Profiles of Extrapulmonary Nontuberculous Mycobacteria Infections and Predictors for Species: A Multicenter Retrospective Study
Abstract
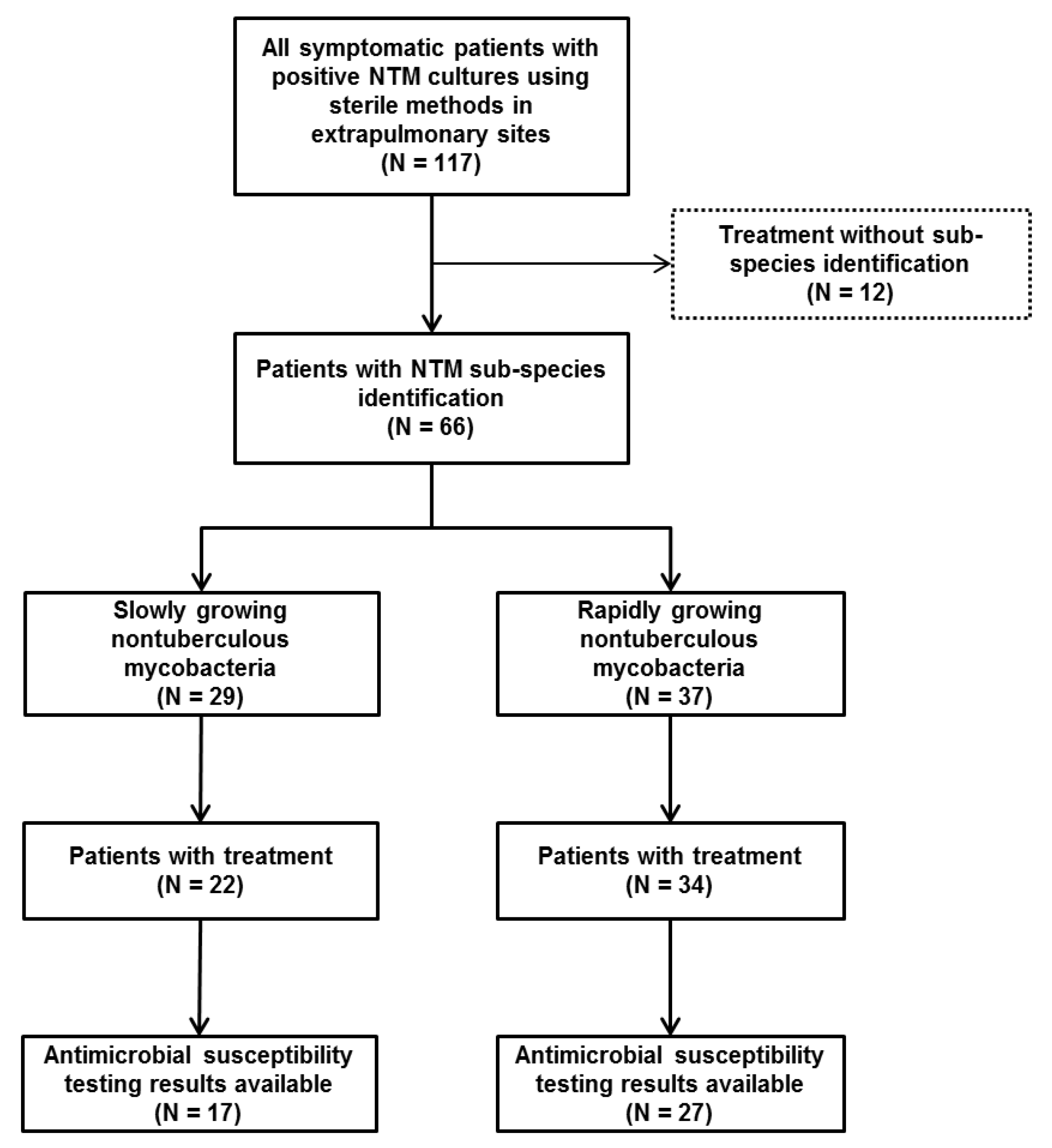
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Diversity and Sample Sites of NTM Infections
2.3. Antimicrobial Susceptibility Testing of NTM Infections
2.4. Antimicrobial Regimens in Patients with NTM Infections
2.5. Comparison of Clinical Characteristics and Predictors between SGM and RGM Infections
3. Discussion
4. Materials and Methods
4.1. Study Design and Case Definitions
4.2. Definitions of Variables
4.3. Specimen Processing and Antimicrobial Susceptibility Testing
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falkinham, J.O. Environmental sources of nontuberculous mycobacteria. Clin. Chest. Med. 2015, 36, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Machado, R.F.; Garcia, J.G.; Schraufnagel, D.E. Nontuberculous mycobacterial disease mortality in the united states, 1999-2010: A population-based comparative study. PLoS ONE 2014, 9, e91879. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tan, C.K.; Chou, C.H.; Hsu, H.L.; Liao, C.H.; Huang, Y.T.; Yang, P.C.; Luh, K.T.; Hsueh, P.R. Increasing incidence of nontuberculous mycobacteria, taiwan, 2000–2008. Emerg. Infect. Dis. 2010, 16, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Marras, T.K.; Mendelson, D.; Marchand-Austin, A.; May, K.; Jamieson, F.B. Pulmonary nontuberculous mycobacterial disease, ontario, canada, 1998–2010. Emerg. Infect. Dis. 2013, 19, 1889–1891. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ats/idsa statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Sheng, W.H.; Hung, C.C.; Yu, C.J.; Lee, L.N.; Hsueh, P.R. Mycobacterium abscessus complex infections in humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef]
- Lyman, M.M.; Grigg, C.; Kinsey, C.B.; Keckler, M.S.; Moulton-Meissner, H.; Cooper, E.; Soe, M.M.; Noble-Wang, J.; Longenberger, A.; Walker, S.R.; et al. Invasive nontuberculous mycobacterial infections among cardiothoracic surgical patients exposed to heater-cooler devices. Emerg. Infect. Dis. 2017, 23, 796–805. [Google Scholar] [CrossRef]
- Schnabel, D.; Esposito, D.H.; Gaines, J.; Ridpath, A.; Barry, M.A.; Feldman, K.A.; Mullins, J.; Burns, R.; Ahmad, N.; Nyangoma, E.N.; et al. Multistate us outbreak of rapidly growing mycobacterial infections associated with medical tourism to the dominican republic, 2013–2014. Emerg. Infect. Dis. 2016, 22, 1340–1347. [Google Scholar] [CrossRef] [Green Version]
