Compost Amendments Based on Vinegar Residue Promote Tomato Growth and Suppress Bacterial Wilt Caused by Ralstonia Solanacearum
Abstract
1. Introduction
2. Results
2.1. Compost Amendments Delay the Disease Symptoms of Tomato Bacterial Wilt
2.2. Growth Indices of Tomato Seedling Exposed to RS Inoculation
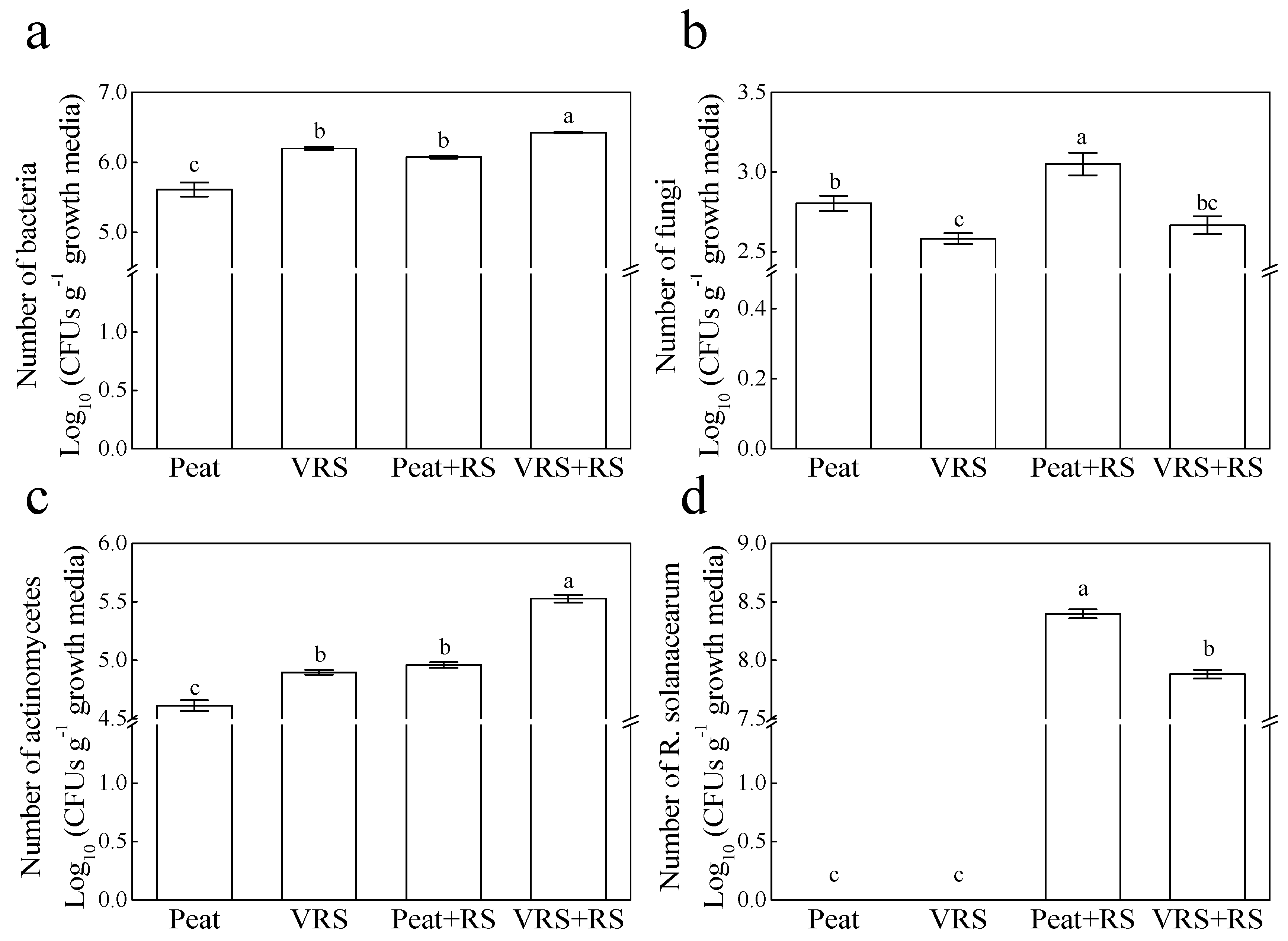
2.3. Enumeration of Culturable Microbial Community and RS Populations
2.4. Microbial Activity of Substrates after RS Inoculation
2.5. ROS Accumulation of Tomato Seedling after RS Inoculation
2.6. Electrolyte Leakage and MDA Content of Tomato Seedlings Exposed to RS Inoculation
2.7. Studies of Tomato Defense Enzymes after RS Inoculation
2.8. The Expression Patterns of Defense Marker Genes
3. Discussion
4. Materials and Methods
4.1. Plant, Bacterial Strains, and Growth Condition
4.2. Pot Experiment and Inoculation with RS
4.3. Monitoring and Evaluation of Disease Symptoms
4.4. Analysis of Basic Physical and Chemical Properties of the Matrix
4.5. Evaluation of the Plants’ Growth-Promoting Properties In Vivo
4.6. Analysis of Microbial Community Populations and Soil Microbial Activities
4.7. Determination of Electrolyte Leakage and Lipid Peroxidation
4.8. Analysis of ROS Accumulation in Pathogen Inoculated Leaves
4.9. Assay of Enzyme Activities
4.10. Analysis of Defense-Related Genes Expression
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslam, M.N.; Mukhtar, T.; Hussain, M.A.; Raheel, M. Assessment of resistance to bacterial wilt incited by Ralstonia solanacearum in tomato germplasm. J. Plant Dis. Prot. 2017, 124, 585–590. [Google Scholar] [CrossRef]
- Wicker, E.; Grassart, L.; Coranson-Beaudu, R.; Mian, D.; Guilbaud, C.; Fegan, M.; Prior, P. Ralstonia solanacearum strains from Martinique (French west indies) exhibiting a new pathogenic potential. Appl. Environ. Microbiol. 2007, 73, 6790–6801. [Google Scholar] [CrossRef] [PubMed]
- Artal, R.B.; Gopalakrishnan, C.; Thippeswamy, B. An efficient inoculation method to screen tomato, brinjal and chilli entries for bacterial wilt resistance. Pest Manag. Hortic. Ecosyst. 2013, 18, 70–73. [Google Scholar]
- Yadessa, G.B.; Van Bruggen, A.H.C.; Ocho, F.L. Effects of different soil amendments on bacterial wilt caused by Ralstonia solanacearum and on the yield of tomato. J. Plant Pathol. 2010, 92, 439–450. [Google Scholar]
- Soumaré, M.; Tack, F.M.G.; Verloo, M.G. Effects of a municipal solid waste compost and mineral fertilization on plant growth in two tropical agricultural soils of Mali. Bioresour. Technol. 2003, 86, 15–20. [Google Scholar] [CrossRef]
- Deborah, A.N.; Lynn, F.; Thomas, R.W. Ecoenzymes as indicators of compost to suppress Rhizoctonia solani. Compost Sci. Util. 2017, 25, 251–261. [Google Scholar]
