Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry
Abstract
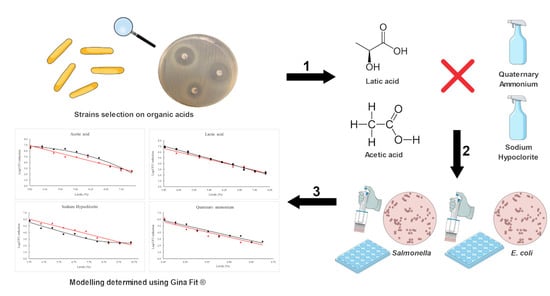
:1. Introduction
2. Results
2.1. Organic Acid in DiskxDiffusion Tests
2.2. Inactivation Modeling Using Organic Acid and Sanitizer Treatments
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Selection of Resistant Strains in Organic Acid Using Disk Diffusion Method
4.3. Organic Acid and Sanitizer Treatments and Enumeration of Survival Cells
4.4. Statistical Analyses and Mathematical Modeling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cunha-Neto, A.D.; Carvalho, L.A.; Castro, V.S.; Barcelos, F.G.; Carvalho, R.C.T.; Rodrigues, D.D.P.; Conte, C.A., Jr.; Figueiredo, E. Salmonella anatum, S. infantis and S. schwarzengrund in Brazilian Cheeses: Occurrence and antibiotic resistance profiles. Int. J. Dairy Technol. 2019, 73, 296–300. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.; Neto, A.D.C.; Mano, S.B.; Figueiredo, E.E.S.; Conte, C.A., Jr. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, V.S.; Rosario, D.K.; Mutz, Y.; Paletta, A.; Figueiredo, E.; Conte, C.A., Jr. Modelling inactivation of wild-type and clinical Escherichia coli O26 strains using UV-C and thermal treatment and subsequent persistence in simulated gastric fluid. J. Appl. Microbiol. 2019, 127, 1564–1575. [Google Scholar] [CrossRef]
- Castro, V.S.; Carvalho, R.C.; Conte, C.A., Jr.; Figuiredo, E.E.S. Shiga-toxin ProducingEscherichia coli: Pathogenicity, Supershedding, Diagnostic Methods, Occurrence, and Foodborne Outbreaks. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.D.C.; Carvalho, L.A.; Carvalho, R.C.; Rodrigues, D.D.P.; Mano, S.B.; Figueiredo, E.; Conte, C.A., Jr. Salmonella isolated from chicken carcasses from a slaughterhouse in the state of Mato Grosso, Brazil: Antibiotic resistance profile, serotyping, and characterization by repetitive sequence-based PCR system. Poult. Sci. 2018, 97, 1373–1381. [Google Scholar] [CrossRef]
- Blank, T.E.; Lacher, D.W.; Scaletsky, I.C.; Zhong, H.; Whittam, T.S.; Donnenberg, M.S. Enteropathogenic Escherichia coli O157 Strains from Brazil. Emerg. Infect. Dis. 2003, 9, 113–115. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [Green Version]
- Basler, C.; Nguyen, T.-A.; Anderson, T.C.; Hancock, T.; Behravesh, C.B. Outbreaks of Human Salmonella Infections Associated with Live Poultry, United States, 1990–2014. Emerg. Infect. Dis. 2016, 22, 1705–1711. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Pathogenicity Assessment of Shiga Toxin-producing Escherichia coli (STEC) and the Public Health Risk Posed by Contamination of Food with STEC. 2020. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.5967 (accessed on 10 August 2020).
