Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice
Abstract
:1. Introduction
2. Results
2.1. Soil Analysis
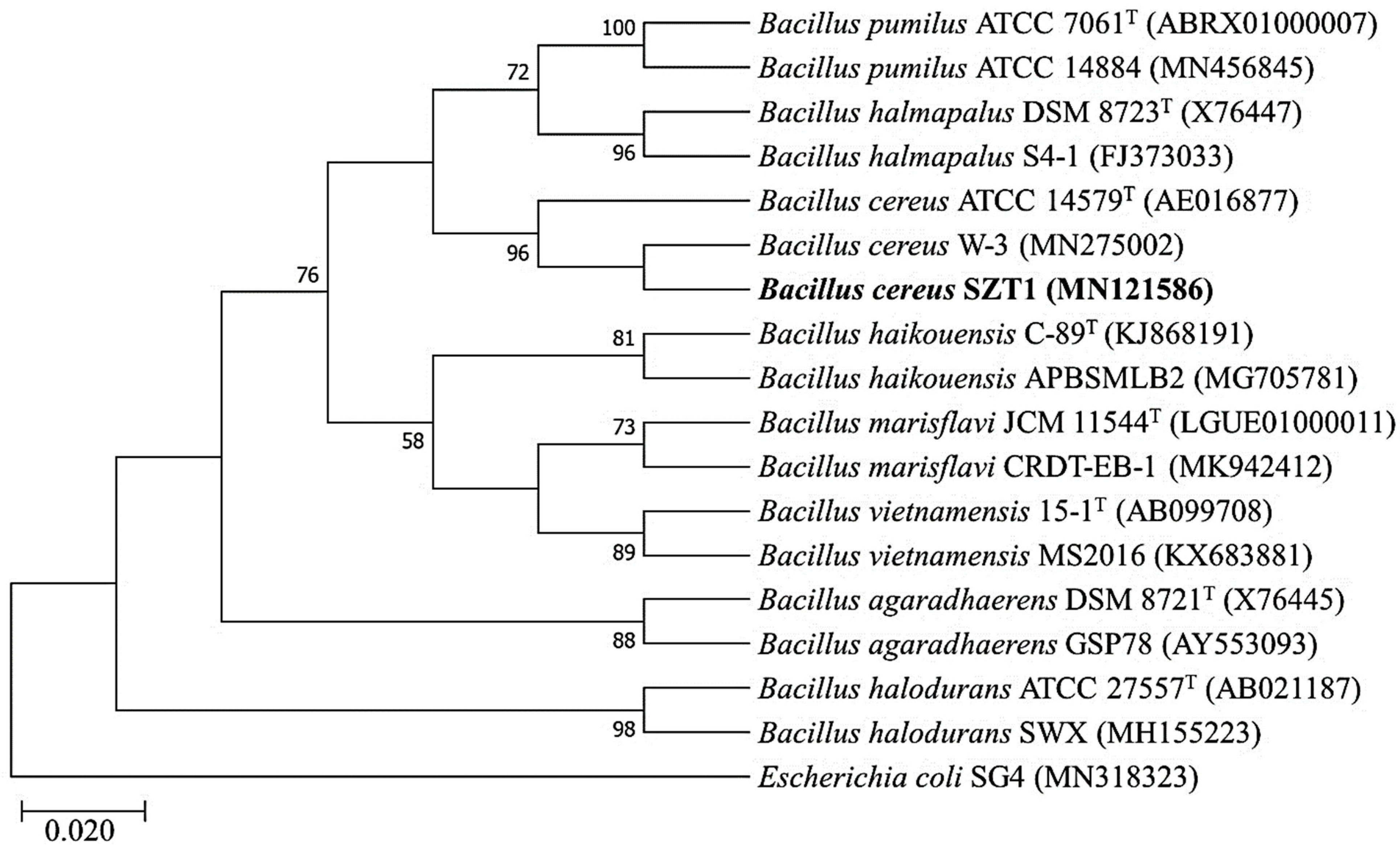
2.2. Identification and Phylogenetic Analysis of B. cereus SZT1
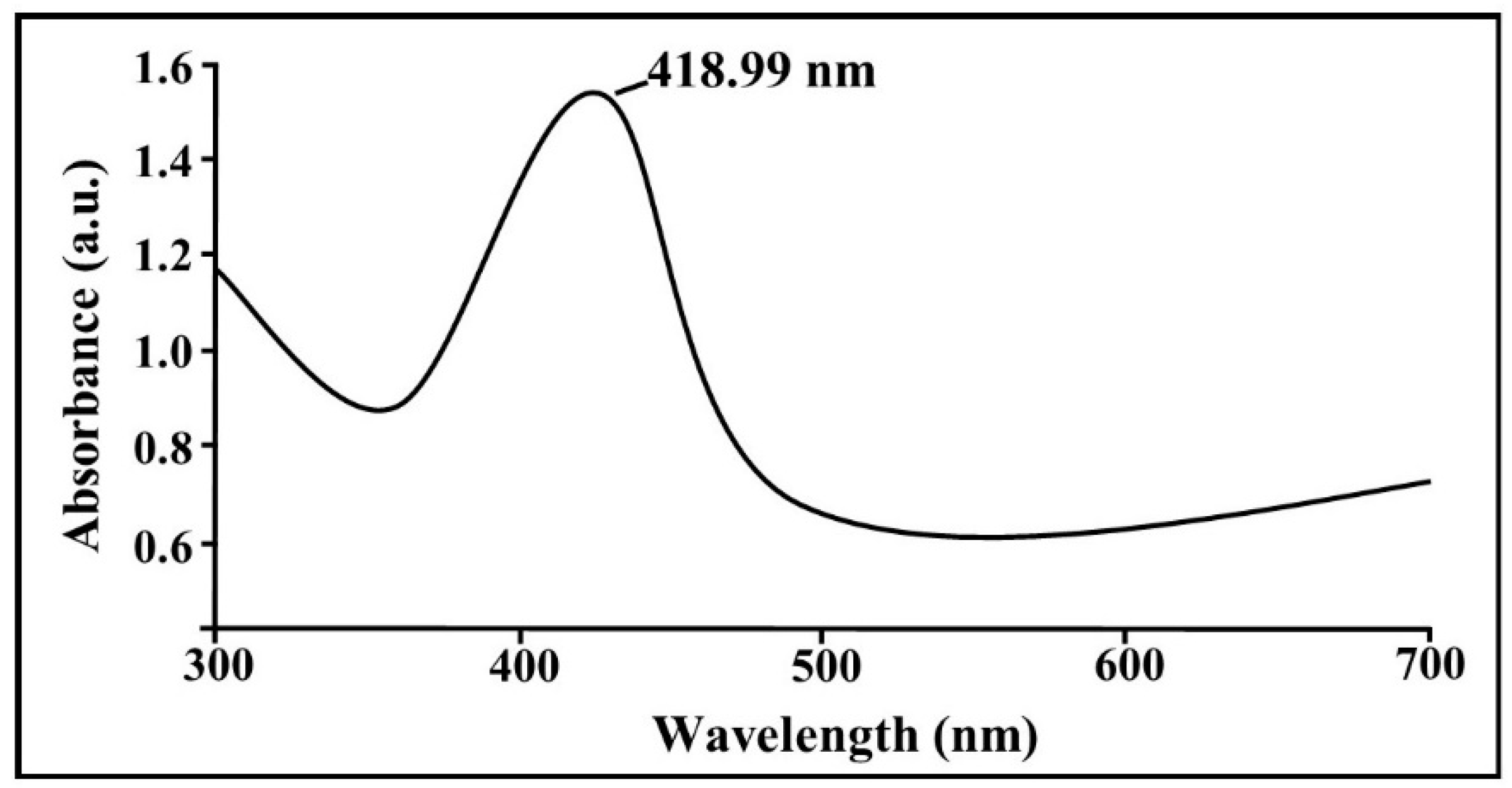
2.3. Biosynthesis of AgNPs by B. cereus SZT1
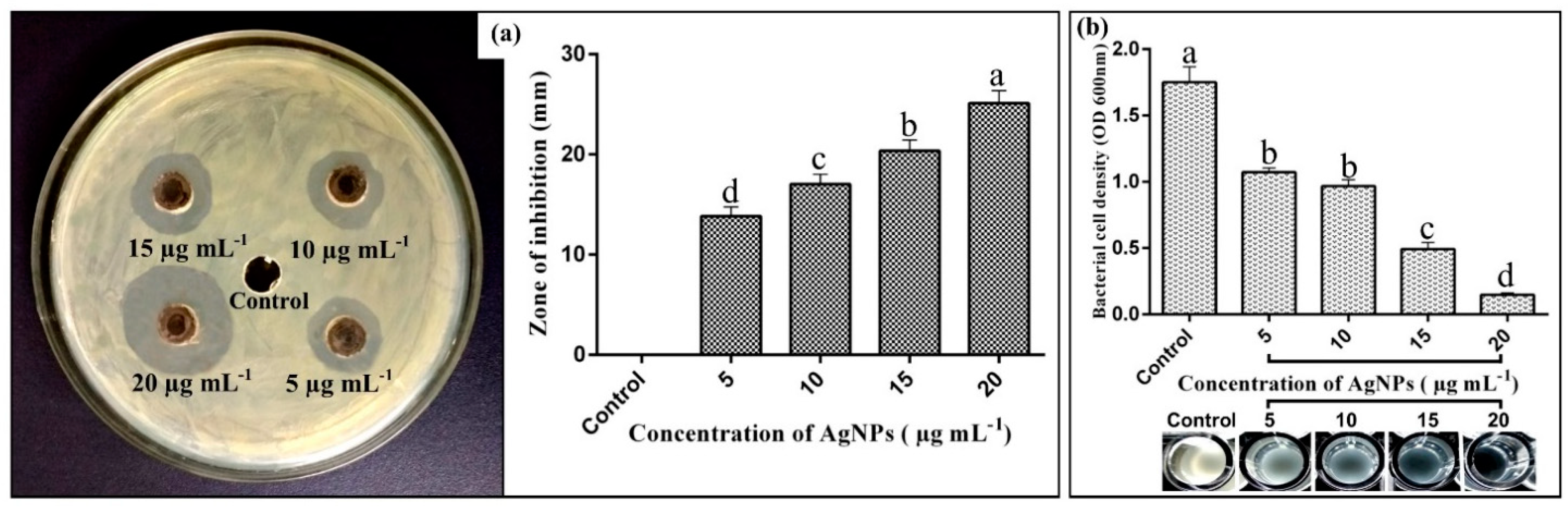
2.4. Antibacterial Activity of Biogenic AgNPs
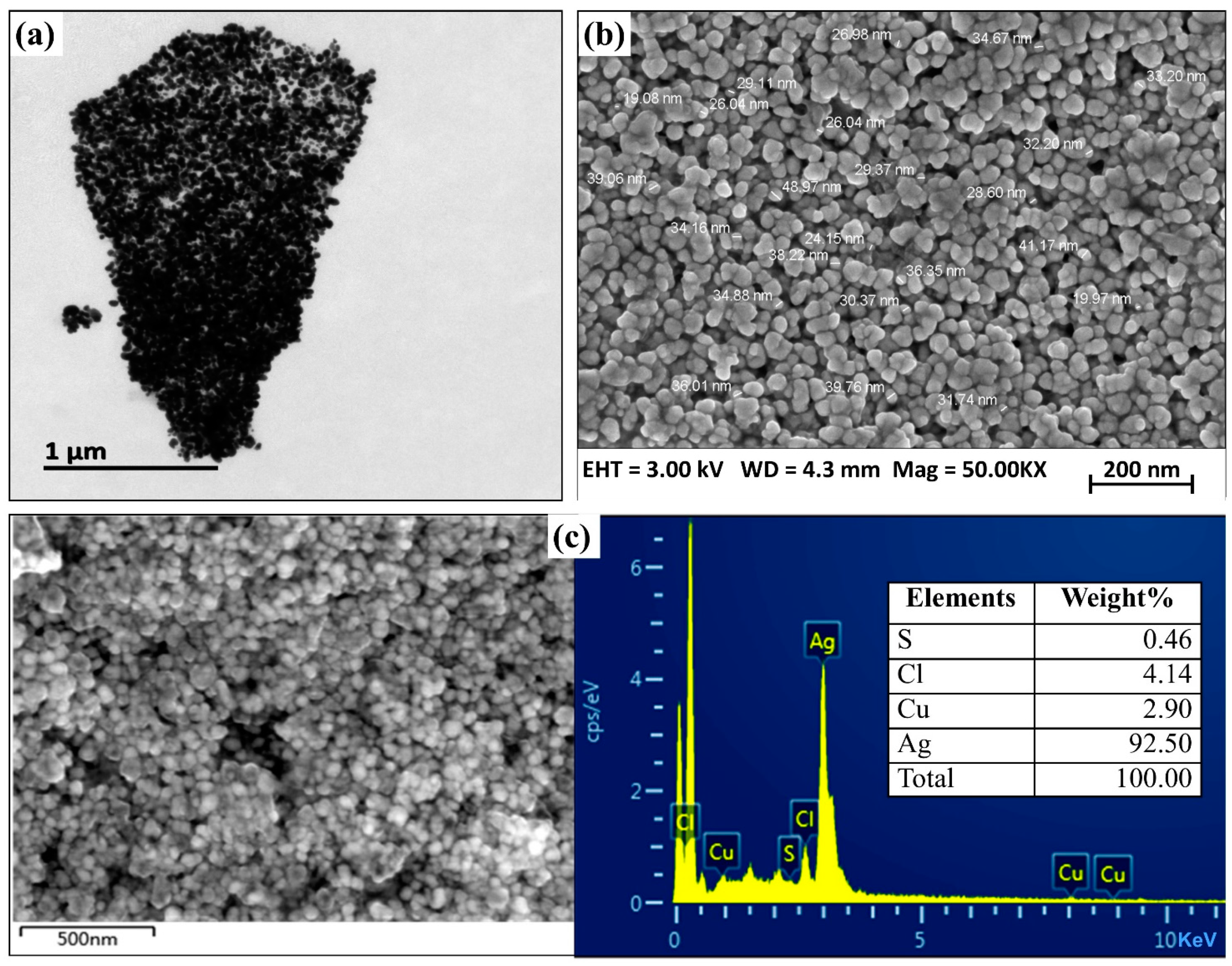
2.5. Characterization of Biogenic AgNPs
2.6. Effect of AgNPs on Lesion Length and Inhibition Rate of Xoo
2.7. Effect of AgNPs on the Growth of Healthy and Diseased Rice Plants
2.8. Effect of AgNPs on the Physiology of Healthy and Diseased Rice Plants
3. Discussion
4. Materials and Methods
4.1. Origin of Strain B. cereus SZT1
4.2. Identification of B. cereus SZT1
4.3. Extracellular Biosynthesis AgNPs
4.4. Antibacterial Activity of Biogenic AgNPs
4.5. Characterization of AgNPs
4.6. Experimental Design of the Pot Plants
4.7. Measurement of Plant Parameters
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, D.; Wei, H.; Guo, B.; Dai, Q.; Wei, C.; Gao, H.; Hu, Y.; Cui, P.; Li, M.; Huo, Z.; et al. The effects of chilling stress after anthesis on the physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2017, 237, 936–941. [Google Scholar] [CrossRef]
- Zahra, S.M.; Wahid, A.; Maqbool, N.; Ibrahim, M.H. Effect of Thiourea on Physiological Performance of Two Salt Affected Rice (Oryza sativa L.) Cultivars. Annu. Res. Rev. Biol. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Xie, K.; Yue, B.; Gong, Y.Y.; Shao, Y.; Shang, X.; Wu, Y. Inorganic arsenic contamination of rice from Chinese major rice-producing areas and exposure assessment in Chinese population. Sci. China Ser. B Chem. 2015, 58, 1898–1905. [Google Scholar] [CrossRef]