- Escuadra, G.M.E.; Usami, T.; Amemiya, Y. Effect of compost amendment on soil microbial community and pathogen cansing fusarium wilt disease of spinach. Hortic. Res. 2008, 62, 21–29. [Google Scholar]
- Varo-Suárez, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; Mulero-Aparicio, A.; Trapero, A. Evaluation of organic amendments from agro-industry waste for the control of verticillium wilt of olive. Plant Pathol. 2017, 67, 860–870. [Google Scholar] [CrossRef]
- Dong, H.C.; Rong, D.J.; Hoon, H.; Yong, W.K.; Yong, C.K.; Park, R.D.; Krishnan, H.B.; Kil, Y.K. Control of late blight (phytophthora capsici) in pepper plant with a compost containing multitude of chitinase-producing bacteria. Biocontrol 2006, 51, 339–351. [Google Scholar]
- Stewart-Wade, S.M. Efficacy of organic amendments used in containerized plant production: Part 1–Compost-based amendments. Sci. Hortic. 2019, 108856. [Google Scholar] [CrossRef]
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13, 100192. [Google Scholar] [CrossRef]
- Islam, T.M.; Toyota, K. Suppression of bacterial wilt of tomato by Ralstonia solanacearum by incorporation of composts in soil and possible mechanisms. Microbes Environ. 2004, 19, 53–60. [Google Scholar] [CrossRef]
- Gorissen, A.; Van Overbeek, L.S.; Van Elsas, J.D. Pig slurry reduces the survival of Ralstonia solanacearum biovar 2 in soil. Can. J. Microbiol. 2004, 50, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Du, N.S.; Shi, L.; Du, L.T.; Yuan, Y.H.; Li, B.; Sang, T.; Sun, J.; Shu, S.; Guo, S.R. Effect of vinegar residue compost amendments on cucumber growth and fusarium wilt. Environ. Sci. Pollut. Res. 2015, 22, 19133–19141. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.S.; Shu, S.; Sun, J.; Li, S.Z.; Guo, S.R. Paenibacillus polymyxansy NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017, 7, 41234–41247. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P. Sustainable Management of Soil-Borne Plant Diseases; ATTRA: New York, NY, USA, 2001. [Google Scholar]
- Du, N.S.; Shi, L.; Yuan, Y.H.; Sun, J.; Shu, S.; Guo, S.R. Isolation of a potential biocontrol agent paenibacillus polymyxa NSY50 from vinegar waste compost and its induction of host defense responses against fusarium wilt of cucumber. Microbiol. Res. 2017, 202, 1–10. [Google Scholar] [CrossRef]
- Noble, R.; Coventry, E. Suppression of soil-borne plant diseases with composts: A review. Biocontrol Sci. Technol. 2010, 15, 3–20. [Google Scholar] [CrossRef]