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Daryaei, H.; Peñaloza, W.; Hildebrandt, I.; Krishnamurthy, K.; Thiruvengadam, P.; Wan, J. Heat inactivation of Shiga toxin-producing Escherichia coli in a selection of low moisture foods. Food Control. 2018, 85, 48–56. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.O.; Jin, T.Z.; Fan, X.; Olanya, O.M.; Juneja, V.K. Inactivation of Salmonella in grape tomato stem scars by organic acid wash and chitosan-allyl isothiocyanate coating. Int. J. Food Microbiol. 2018, 266, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.H.; Lee, S.Y.; Park, H.H.; Lee, E.S.; Min, S.C. Effects of the treatment parameters on the efficacy of the inactivation of Salmonella contaminating boiled chicken breast by in-package atmospheric cold plasma treatment. Int. J. Food Microbiol. 2019, 293, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Beuchat, L.R. Biofilm Formation and Sporulation by Bacillus cereus on a Stainless Steel Surface and Subsequent Resistance of Vegetative Cells and Spores to Chlorine, Chlorine Dioxide, and a Peroxyacetic Acid–Based Sanitizer. J. Food Prot. 2005, 68, 2614–2622. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Leus, I.V.; Weeks, J.W.; Wolloscheck, D.; Rybenkov, V.V.; Zgurskaya, H.I. Synergy between Active Efflux and Outer Membrane Diffusion Defines Rules of Antibiotic Permeation into Gram-Negative Bacteria. mBio 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.-Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef]
- Bağcı, U.; Temiz, A.; Bagci, U. Microbiological Quality of Fresh-Squeezed Orange Juice and Efficacy of Fruit Surface Decontamination Methods in Microbiological Quality. J. Food Prot. 2011, 74, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Inventory of Effective Food Contact Substance (FCS) Notifications. 2017. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FCN&id=1811 (accessed on 10 August 2020).
- Agência Nacional de Vigilância Sanitária. Resolução RDC nº 55, de 16 de dezembro de 2010. Dispõe Sobre o Registro de Produtos Biológicos Novos e Produtos Biológicos e dá Outras Providências. Diário Oficial da União 2010; 17 dez. Available online: http://portal.anvisa.gov.br/documents/10181/2718376/RDC_55_2010_COMP.pdf/bb86b1c8-d410-4a51-a9df-a61e165b9618 (accessed on 2 September 2020).
- López-Gálvez, F.; Allende, A.; Truchado, P.; Martínez-Sánchez, A.; Tudela, J.A.; Selma, M.V.; Gil, M.I. Suitability of aqueous chlorine dioxide versus sodium hypochlorite as an effective sanitizer for preserving quality of fresh-cut lettuce while avoiding by-product formation. Postharvest Biol. Technol. 2010, 55, 53–60. [Google Scholar] [CrossRef]
- Han, J.; Luo, X.; Zhang, Y.; Zhu, L.; Mao, Y.; Dong, P.; Yang, X.; Liang, R.; Hopkins, D.L.; Zhang, Y. Effects of spraying lactic acid and peroxyacetic acid on the bacterial decontamination and bacterial composition of beef carcasses. Meat Sci. 2020, 164, 108104. [Google Scholar] [CrossRef]
- Rosario, D.K.; Duarte, A.L.A.; Madalao, M.C.M.; Libardi, M.C.; Teixeira, L.J.Q.; Conte, C.A., Jr.; Bernardes, P.C. Ultrasound Improves Antimicrobial Effect of Sodium Hypochlorite and Instrumental Texture on Fresh-Cut Yellow Melon. J. Food Qual. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Duarte, A.L.A.; Rosario, D.K.; Oliveira, S.B.S.; De Souza, H.L.S.; De Carvalho, R.V.; Carneiro, J.C.S.; Silva, P.I.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. Int. J. Food Microbiol. 2018, 269, 12–18. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Expert Group for Technical Advice on Organic Production—EGTOP. Final Report on Plant protection (III). Directorate-Général for Agriculture and Rural Development 2016. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/final_report_egtop_on_cleaning_disinfectant_en.pdf (accessed on 2 September 2020).
- Yang, Y.; Mikš-Krajnik, M.; Zheng, Q.; Lee, S.-B.; Lee, S.-C.; Yuk, H.-G. Biofilm formation of Salmonella Enteritidis under food-related environmental stress conditions and its subsequent resistance to chlorine treatment. Food Microbiol. 2016, 54, 98–105. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Qiu, W.; Zhou, X.-D.; Li, H.; Lu, J.-Z.; Xu, H.H.; Peng, X.; Li, M.-Y.; Feng, M.-Y.; Cheng, L.; et al. Quaternary ammonium-induced multidrug tolerant Streptococcus mutans persisters elevate cariogenic virulence in vitro. Int. J. Oral Sci. 2017, 9, e7. [Google Scholar] [CrossRef] [Green Version]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Mani-López, E.; Garcia, H.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Generally Recognized as Safe (GRAS). 2019. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 10 August 2020).