- Hermansen, T.S.; Ravn, P.; Svensson, E.; Lillebaek, T. Nontuberculous mycobacteria in denmark, incidence and clinical importance during the last quarter-century. Sci. Rep. 2017, 7, 6696. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, P.M.; Hedberg, K.; Saulson, A.; McNelly, E.; Winthrop, K.L. Nontuberculous mycobacterial disease prevalence and risk factors: A changing epidemiology. Clin. Infect. Dis. 2009, 49, e124–e129. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.R.; Kasperbauer, S. Management of extrapulmonary nontuberculous mycobacterial infections. Semin. Respir. Crit. Care Med. 2018, 39, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Kothavade, R.J.; Dhurat, R.S.; Mishra, S.N.; Kothavade, U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Henkle, E.; Hedberg, K.; Schafer, S.D.; Winthrop, K.L. Surveillance of extrapulmonary nontuberculous mycobacteria infections, oregon, USA, 2007–2012. Emerg. Infect. Dis. 2017, 23, 1627–1630. [Google Scholar] [CrossRef]
- Morimoto, K.; Hasegawa, N.; Izumi, K.; Namkoong, H.; Uchimura, K.; Yoshiyama, T.; Hoshino, Y.; Kurashima, A.; Sokunaga, J.; Shibuya, S.; et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in japan from 2012 to 2013. Ann. Am. Thorac. Soc. 2017, 14, 49–56. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Benwill, J.L.; Wallace, R.J., Jr. Mycobacterium abscessus. “Pleased to meet you, hope you guess my name...”. Ann. Am. Thorac. Soc. 2015, 12, 436–439. [Google Scholar] [CrossRef]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An ntm-net collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in u.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Santiago, T.M.; Drage, L.A. Nontuberculous mycobacteria: Skin and soft tissue infections. Dermatol. Clin. 2015, 33, 563–577. [Google Scholar] [CrossRef]
- Shu, C.C.; Wang, J.Y.; Yu, C.J.; Lee, L.N. Mycobacterial arthritis of large joints. Ann. Rheum. Dis. 2009, 68, 1504–1505. [Google Scholar] [CrossRef]
- Blanc, P.; Dutronc, H.; Peuchant, O.; Dauchy, F.A.; Cazanave, C.; Neau, D.; Wirth, G.; Pellegrin, J.L.; Morlat, P.; Mercie, P.; et al. Nontuberculous mycobacterial infections in a french hospital: A 12-year retrospective study. PLoS ONE 2016, 11, e0168290. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.C.; Cassidy, P.M.; Perkins, K.M.; Crist, M.B.; Cieslak, P.R.; Leman, R.L. Extrapulmonary nontuberculous mycobacterial disease surveillance—Oregon, 2014–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 854–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.H.; Tsai, T.F.; Hsueh, P.R. Characteristics of skin and soft tissue infection caused by non-tuberculous mycobacteria in taiwan. Int. J. Tuberc. Lung Dis. 2011, 15, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, S.Y.