- Vitale, J.; Godsey, C.; Edwards, J.; Taylor, R. The adoption of conservation tillage practices in Oklahoma: Findings from a producer survey. J. Soil Water Conserv. 2011, 66, 250–264. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Mehta, C.M.; Palni, U.; Franke-Whittle, I.H.; Sharma, A.K. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manag. 2014, 34, 607–622. [Google Scholar] [CrossRef]
- Van der Gaag, D.; Van Noort, F.R.; Stapel-Cuijpers, L.H.M.; De Kreij, C.; Termorshuizen, A.J.; Van Rijn, E.; Zmora-Nahum, S.; Chen, Y. The use of green waste compost in peat-based potting mixtures: Fertilization and suppressiveness against soilborne diseases. Sci. Hortic. 2007, 114, 289–297. [Google Scholar] [CrossRef]
- Schönfeld, J.; Gelsomino, A.; Overbeek, L.S.V.; Gorissen, A.; Smalla, K.; Elsas, J.D.V. Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2 and indigenous bacteria in soil. FEMS Microbiol. Ecol. 2003, 43, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Sun, C.L.; Liu, S.R.; Chai, R.S.; Huang, W.Q.; Liu, X.X.; Tang, C.X.; Zhang, Y.S. Bioorganic fertilizer enhances soil suppressive capacity against bacterial wilt of tomato. PLoS ONE 2015, 10, 0121304–0121320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Park, R.D.; Kim, Y.W.; Shim, J.H.; Chae, D.H.; Rim, Y.S.; Sohn, B.K.; Kim, T.H.; Kim, K.Y. Effect of food waste compost on microbial population, soil enzyme activity and lettuce growth. Bioresour. Technol. 2004, 93, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Brussaard, L.; De Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Ding, C.Y.; Shen, Q.R.; Zhang, R.F.; Chen, W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 2012, 366, 453–466. [Google Scholar] [CrossRef]
- Yuan, S.F.; Wang, L.L.; Wu, K.; Shi, J.X.; Wang, M.S.; Yang, X.M.; Shen, Q.R.; Shen, B. Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl. Soil Ecol. 2014, 75, 86–94. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.S.; Yuan, Y.H.; Shu, S.; Sun, J.; Guo, S.R. Vinegar residue compost as a growth substrate enhances cucumber resistance against the Fusarium wilt pathogen Fusarium oxysporum by regulating physiological and biochemical responses. Environ. Sci. Pollut. Res. 2016, 23, 18277–18287. [Google Scholar] [CrossRef]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The plant growth-promoting rizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Am. Phytopathol. Soc. 2011, 22, 991–1004. [Google Scholar]
- Wang, X.L.; Wang, L.; Wang, J.; Jin, P.; Liu, H.X.; Zheng, Y.H. Bacillus cereus AR156-induced resistance to colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 2014, 9, e112494. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, T.; Bhaskaran, R.; Muthusamy, M. Pseudomonas fluorescens induced enzymological changes in banana roots (Cv. Rasthali) against Fusarium wilt disease. Plant Pathol. J. 2004, 3, 72–80. [Google Scholar]