- Amarowicz, R.; Pegg, R.B.; Bautista, D.A. Antibacterial activity of green tea polyphenols against Escherichia coliK 12. Food/Nahrung 2000, 44, 60–62. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Akbas, M.Y.; Cag, S. Use of organic acids for prevention and removal of Bacillus subtilis biofilms on food contact surfaces. Food Sci. Technol. Int. 2016, 22, 587–597. [Google Scholar] [CrossRef]
- Bragg, R.; Jansen, A.; Coetzee, M.; Van Der Westhuizen, W.; Boucher, C. Bacterial Resistance to Quaternary Ammonium Compounds (QAC) Disinfectants. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: New Delhi, India, 2014; Volume 808, pp. 1–13. [Google Scholar]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the Wide Use of Quaternary Ammonium Compounds Enhance the Selection and Spread of Antimicrobial Resistance and Thus Threaten Our Health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef]
- Deng, W.; Quan, Y.; Yang, S.; Guo, L.; Zhang, X.; Liu, S.; Chen, S.; Zhou, K.-Y.; He, L.; Li, B.; et al. Antibiotic Resistance in Salmonella from Retail Foods of Animal Origin and Its Association with Disinfectant and Heavy Metal Resistance. Microb. Drug Resist. 2018, 24, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagedar, A.; Singh, J. Evaluation of antibiofilm effect of benzalkonium chloride, iodophore and sodium hypochlorite against biofilm of Pseudomonas aeruginosa of dairy origin. J. Food Sci. Technol. 2014, 52, 5317–5322. [Google Scholar] [CrossRef] [Green Version]
- Vetas, D.; Dimitropoulou, E.; Mitropoulou, G.; Kourkoutas, Y.; Giaouris, E. Disinfection efficiencies of sage and spearmint essential oils against planktonic and biofilm Staphylococcus aureus cells in comparison with sodium hypochlorite. Int. J. Food Microbiol. 2017, 257, 19–25. [Google Scholar] [CrossRef]
- Vogeleer, P.; Tremblay, Y.D.; Jubelin, G.; Jacques, M.; Harel, J. Biofilm-Forming Abilities of Shiga Toxin-Producing Escherichia coli Isolates Associated with Human Infections. Appl. Environ. Microbiol. 2015, 82, 1448–1458. [Google Scholar] [CrossRef] [Green Version]
- Borges, K.A.; Furian, T.Q.; De Souza, S.N.; Menezes, R.; De Lima, D.A.; Fortes, F.B.B.; Salle, C.T.P.; Moraes, H.L.S.; Nascimento, V.P. Biofilm formation by Salmonella Enteritidis and Salmonella Typhimurium isolated from avian sources is partially related with their in vivo pathogenicity. Microb. Pathog. 2018, 118, 238–241. [Google Scholar] [CrossRef]
- Köhler, A.T.; Rodloff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Efficacy of sodium hypochlorite against multidrug-resistant Gram-negative bacteria. J. Hosp. Infect. 2018, 100, e40–e46. [Google Scholar] [CrossRef]
- Salim, A.P.A.; Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Simoes, J.S.; Panzenhagen, P.H.N.; Costa, M.P.; Franco, R.M.; Silva, T.J.P.; Conte, C.A., Jr. Inhibitory effect of acid concentration, aging, and different packaging onEscherichia coliO157:H7 and on color stability of beef. J. Food Process. Preserv. 2017, 42, e13402. [Google Scholar] [CrossRef]
- Food Safety and Inspection Service (FSIS). Safe and Suitable Ingredients September 2019. Available online: https://www.fsis.usda.gov/wps/wcm/connect/ce40e7ae-3d55-419e-9c68-a1b6fefcd4de/7120.1_table_2.pdf?MOD=AJPERES (accessed on 2 September 2020).