- Lutz, D.W.; Samir, K. Dimensions of global population projections: What do we know about future population trends and structures? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2779–2791. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Xạ, T.V.; Hao, L.T. Disease-reducing effects of aqueous leaf extract of Kalanchoe pinnata on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae involve induced resistance. Physiol. Mol. Plant Pathol. 2017, 100, 57–66. [Google Scholar] [CrossRef]
- Ryan, R.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding bacterium—Plant interactions. Nat. Rev. Genet. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Chithrashree; Udayashankar, A.; Nayaka, S.C.; Reddy, M.; Srinivas, C. Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2011, 59, 114–122. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Giàu, N.Đ.N.; Tuấn, T.Q. Effects of Serratia nematodiphila CT-78 on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2016, 103, 1–10. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Ji, G.-H.; Wei, L.-F.; He, Y.-Q.; Wu, Y.-P.; Bai, X.-H. Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Biol. Control 2008, 45, 288–296. [Google Scholar] [CrossRef]
- Yasmin, S.; Zaka, A.; Imran, A.; Zahid, M.A.; Yousaf, S.; Rasul, G.; Arif, M.; Mirza, M.S. Plant Growth Promotion and Suppression of Bacterial Leaf Blight in Rice by Inoculated Bacteria. PLoS ONE 2016, 11, e0160688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shakh, A.S.; Kakar, K.U.; Wang, X.; Almoneafy, A.; Ojaghian, M.R.; Li, B.; Anjum, S.I.; Xie, G.-L. Controlling bacterial leaf blight of rice and enhancing the plant growth with endophytic and rhizobacterialBacillusstrains. Toxicol. Environ. Chem. 2015, 97, 766–785. [Google Scholar] [CrossRef]
- Metch, J.W.; Burrows, N.; Murphy, C.J.; Pruden, A.; Vikesland, P. Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design. Nat. Nanotechnol. 2018, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Uttam, K. Rapid analyses of stress of copper oxide nanoparticles on wheat plants at an early stage by laser induced fluorescence and attenuated total reflectance Fourier transform infrared spectroscopy. Vib. Spectrosc. 2017, 92, 135–150. [Google Scholar] [CrossRef]