- Umesha, S. Note: Phenylalanine ammonia lyase activity in tomato seedlings and its relationship to bacterial canker disease resistance. Phytoparasitica 2006, 34, 68–71. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Wang, R.; Shen, W.B.; Liu, L.L.; Jiang, L.; Liu, Y.Q.; Su, N.; Wan, J.M. A novel lipoxygenase gene from developing rice seeds confers dual position specificity and responds to wounding and insect attack. Plant Mol. Biol. 2008, 66, 401–414. [Google Scholar] [CrossRef]
- Deslandes, L.; Olivier, J.; Theulieres, F.; Hirsch, J.; Feng, D.X.; Bittnereddy, P.; Beynon, J.; Marco, Y. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc. Natl. Acad. Sci. USA 2002, 99, 2404–2409. [Google Scholar] [CrossRef]
- Tasset, C.; Bernoux, M.; Jauneau, A.; Pouzet, C.; Briere, C.; Kieffer-Jacquinod, S.; Rivas, S.; Marco, Y.; Deslandes, L. Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1-R-mediated immunity in Arabidopsis. PLoS Pathog. 2010, 6, e1001202. [Google Scholar] [CrossRef]
- Tan, S.Y.; Dong, Y.; Liao, H.P.; Huang, J.F.; Song, S.; Xu, Y.C.; Shen, Q.R. Antagonistic bacterium Bacillus amyloliquefaciens induces resistance and controls the bacterial wilt of tomato. Pest Manag. Sci. 2013, 69, 1245–1252. [Google Scholar]
- Makandar, R.; Nalam, V.J.; Chowdhury, Z.; Sarowar, S.; Klossner, G.; Lee, H.; Burdan, D.; Trick, H.N.; Gobbato, E.; Parker, J.E.; et al. The combined action of enhanced disease susceptibility1, phytoalexin deficient4, and senescence-associated101 promotes salicylic defenses to limit Fusarium graminearum infection in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2015, 28, 943–953. [Google Scholar] [CrossRef]
- Ito, M.; Ohnishi, K.; Hikichi, Y.; Kiba, A. Molecular chaperons and co-chaperons, Hsp90, RAR1, and SGT1 negatively regulate bacterial wilt disease caused by Ralstonia solanacearum in Nicotiana benthamiana. Plant Signal. Behav. 2015, 10, e970410. [Google Scholar] [CrossRef]
- Zhang, J.H.; Guo, Y.S.; Tian, G.M.; Zhang, J.; Yao, J.H. Effects of three composts on growth and bacterial wilt of tomato. Plant Nutr. Fertil. Sci. 2010, 18, 1185–1192. [Google Scholar]
- Milling, A.; Babujee, L.; Allen, C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS ONE 2011, 6, 15853–15863. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, P.R.; Matta, F.B.; Harkess, R.L. Physical and chemical properties of substrates containing earthworm castings and effects on marigold growth. Hort Sci. 2006, 41, 1473–1476. [Google Scholar] [CrossRef]
- Shi, L.H.; Zhang, Z.G.; Liu, D.M.; Li, W.Q.; Wen, J.R.B. Comparison of physiochemical properties between spent mushroom compost and peat substrate and adjustment. Trans. Chin. Soc. Agric. Eng. 2008, 24, 199–203. [Google Scholar]