- Huang, Y.; Chen, H. Effect of organic acids, hydrogen peroxide and mild heat on inactivation of Escherichia coli O157:H7 on baby spinach. Food Control. 2011, 22, 1178–1183. [Google Scholar] [CrossRef]
- Bang, I.S.; Kim, B.H.; Foster, J.W.; Park, Y.K. OmpR Regulates the Stationary-Phase Acid Tolerance Response of Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2000, 182, 2245–2252. [Google Scholar] [CrossRef] [Green Version]
- Hamdallah, I.; Torok, N.; Bischof, K.M.; Majdalani, N.; Chadalavada, S.; Mdluli, N.; Creamer, K.E.; Clark, M.; Holdener, C.; Basting, P.J.; et al. Experimental Evolution of Escherichia coli K-12 at High pH and with RpoS Induction. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Chapman, B.; Ross, T. Escherichia coli and Salmonella enterica Are Protected against Acetic Acid, but Not Hydrochloric Acid, by Hypertonicity. Appl. Environ. Microbiol. 2009, 75, 3605–3610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adil, S.; Banday, M.T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of Dietary Supplementation of Organic Acids on Performance, Intestinal Histomorphology, and Serum Biochemistry of Broiler Chicken. Vet. Med. Int. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, F.; Seribelli, A.A.; Medeiros, M.I.C.; Rodrigues, D.D.P.; Varani, A.M.; Luo, Y.; Allard, M.W.; Falcão, J.P. Phylogenetic and antimicrobial resistance gene analysis of Salmonella Typhimurium strains isolated in Brazil by whole genome sequencing. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.C.; Filho, R.P.; Kuaye, A.P.Y.; Andrade, L.N.; Chang, Y.-F.; Darini, A.L.D.C. Virulence potential of commensal multidrug resistant Escherichia coli isolated from poultry in Brazil. Infect. Genet. Evol. 2018, 65, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agência Nacional de Vigilância Sanitária. Portaria n° 326, de 30 de julho de 1997. Regulamento Técnico Sobre Condições Higiênico-sanitárias e de Boas Práticas de Fabricação para Estabelecimentos Produtores/Industrializadores de Alimentos. Diário Oficial da União 1997. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/svs1/1997/prt0326_30_07_1997.html (accessed on 10 August 2020).
- Agência Nacional de Vigilância Sanitária. Resolução RDC n° 110, de 6 de setembro de 2016. Dispõe sobre regulamento técnico para produtos saneantes categorizados como água sanitária e dá outras providências. Diário Oficial da União 2016. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/23530048/do1-2016-09-08-resolucao-rdc-n-110-de-6-de-setembro-de-2016-23530032 (accessed on 2 September 2020).
- Regulation E.U. No 101/2013 of 4 February 2013 Concerning the Use of Lactic Acid to Reduce Microbiological Surface Contamination on Bovine Carcases. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:034:0001:0003:EN:PDF (accessed on 2 September 2020).
- Castro, V.S.; Vieira, B.; Neto, A.D.C.; Figueiredo, E.; Conte, C.A., Jr. Acetic Acid Increased the Inactivation of Multi-drug Resistant Non-typhoidal Salmonella by Large-Scaffold Antibiotic. Indian J. Microbiol. 2019, 59, 508–513. [Google Scholar] [CrossRef]
