66.7%) | 3 (37.5%) | 10 (47.6%) | 0.5 |
| Digestive diseases, (n, %) | 3 (8.1%) | 0 | 0 | 3 (14.3%) | 0.9 |
| Respiratory diseases, (n, %) | 3 (8.1%) | 1 (16.7%) | 1 (12.5%) | 1 (4.8%) | 0.6 |
| Neoplastic diseases, (n, %) | 2 (5.4%) | 0 | 0 | 2 (9.5%) | 0.9 |
| Characteristics | HEV GT3 (n = 37) | HEV GT3c (n = 6) | HEV GT3e (n = 8) | HEV GT3f (n = 21) | p-Value |
|---|---|---|---|---|---|
| Duration of hospitalization, days | 9 (7–11) | 9.5 (8.5–13.25) | 8 (7–8.75) | 10 (6.5–16.5) | 0.28 |
| Duration of symptoms before hospitalization, days | 7 (5–9.5) | 5.5 (2.7–15) | 9 (2.7–13.7) | 7 (5–8) | 0.80 |
| Clinical hepatitis | |||||
| Icteric | 15 (40.5%) | 2 (33.3%) | 3 (37.5%) | 9 (42.8%) | 0.9 |
| Anicteric | 22 (59.5%) | 4 (66.7%) | 5 (62.5%) | 12 (57.1%) | |
| Severe acute hepatitis and/or liver decompensation | 13 (36.1%) | 3 (50%) | 1 (12.5%) | 9 (42.8%) | 0.2 |
| Symptoms | |||||
| Asthenia | 18 (48.6%) | 3 (50% | 4 (50%) | 9 (42.8%) | 0.9 |
| Fever | 12 (32.4%) | 1 (16.7%) | 2 (25%) | 9 (42.8%) | 0.4 |
| Nausea/vomiting | 15 (40.5%) | 2 (33.3%) | 4 (50%) | 7 (33.3%) | 0.7 |
| Diarrhea | 4 (10.8%) | 1 (16.7%) | 1 (12.5%) | 2 (9.5%) | 0.9 |
| Epigastric pain | 13 (35.1%) | 3 (50%) | 6 (75%) | 4 (19%) | 0.01 |
| Arthralgia | 4 (10.8%) | 0 | 3 (37.5%) | 1 (4.8%) | |
| Extra-hepatic manifestations | 11 (29.7%) | 3 (50%) | 0 | 7 (33.3%) | 0.2 |
| Laboratory parameters at peak | |||||
| AST, U/L | 1167 (412.5–2088) | 1587.5 (613.25–3608.25) | 1210.5 (178.25–1912.7) | 1228 (284.5–2211.5) | 0.7 |
| ALT, U/L | 1751 (1.026–2810) | 2100.5 (1013–3629.75) | 1932.5 (525.5–2930) | 1591 (995.5–2736.5) | 0.6 |
| Total bilirubin, mg/dL | 7 (2.3–12.2) | 6.03 (1.4–17.1) | 3.5 (1.4–7.2) | 10 (2.8–19.2) | 0.3 |
| γGT, U/L | 265 (164.5–401.5) | 437 (168.7–646) | 399.5 (202–578.5) | 242 (148.5–336.5) | 0.1 |
| ALP | 317.5 (203.5–577.25) | 261 (165.5–539.7) | 403 (231.7–661.7) | 276 (204–465.7) | 0.6 |
| INR | 1.17 (1.08–1.5) | 1.6 (1.17–2.89) | 1.15 (1.03–1.21) | 1.15 (1.1–1.5) | 0.03 |
| Primer | Location, nt * | Sequence, 5′ -> 3′ | Amplicon Length, bp | |
|---|---|---|---|---|
| 1 round ORF1 | 1679 | 36–56 | CCAYCAGTTYATHAAGGCTCC | 348 bp |
| 1680 | 383–367 | TACCAVCGCTGRACRTC | ||
| 2 round ORF1 | 1681 | 53–71 | CTCCTGGCRTYACWACTGC | 172 bp |
| 1682 | 224–205 | GGRTGRTTCCAIARVACYTC | ||
| 1 round ORF2 | HE44 | 5934–5912 | CAAGGHTGGCGYTCKGTTGAGAC | 484 bp |
| HE40 | 6417–6395 | CCCTTRTCCTGCTGAGCRTTCTC | ||
| 2 round ORF2 | HE110 | 5922–5942 | GYTCKGTTGAGACCTCYGGGT | 457 bp |
| HE41 | 6378–6356 | TTMACWGTCRGCTCGCCATTGGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minosse, C.; Biliotti, E.; Lapa, D.; Rianda, A.; Marchili, M.; Luzzitelli, I.; Capobianchi, M.R.; McPhee, F.; Garbuglia, A.R.; D’Offizi, G. Clinical Characteristics of Acute Hepatitis E and Their Correlation with HEV Genotype 3 Subtypes in Italy. Pathogens 2020, 9, 832. https://doi.org/10.3390/pathogens9100832
Minosse C, Biliotti E, Lapa D, Rianda A, Marchili M, Luzzitelli I, Capobianchi MR, McPhee F, Garbuglia AR, D’Offizi G. Clinical Characteristics of Acute Hepatitis E and Their Correlation with HEV Genotype 3 Subtypes in Italy. Pathogens. 2020; 9(10):832. https://doi.org/10.3390/pathogens9100832
Chicago/Turabian StyleMinosse, Claudia, Elisa Biliotti, Daniele Lapa, Alessia Rianda, Mauro Marchili, Ilaria Luzzitelli, Maria Rosaria Capobianchi, Fiona McPhee, Anna Rosa Garbuglia, and Gianpiero D’Offizi. 2020. "Clinical Characteristics of Acute Hepatitis E and Their Correlation with HEV Genotype 3 Subtypes in Italy" Pathogens 9, no. 10: 832. https://doi.org/10.3390/pathogens9100832