- Saez-Plaza, P.; Michalowski, T.; Jose Navas, M.; Garcia Asuero, A.; Wybraniec, S. An overview of the kjeldahl method of nitrogen determination. Part I. early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Sciubba, L.; Cavani, L.; Marzadori, C.; Ciavatta, C. Effect of biosolids from municipal sewage sludge composted with rice husk on soil functionality. Biol. Fertil. Soils 2013, 49, 597–608. [Google Scholar] [CrossRef]
- Cai, J.H.; Li, J.F.; Wei, X.Y.; Zhang, L. Dynamic change in biomass, root vigor and replacement rate during the green leaf period of lycoris radiat. J. Nanjing For. Univ. 2018, 42, 55–59. [Google Scholar]
- Wu, W.X.; Ye, Q.F.; Min, H.; Duan, X.J.; Jin, W.M. Bt-transgenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol. Biochem. 2004, 36, 289–295. [Google Scholar]
- French, E.B.; Gutarra, L.; Aley, P.; Elphinstone, J. Bacterial wilt-culture media of Ralstonia solanacearum isolation, identification and maintenance. Fitopatologoa 1995, 30, 126–130. [Google Scholar]
- Hou, X.Q.; Wang, X.J.; Li, R.; Jia, Z.K.; Liang, L.Y.; Wang, J.P.; Nie, J.F.; Chen, X.; Wang, Z. Effects of different manure application rates on soil properties, nutrient use, and crop yield during dryland maize farming. Soil Res. 2012, 50, 507–514. [Google Scholar] [CrossRef]
- Antonious, G.F. Impact of soil management and two botanical insecticides on urease and invertase activity. J. Environ. Sci. Health 2003, 38, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Xue, D.; Huang, X.D. The impact of sewage sludge compost on tree peony growth and soil microbiological, and biochemical properties. Chemosphere 2013, 93, 583–589. [Google Scholar] [CrossRef]
- Hayano, K. A method for the determination of β-glucosidase activity in soil. Soil Sci. Plant Nutr. 1973, 19, 103–108. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- KoÇ, A. Effect of plant growth-promoting bacteria and arbuscular mycorrhizal fungi on lipid peroxidation and total phenolics of strawberry (Fragaria×ananassa’San Andreas’) under salt stress. Turk. J. Agric. For. 2015, 39, 992–998. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 292–298. [Google Scholar]
- Wu, J.Q.; Shu, S.; Li, C.C.; Sun, J.; Guo, S.R. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Biochem. 2018, 128, 152–162. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.S.; Cunningham, B.A. Improved peroxidase assay method using leuco 2, 3’, 6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis. Anal. Biochem. 1969, 27, 292–299. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.A.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2013, 23, 33–39. [Google Scholar] [CrossRef]