- Zhitnitsky, D.; Rose, J.; Lewinson, O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, E.C.C.; Castro, V.S.; Neto, A.D.C.; Dos Santos, L.F.; Vallim, D.C.; Lisbôa, R.D.C.; Carvalho, R.C.; Conte, C.A., Jr.; Figueiredo, E. Escherichia coli O26 and O113:H21 on Carcasses and Beef from a Slaughterhouse Located in Mato Grosso, Brazil. Foodborne Pathog. Dis. 2018, 15, 653–659. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Hippurate hydrolysis test. In Biochemical Tests for the Identification of Medical Bacteria, 2nd ed.; Williams and Wilkins: Baltimore, MD, USA, 1980; pp. 141–162. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Bigelow, W.D.; Esty, J.R. The Thermal Death Point in Relation to Time of Typical Thermophilic Organisms. J. Infect. Dis. 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Geeraerd, A.; Herremans, C.; Van Impe, J. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Besten, H.M.W.D.; Mataragas, M.; Moezelaar, R.; Abee, T.; Zwietering, M.H. Quantification of the Effects of Salt Stress and Physiological State on Thermotolerance of Bacillus cereus ATCC 10987 and ATCC 14579. Appl. Environ. Microbiol. 2006, 72, 5884–5894. [Google Scholar] [CrossRef] [Green Version]



| Bacteria | Treatment | Model | 4-Log Reduction | R2 Adj | Kmax | Delta | MSE |
|---|---|---|---|---|---|---|---|
| Salmonella | Lactic acid | Log Linear | 3.92 | 0.97 | 2.43 ± 0.13 | - | 0.0511 |
| Escherichia coli | Lactic acid | Log Linear | 3.92 | 0.98 | 2.75 ± 0.11 | - | 0.0353 |
| Salmonella | Acetic acid | Log Linear | 3.92 | 0.96 | 2.52 ± 0.16 | - | 0.0803 |
| Escherichia coli | Acetic acid | Weibull | 7.37 | 0.95 | - | 5.03 ± 0.63 | 0.1162 |
| Salmonella | Sodium hypochlorite | Log Linear | 6.95 | 0.94 | 0.20 ± 0.02 | - | 0.0971 |
| Escherichia coli | Sodium hypochlorite | Log Linear/Tail | 5.43 | 0.96 | 0.25 ± 0.03 | - | 0.0524 |
| Salmonella | Quaternary ammonium | Log Linear | 0.45 | 0.88 | 0.54 ± 0.06 | - | 0.1774 |
| Escherichia coli | Quaternary ammonium | Log Linear | 0.45 | 0.94 | 0.52 ± 0.04 | - | 0.0765 |
| Strain | Resistance Profile 1 | Isolation Source | Reference |
|---|---|---|---|
| S.O:4,5 (S45-1) | AMP, ATM, CFL, CTF, GEN, SUL, SUT, TRI | Chicken meat | [5] |
| S.Agona (SAg-2) | AMP, ATM, CFL, CFO, SUL, SUT, TRI | Chicken meat | [5] |
| S.Abony (SAb-3) | AMP, ATM, CFL, CFO, CTF, SUL, TRI | Chicken meat | [5] |
| S.Infantis (SI-4) | AMP, ATM, CFL, CFO, SUL, TRI | Chicken meat | [5] |
| S.Shwarzengrund (SS-5) | SUL, SUT, TRI | Chicken meat | [5] |
| S.Typhimurium (ATCC) | - | Clinical strain | ATCC–23564 |
| E. coliO26 (E26-1) | - | Rectal swab of bovine | [57] |
| E. coliO26 (E26-2) | - | Hide swab | [57] |
| E. coliO26 (E26-3) | - | Rectal swab of bovine | [57] |
| E. coliO113:H21 (E113-4) | STR | Retail beef | [57] |
| E. coliO113:H21 (E113-5) | - | Carcass swab | [57] |
| E. coliO26 (ATCC) | - | Clinical strain | ATCC–2196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, V.S.; Mutz, Y.d.S.; Rosario, D.K.A.; Cunha-Neto, A.; Figueiredo, E.E.d.S.; Conte-Junior, C.A. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens 2020, 9, 849. https://doi.org/10.3390/pathogens9100849
Castro VS, Mutz YdS, Rosario DKA, Cunha-Neto A, Figueiredo EEdS, Conte-Junior CA. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens. 2020; 9(10):849. https://doi.org/10.3390/pathogens9100849
Chicago/Turabian StyleCastro, Vinicius Silva, Yhan da Silva Mutz, Denes Kaic Alves Rosario, Adelino Cunha-Neto, Eduardo Eustáquio de Souza Figueiredo, and Carlos Adam Conte-Junior. 2020. "Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry" Pathogens 9, no. 10: 849. https://doi.org/10.3390/pathogens9100849
APA StyleCastro, V. S., Mutz, Y. d. S., Rosario, D. K. A., Cunha-Neto, A., Figueiredo, E. E. d. S., & Conte-Junior, C. A. (2020). Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens, 9(10), 849. https://doi.org/10.3390/pathogens9100849