- Sadegh, H.R.; Ali, G.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Mika, S.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Majeed, S.; Danish, M.; Zahrudin, A.H.B.; Dash, G.K. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int. J. Mod. Sci. 2018, 4, 86–92. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W.; Siddiqui, R.H. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef]
- Dakhil, A.S. Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats. J. King Saud Univ. Sci. 2017, 29, 462–467. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Cáceres, R.R.; Stephano-Hornedo, J.L.; Lugo, J.; Vaca, R.; Del Aguila, P.; Yañez-Ocampo, G.; Mora-Herrera, M.E.; Díaz, L.M.C.; Cipriano-Salazar, M.; Alaba, P.A.; et al. Bactericidal effect of silver nanoparticles against propagation of Clavibacter michiganensis infection in Lycopersicon esculentum Mill. Microb. Pathog. 2018, 115, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Marpu, S.; Kolailat, S.S.; Korir, D.; Kamras, B.L.; Chaturvedi, R.; Joseph, A.; Smith, C.M.; Palma, M.C.; Shah, J.; Omary, M.A. Photochemical formation of chitosan-stabilized near-infrared-absorbing silver Nanoworms: A “Green” synthetic strategy and activity on Gram-negative pathogenic bacteria. J. Colloid Interface Sci. 2017, 507, 437–452. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Orozco, M.F.S.; Martínez, N.N.; Castañón, G.A.M.-; Méndez, F.T.; Patiño-Marín, N.; Ruiz, F. Detection of Genes Related to Resistance to Silver Nanoparticles in Bacteria from Secondary Endodontic Infections. J. Nanomater. 2019, 2019, 1–7. [Google Scholar]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented Silver Resistance in Clinically Isolated Enterobacteriaceae: Major Implications for Burn and Wound Management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef] [Green Version]