- González, E.M.; Ancos, B.D.; Cano, M.P. Partial characterization of polyphenol oxidase activity in raspberry fruits. J. Agric. Food Chem. 1999, 47, 4068. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef]







| Treatment | Plant Height (cm) | Stem Diameter (mm) | Leaf Area (cm2) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| Peat | 15 ± 0.24 b | 3.81 ± 0.03 b | 20.84 ± 0.85 b | 4.23 ± 0.32 b | 0.86 ± 0.03 b | 0.36 ± 0.03 b | 0.07 ± 0.003 b |
| VRS | 16.2 ± 0.12 a | 3.95 ± 0.01 a | 25.17 ± 0.21 a | 5.20 ± 0.36 a | 1.05 ± 0.08 a | 0.45 ± 0.03 a | 0.10 ± 0.003 a |
| Peat + RS | 9.84 ± 0.26 d | 3.41 ± 0.02 d | 10.09 ± 0.63 d | 2.06 ± 0.02 d | 0.59 ± 0.02 d | 0.21 ± 0.006 c | 0.04 ± 0.003 d |
| VRS + RS | 11.31 ± 0.50 c | 3.59 ± 0.03 c | 13.32 ± 0.61 c | 3.06 ± 0.09 c | 0.74 ± 0.03 c | 0.26 ± 0.006 c | 0.06 ± 0.003 c |
| Treatment | Total Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Mean Diameter (mm) | Tips of Number | Root Vigor (mg·g−1·h−1) |
|---|---|---|---|---|---|---|
| Peat | 461.6 ± 8.7 b | 54.42 ± 4.17 b | 0.52 ± 0.08 ab | 0.38 ± 0.03 c | 1164 ± 54 b | 74.51 ± 2.72 a |
| VRS | 611.9 ± 22.6 a | 69.29 ± 1.22 a | 0.63 ± 0.04 a | 0.36 ± 0.02 bc | 1370 ± 7 a | 84.69 ± 3.68 a |
| Peat + RS | 258.6 ± 20.2 c | 37.46 ± 0.28 c | 0.44 ± 0.03 b | 0.47 ± 0.04 a | 597 ± 33 c | 27.23 ± 1.89 c |
| VRS + RS | 274.0 ± 7.69 c | 37.85 ± 0.37 c | 0.42 ± 0.01 b | 0.44 ± 0.01 ab | 637 ± 84 c | 62.13 ± 3.15 b |
| Treatment | Invertase (mg glucose g−1 h−1) | Urease (mg NH4 + -N g−1 h−1) | Proteinase (mg glycine kg−1 h−1) | Catalase (mL (0.1 M KMnO4) g−1 h−1) | Phosphatase (mg phenol g−1 h−1) | β-glucosidase (μg hydrolyzed p-nitrophenol g−1 h−1) | FDA hydrolysis (μg FDA g−1 h−1) |
|---|---|---|---|---|---|---|---|
| Peat | 11.20 ± 2.08 bc | 0.91 ± 0.03 c | 2.54 ± 0.22 c | 2.96 ± 0.008 b | 0.49 ± 0.01 a | 706.8 ± 19.59 c | 925.6 ± 8.49 c |
| VRS | 39.99 ± 4.42 a | 1.95 ± 0.09 a | 10.79 ± 0.35 a | 3.15 ± 0.006 a | 0.57 ± 0.006 a | 1532.3 ± 15.69 a | 1387.1 ± 22.86 a |
| Peat + RS | 4.82 ± 0.27 c | 0.10 ± 0.03 c | 0.72 ± 0.06 d | 2.86 ± 0.057 b | 0.38 ± 0.04 b | 654.2 ± 54.09 c | 911.7 ± 12.14 c |
| VRS + RS | 13.89 ± 1.68 b | 1.25 ± 0.06 b | 6.98 ± 0.06 b | 3.15 ± 0.006 a | 0.52 ± 0.005 a | 1379.2 ± 12.66 b | 955.8 ± 13.69 b |
| Peat | VRS | |
|---|---|---|
| pH | 5.16 ± 0.04 | 5.88 ± 0.16 |
| EC (ms·cm−1) | 0.77 ± 0.12 | 1.62 ± 0.30 |
| Total porosity (%) | 80.5 ± 0.51 | 80.1 ± 0.44 |
| Aeration porosity (%) | 1.72 ± 0.57 | 9.28 ± 0.08 |
| Water-holding porosity (%) | 78.8 ± 0.58 | 70.9 ± 0.46 |
| Aeration porosity/Water-holding porosity | 2.18 ± 0.74 | 13.1 ± 0.16 |