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Kang, C.I.; Chung, D.R.; Peck, K.R.; Shin, S.J.; Koh, W.J. Mycobacteriological characteristics and treatment outcomes in extrapulmonary mycobacterium abscessus complex infections. Int. J. Infect. Dis. 2017, 60, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, M.A.; Ambrose, L.R.; Lipman, M.C.; Lowe, D.M. “Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Honda, J.R.; Alper, S.; Bai, X.; Chan, E.D. Acquired and genetic host susceptibility factors and microbial pathogenic factors that predispose to nontuberculous mycobacterial infections. Curr. Opin. Immunol. 2018, 54, 66–73. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; Ward, S.C.; Crist, C.J.; Mann, L.B.; Wilson, R.W. Activities of linezolid against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 2001, 45, 764–767. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Chen, C.Y.; Huang, C.T.; Ruan, S.Y.; Chou, C.H.; Lai, C.C.; Liao, C.H.; Tan, C.K.; Huang, Y.T.; Yu, C.J.; et al. Skin and soft-tissue infection caused by non-tuberculous mycobacteria in taiwan, 1997–2008. Epidemiol. Infect. 2011, 139, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Uslan, D.Z.; Kowalski, T.J.; Wengenack, N.L.; Virk, A.; Wilson, J.W. Skin and soft tissue infections due to rapidly growing mycobacteria: Comparison of clinical features, treatment, and susceptibility. Arch. Dermatol. 2006, 142, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Kham-Ngam, I.; Chetchotisakd, P.; Ananta, P.; Chaimanee, P.; Sadee, P.; Reechaipichitkul, W.; Faksri, K. Epidemiology of and risk factors for extrapulmonary nontuberculous mycobacterial infections in northeast thailand. Peer J. 2018, 6, e5479. [Google Scholar] [CrossRef] [Green Version]
- Chongwe, G.; Michelo, C.; Kelly, P. Diagnostic yield of nontuberculous mycobacteria in patients booked for endoscopy at the university teaching hospital, lusaka. BMC Res. Notes 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.J.; Choi, W.S.; Lee, S.Y.; Kim, K.S. The definition of past tuberculosis affects the magnitude of association between pulmonary tuberculosis and respiratory dysfunction: Korea national health and nutrition examination survey, 2008–2012. J. Korean Med. Sci. 2017, 32, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A.C. Immunosuppressive medications. Clin. J. Am. Soc. Nephrol. 2016, 11, 332–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Hu, D.; Yu, X.; Li, F.; Chen, E.; Wang, X.; Huang, D.; Lin, Z.; Lin, J. An outbreak of mycobacterium tuberculosis infection associated with acupuncture in a private clinic of zhejiang province, China, 2012. Int. J. Infect. Dis. 2014, 29, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piersimoni, C.; Scarparo, C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg. Infect. Dis. 2009, 15, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis, 3rd ed.; Korea Centers for Disease Control and Prevention: Cheongju, Korea, 2017.