- Adriano, J.S.; Oyong, G.G.; Cabrera, E.C.; Janairo, J.I. Screening of Silver-Tolerant Bacteria from a Major Philippine Landfill as Potential Bioremediation Agents. Ecol. Chem. Eng. S 2018, 25, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Rostami, H.; Khosravi, F.; Mohseni, M.; Rostami, A.A. Biosynthesis of Ag nanoparticles using isolated bacteria from contaminated sites and its application as an efficient catalyst for hydrazine electrooxidation. Int. J. Biol. Macromol. 2018, 107, 343–348. [Google Scholar] [CrossRef]
- Wang, C.; Kim, Y.-J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–6. [Google Scholar]
- Omole, R.; Torimiro, N.; Alayande, S.O.; Ajenifuja, E. Silver nanoparticles synthesized from Bacillus subtilis for detection of deterioration in the post-harvest spoilage of fruit. Sustain. Chem. Pharm. 2018, 10, 33–40. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef] [Green Version]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Mo, J. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilisto control filarial vectorCulex pipiens pallensand its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degrassi, G.; Antisari, L.V.; Venturi, V.; Carbone, S.; Gatti, A.M.; Gambardella, C.; Falugi, C.; Vianello, G. Impact of engineered nanoparticles on virulence of xanthomonas oryzae pv. oryzae and on rice sensitivity at its infection. EQA-Int. J. Environ. Qual. 2014, 16, 21–33. [Google Scholar]
- Nasir, M.; Iqbal, B.; Hussain, M.; Mustafa, A.; Ayub, M. Chemical management of bacterial leaf blight disease in rice. J. Agric. Res. 2019, 57, 99–103. [Google Scholar]
- Chikte, R.G.; Paknikar, K.; Rajwade, J.M.; Sharma, J. Nanomaterials for the control of bacterial blight disease in pomegranate: Quo vadis? Appl. Microbiol. Biotechnol. 2019, 103, 4605–4621. [Google Scholar] [CrossRef]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Ejaz, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Ahmad, M.S.; Hussain, M.; Iqbal, M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018, 12, 927–932. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual; ICARDA: Aleppo, Syria, 2001. [Google Scholar]
- Wilson, K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2.4.1–2.4.5. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Juibari, M.M.; Abbasalizadeh, S.; Jouzani, G.S.; Noruzi, M. Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater. Lett. 2011, 65, 1014–1017. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De Haseth, J.A. Fourier Transform Infrared Spectrometry; Wiley: Hoboken, NJ, USA, 2007; Volume 171. [Google Scholar]
- Shankar, S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis—antiproliferative effect against prostate cancer cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2013, 4, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, L.-N.; Wu, H.; Zang, H.; Gao, S.; Yang, Y.; Xie, S.; Gao, X. Comparative proteomic analysis of rice seedlings in response to inoculation with Bacillus cereus. Lett. Appl. Microbiol. 2013, 56, 208–215. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzym. 1955, 2, 764–775. [Google Scholar]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]


| Soil Properties | Value | Soil Properties | Value |
|---|---|---|---|
| EC (dS m−1) | 8.78 | Available K (mg kg−1) | 142 |
| pH | 8.5 | Ag (mg kg−1) | 65 |
| Organic matter (%) | 1.43 | Zn (mg kg−1) | 211 |
| Organic C (g kg−1) | 4.32 | Cd (mg kg−1) | 6.9 |
| Total N (g kg−1) | 0.62 | Ni (mg kg−1) | 104 |
| Available P (mg kg−1) | 4.3 | Pb (mg kg−1) | 273 |
| Treatments ∗ | Lesion Length (cm) | Inhibition Rate % |
|---|---|---|
| Diseased Rice Plants | ||