- Lee, S.K.; Lee, E.J.; Kim, S.K.; Chang, J.; Jeong, S.H.; Kang, Y.A. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand. J. Infect. Dis. 2012, 44, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]

| Variables | N (%) |
|---|---|
| Age (years) | 53.1 ± 15.6 |
| Female sex, n (%) | 71 (60.7) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 24 (20.5) |
| HIV infection | 1 (0.9) |
| Solid organ cancer | 10 (8.5) |
| Hematologic malignancy | 4 (3.4) |
| History of previous tuberculosis | 6 (5.1) |
| Chronic kidney disease | 14 (12.0) |
| Chronic liver disease | 4 (3.4) |
| Connective tissue disease | 5 (4.3) |
| Immunosuppressive treatment | 16 (13.7) |
| Antibiotics treatment within 30 days | 40 (34.2) |
| Predisposing factors, n (%) | |
| Any surgery | 20 (17.1) |
| Orthopedic Surgery | 4 (3.4) |
| Plastic surgery | 4 (3.4) |
| Other surgery | 12 (10.3) |
| Local injection | 17 (14.5) |
| History of acupuncture | 6 (5.1) |
| Trauma | 6 (5.1) |
| Indwelling catheter | 4 (3.4) |
| Unknown | 64 (54.7) |
| Laboratory findings | |
| ESR (mm/h) | 33.0 (16.0–57.0) |
| CRP (mg/L) | 11.55 (2.90–57.05) |
| IGRA positivity, n (%) | 11/23 (47.8) |
| Rapidly Growing Mycobacteria | Slowly Growing Mycobacteria | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. fortuitum Complex (N = 9) | M. abscessus subsp. abscessus (N = 6) | M. abscessus subsp. massiliense (N = 7) | M. chelonae (N = 5) | M. intracellulare (N = 15) | M. kansasii (N = 1) | M. avium (N = 1) | |||||||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | |
| Amikacin | 8 | 0 | 1 | 4 | 0 | 1 | 7 | 0 | 0 | 2 | 1 | 2 | |||||||||
| Cefoxitin | 2 | 6 | 0 | 2 | 2 | 0 | 6 | 1 | 0 | 0 | 0 | 5 | |||||||||
| Ciprofloxacin | 8 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | 5 | 1 | 1 | 3 | |||||||||
| Clarithromycin | 4 | 1 | 3 | 1 | 0 | 3 | 6 | 0 | 1 | 4 | 1 | 15 | 0 | 0 | 1 | 0 | 0 | ||||
| Doxycycline | 4 | 0 | 4 | 0 | 0 | 4 | 0 | 1 | 6 | 2 | 0 | 3 | |||||||||
| Imipenem | 6 | 2 | 0 | 2 | 1 | 0 | 5 | 2 | 0 | 0 | 4 | 1 | |||||||||
| Moxifloxacin | 9 | 0 | 0 | 0 | 1 | 3 | 2 | 2 | 3 | 0 | 1 | 4 | 2 | 4 | 9 | 1 | 0 | 0 | 0 | 1 | 0 |
| TMPSMX | 3 | 0 | 5 | 0 | 0 | 4 | 4 | 0 | 3 | 0 | 0 | 5 | |||||||||
| Tobramycin | 3 | 1 | 1 | ||||||||||||||||||
| Linezolid | 6 | 2 | 0 | 3 | 0 | 0 | 6 | 0 | 1 | 4 | 0 | 1 | 0 | 8 | 7 | 0 | 0 | 1 | |||
| Isoniazid | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | ||||||||||||
| Pyrazinamide | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | ||||||||||||
| Rifabutin | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||
| Ethambutol | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||
| Rifampicin | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ||||||||||||
| Rapidly Growing Mycobacteria | Slowly Growing Mycobacteria | |||||||
|---|---|---|---|---|---|---|---|---|
| M. fortuitum complex | M. abscessus subsp. abscessus | M. abscessus subsp. massiliense | M. chelonae | M. intracellulare | M. kansasii | M. gordonae | M. marinum | |
| No of patients * | 13 | 8 | 7 | 6 | 18 | 2 | 1 | 1 |
| Median treatment duration (months) | 9.0 | 12.0 | 10.5 | 10.5 | 12.0 | 18.0 | 14.0 | 6.0 |
| Mean numbers of antimicrobial used % | 3.0 | 3.1 | 2.6 | 2.8 | 2.9 | 3.5 | 4.0 | 4.0 |
| Amikacin | 4 | 2 | 0 | 0 | 2 | 0 | 0 | 1 |
| Cefoxitin | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| Ethambutol | 4 | 1 | 1 | 1 | 13 | 2 | 1 | 0 |
| Fluoroquinolone x | 10 | 5 | 4 | 4 | 7 | 0 | 1 | 1 |
| Imipenem | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 |
| Isoniazid | 3 | 1 | 0 | 0 | 3 | 2 | 0 | 0 |
| Linezolid | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Macrolide ‡ | 8 | 8 | 7 | 6 | 15 | 1 | 1 | 1 |