| Control 2 | 8.64 (0.50)a | - |
| 25 mg L−1 | 5.92 (0.70)b | 31.36 (8.72)c |
| 50 mg L−1 | 4.40 (0.40)c | 49.12 (1.73)b |
| 75 mg L−1 | 3.49 (0.33)d | 59.34 (6.13)b |
| 100 mg L−1 | 2.38 (0.34)e | 72.51 (2.34)a |
| Treatments ∗ | Root Length (cm) | Shoot Length (cm) | Root FW (mg) | Shoot FW (mg) | Root DW (mg) | Shoot DW (mg) |
|---|---|---|---|---|---|---|
| Healthy Rice Plants | ||||||
| Control 1 | 5.44 (0.28)c | 26.99 (0.45)ab | 140.30 (1.95)a | 794.59 (28.16)a | 40.43 (1.90)bc | 158.88 (10.22)a |
| 25 mg L−1 | 5.87 (0.64)bc | 27.04 (0.46)ab | 140.77 (3.67)a | 795.78 (27.56)a | 42.43 (3.83)abc | 161.38 (9.26)a |
| 50 mg L−1 | 6.03 (0.38)abc | 28.07 (0.97)a | 143.07 (6.42)a | 806.52 (15.73)a | 43.50 (3.90)ab | 161.52 (8.90)a |
| 75 mg L−1 | 6.14 (0.03)ab | 28.19 (0.95)a | 143.16 (4.59)a | 808.56 (16.39)a | 44.24 (5.58)ab | 164.68 (11.12)a |
| 100 mg L−1 | 6.32 (0.10)ab | 28.25 (0.77)a | 143.21 (4.47)a | 808.39 (16.55)a | 44.97 (2.79)a | 165.14 (9.54)a |
| Diseased Rice Plants | ||||||
| Control 2 | 3.47 (0.26)e | 21.64 (1.00)d | 92.40 (4.32)d | 539.47 (23.20)e | 20.72 (1.50)e | 123.52 (5.81)c |
| 25 mg L−1 | 4.22 (0.11)d | 23.52 (0.88)c | 99.49 (5.09)cd | 590.32 (26.72)d | 24.26 (2.72)e | 134.94 (5.71)bc |
| 50 mg L−1 | 4.80 (0.52)d | 26.17 (1.31)b | 107.31 (6.35)c | 626.02 (25.39)c | 29.05 (1.95)d | 137.17 (4.07)b |
| 75 mg L−1 | 5.41 (0.30)c | 26.67 (0.49)b | 128.21 (10.23)b | 725.81 (35.21)b | 32.72 (1.40)d | 153.51 (5.35)a |
| 100 mg L−1 | 6.59 (0.37)a | 27.17 (0.69)ab | 138.27 (5.06)a | 790.21 (39.03)a | 39.39 (2.01)c | 156.51 (5.79)a |
| Treatments ∗ | MDA (ƞmol g−1 DW) | H2O2 (ƞg g−1 DW) | Proline (µg g−1 DW) | CAT (U mg−1 protein) | POD (U mg−1 protein) | Total Phenolics (mg g−1 FW) |
|---|---|---|---|---|---|---|
| Healthy Rice Plants | ||||||
| Control 1 (none) | 3.85 (0.23)b | 12.58 (1.09)d | 2.38 (0.10)c | 26.99 (0.87)f | 24.14 (0.62)e | 17.73 (1.32)h |