| Pyrazinamide | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Rifamycin & | 5 | 2 | 1 | 1 | 16 | 2 | 1 | 0 |
| Tetracycline # | 4 | 3 | 0 | 2 | 1 | 0 | 0 | 1 |
| TMPSMX | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Univariate Analysis | |||
|---|---|---|---|
| Slowly Growing NTM (N = 29) | Rapidly Growing NTM (N = 37) | p Value | |
| Age (years) | 58.17 ± 15.36 | 51.84 ± 15.59 | 0.104 |
| Female sex, n (%) | 11 (37.9) | 30 (81.1) | <0.001 |
| Isolated sites, n (%) | <0.001 a | ||
| Skin and soft tissue | 2 (6.9) | 26 (70.3) | |
| Bone and joint infection | 18 (62.1) | 8 (21.6) | |
| Others | 9 (31.0) | 3 (8.1) | |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 6 (20.7) | 11 (29.7) | 0.580 |
| Solid organ cancer | 1 (3.4) | 2 (5.4) | >0.999 |
| Hematologic malignancy | 1 (3.4) | 2 (5.4) | >0.999 |
| History of tuberculosis | 3 (10.3) | 2 (5.4) | 0.647 |
| Chronic kidney disease | 2 (6.9) | 4 (10.8) | 0.688 |
| Chronic liver disease | 1 (3.4) | 1 (2.7) | >0.999 |
| Connective tissue disease | 1 (3.4) | 3 (8.1) | 0.625 |
| Immunosuppressive treatment | 3 (10.3) | 7 (18.9) | 0.493 |
| Antibiotics within 30 days | 9 (31.0) | 19 (51.4) | 0.160 |
| Predisposing factors, n (%) | 0.480 a | ||
| History of acupuncture | 1 (3.4) | 3 (8.1) | |
| Local injection | 4 (13.8) | 10 (27.0) | |
| Any surgery | 5 (17.2) | 9 (24.3) | |
| Orthopedic Surgery | 3 (10.3) | 1 (2.7) | |
| Plastic surgery | 0 (0.0) | 3 (8.1) | |
| Other surgery | 2 (6.9) | 5 (13.5) | |
| Trauma | 2 (6.9) | 2 (5.4) | |
| Indwelling catheter | 0 (0.0) | 2 (5.4) | |
| Laboratory findings | |||
| ESR (mm/h) | 41.0 (11.5–64.0) | 32.0 (22–60) | 0.692 |
| CRP (mg/L) | 10.0 (3.4–40.9) | 12.4 (1.8–59.8) | 0.913 |
| IGRA positivity, n (%) | 3/7 (42.9) | 4/9 (44.4) | >0.999 |
| Treatment | |||
| Surgical treatment, n (%) | 17 (58.6) | 23 (62.2) | 0.770 |
| Duration of medical treatment (months) | 12.0 (6.0–14.8) | 9.0 (6.0–13.5) | 0.683 |
| Treatment outcomes, n (%) | |||
| Cured | 22 (75.9) | 27 (73) | 0.790 |
| Died | 1 (3.4) | 2 (5.4) | 1.000 |
| Loss to follow up | 6 (20.7) | 8 (21.6) | 0.927 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 0.97 (0.94, 1.00) | 0.110 | 0.99 (0.94, 1.04) | 0.700 |
| Sex | ||||
| Female | 1 (ref.) | 1 (ref.) | ||
| Male | 7.01 (2.40, 22.63) | <0.001 | 5.30 (1.35, 24.26) | 0.020 |
| Isolated sites | ||||
| Skin and soft tissue | 1 (ref.) | 1 (ref.) | ||
| Bone and joint | 29.25 (6.65, 211.69) | <0.001 | 18.10 (3.28, 157.07) | 0.002 |
| Others | 1.33 (0.30, 7.14) | 0.720 | 2.13 (0.36, 16.67) | 0.430 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jung, I.Y.; Song, J.E.; Kim, E.J.; Kim, J.H.; Lee, W.J.; Seong, H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; et al. Profiles of Extrapulmonary Nontuberculous Mycobacteria Infections and Predictors for Species: A Multicenter Retrospective Study. Pathogens 2020, 9, 949. https://doi.org/10.3390/pathogens9110949
Kim JH, Jung IY, Song JE, Kim EJ, Kim JH, Lee WJ, Seong H, Ahn JY, Jeong SJ, Ku NS, et al. Profiles of Extrapulmonary Nontuberculous Mycobacteria Infections and Predictors for Species: A Multicenter Retrospective Study. Pathogens. 2020; 9(11):949. https://doi.org/10.3390/pathogens9110949
Chicago/Turabian StyleKim, Jung Ho, In Young Jung, Je Eun Song, Eun Jin Kim, Jun Hyoung Kim, Woon Ji Lee, Hye Seong, Jin Young Ahn, Su Jin Jeong, Nam Su Ku, and et al. 2020. "Profiles of Extrapulmonary Nontuberculous Mycobacteria Infections and Predictors for Species: A Multicenter Retrospective Study" Pathogens 9, no. 11: 949. https://doi.org/10.3390/pathogens9110949