| 25 mg L−1 | 3.22 (0.18)cd | 11.56 (0.20)e | 2.81 (0.19)bc | 27.72 (1.68)f | 26.79 (1.50)d | 24.26 (1.64)f |
| 50 mg L−1 | 3.08 (0.07)d | 9.72 (0.19)ef | 3.64 (0.23)a | 33.30 (1.56)de | 28.12 (1.54)d | 32.34 (1.07)d |
| 75 mg L−1 | 2.15 (0.13)e | 8.86 (0.12)f | 3.65 (0.15)a | 37.96 (1.66)b | 34.13 (0.59)ab | 37.66 (1.02)c |
| 100 mg L−1 | 1.45 (0.08)f | 8.39 (0.24)g | 3.79 (0.15)a | 43.12 (1.54)a | 35.15 (0.54)a | 42.90 (1.11)a |
| Diseased Rice Plants | ||||||
| Control 2 (pathogen) | 4.73 (0.40)a | 21.64 (1.00)a | 2.42 (0.37)c | 22.56 (1.32)g | 21.64 (1.00)f | 18.73 (1.15)gh |
| 25 mg L−1 | 4.58 (0.23)a | 18.03 (0.65)b | 2.57 (0.27)bc | 23.75 (1.15)g | 23.40 (1.01)e | 20.52 (1.06)g |
| 50 mg L−1 | 3.51 (0.06)bc | 14.78 (0.53)c | 2.60 (0.07)bc | 30.99 (1.48)e | 28.18 (0.52)d | 27.31 (1.20)e |
| 75 mg L−1 | 3.11 (0.11)c | 13.24 (0.78)d | 2.68 (0.26)bc | 34.36 (1.06)cd | 32.18 (1.21)c | 32.42 (0.95)d |
| 100 mg L−1 | 2.43 (0.17)e | 8.63 (0.43)fg | 2.98 (0.71)b | 36.09 (1.50)bc | 33.14 (0.57)bc | 39.99 (1.48)b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. https://doi.org/10.3390/pathogens9030160
Ahmed T, Shahid M, Noman M, Niazi MBK, Mahmood F, Manzoor I, Zhang Y, Li B, Yang Y, Yan C, et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens. 2020; 9(3):160. https://doi.org/10.3390/pathogens9030160
Chicago/Turabian StyleAhmed, Temoor, Muhammad Shahid, Muhammad Noman, Muhammad Bilal Khan Niazi, Faisal Mahmood, Irfan Manzoor, Yang Zhang, Bin Li, Yong Yang, Chengqi Yan, and et al. 2020. "Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice" Pathogens 9, no. 3: 160. https://doi.org/10.3390/pathogens9030160
APA StyleAhmed, T., Shahid, M., Noman, M., Niazi, M. B. K., Mahmood, F., Manzoor, I., Zhang, Y., Li, B., Yang, Y., Yan, C., & Chen, J. (2020). Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens, 9(3), 160. https://doi.org/10.3390/pathogens9030160