| Bulk density (g·cm-3) | 18.7 ± 0.05 | 12.2 ± 0.26 |
| Total N (mg·g−1) | 13.0 ± 0.70 | 18.4 ± 1.11 |
| Total P (mg·g−1) | 1.00 ± 0.05 | 4.71 ± 0.14 |
| Total K (mg·g−1) | 1.12 ± 0.20 | 4.30 ± 1.50 |
| Na (mg·g−1) | 0.46 ± 0.05 | 1.08 ± 0.34 |
| Ca (mg·g−1) | 3.31 ± 0.77 | 7.93 ± 2.01 |
| Mg (mg·g−1) | 0.42 ± 0.15 | 2.26 ± 0.76 |
| Gene Full Name | Gene Acronym | Accession Numbers | Forward Primer | Reverse Primer |
|---|---|---|---|---|
| Superoxide dismutase | SOD | Solyc02g082590 | 5′-ATAGGAAGCCATACGATA-3′ | 5′-ATCACCGCATATTGTAAT-3′ |
| Peroxidase 3 | POD3 | Solyc07g052510 | 5′-CTGGTAGAAGAGATGGAA-3′ | 5′-CGAAGGATTGTTGTAGTC-3′ |
| Catalase | CAT | Solyc12g094620 | 5′-ATTCCTTCTTGTGTCTTG-3′ | 5′-TGTTGATGTATCTGTCTTG-3′ |
| Ascorbate peroxidase | APX | Solyc06g005150 | 5′-CCTATGATGTGTGTTCCA-3′ | 5′-AAGAGTCTGAGAGCAATG-3′ |
| Phenylalanine ammonia lyase 5 | PAL5 | Solyc09g007910 | 5′-CGGTGAGGAGATTGATAA-3′ | 5′-TTAGCAGATTGGAATAGGA-3′ |
| Polyphenol oxidase | PPO | Solyc08g074680 | 5′-TACTACTACAACGCTCAA-3′ | 5′-AACCAAGAAGAACATTCC-3′ |
| Lipoxygenase | LOX | Solyc01g099190 | 5′-TTGGCTTATACTCTTACG-3′ | 5′-GAATACCTTGTCTGGATT-3′ |
| 1-aminocyclopropane−1-carboxylate oxidase 1 | ACO1 | Solyc07g049530 | 5′-TTGACGAAGAATACAGAGA-3′ | 5′-ATGGTGGATAGTTGCTAA-3′ |
| Mitogen-activated protein kinase 3 | MAPK3 | Solyc06g005170 | 5′-ATGGTTGATGCTAATATGG-3′ | 5′-AGGAGGTTGATACTTGTT-3′ |
| A key regulator of the SA-mediated systemic-acquired resistance pathway | NPR1 | Solyc10g079750 | 5′- GCGATATTCCAACCTATA-3′ | 5′-TAGATTCAAATACACCATTC-3′ |
| Enhanced disease susceptibility 1 | EDS1 | Solyc06g071280 | 5′-AATGATGCTTGCTCCTCTT-3′ | 5′-GCCTCGTGCTGATAATACT-3′ |
| Ribosome biogenesis regulatory protein homolog | RRS1 | Solyc12g006550 | 5′-TTGGTGAAGGAGTGTCTA-3′ | 5′-TCTGTTGAAGGTAAGTTGAA-3′ |
| Heat shock protein 90 | HSP90 | Solyc06g036290 | 5′-TGTTGTTGACTCTGATGATT-3′ | 5′-GTTCTTCCTAATGACCTTGA-3′ |
| Pathogenesis-related protein 1a | PR1a | Solyc10g048080 | 5′-GCTCATCCAAATAGTATCC-3′ | 5′-GGTCTAACTCCCACATTA-3′ |
| Pathogenesis-related protein 1b | PR1b | Solyc10g048100 | 5′-ATTCTCATGGTCAGTATT-3′ | 5′-GGTAATAGTATTGTTTCTCA-3′ |
| Proteinase inhibitor II | Pin2 | Solyc03g020080 | 5′-TGATGCCAAGGCTTGTACTAGAGA-3′ | 5′-AGCGGACTTCCTTCTGAACGT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Shah Jahan, M.; Wang, Y.; Sun, J.; Shu, S.; Guo, S. Compost Amendments Based on Vinegar Residue Promote Tomato Growth and Suppress Bacterial Wilt Caused by Ralstonia Solanacearum. Pathogens 2020, 9, 227. https://doi.org/10.3390/pathogens9030227
He M, Shah Jahan M, Wang Y, Sun J, Shu S, Guo S. Compost Amendments Based on Vinegar Residue Promote Tomato Growth and Suppress Bacterial Wilt Caused by Ralstonia Solanacearum. Pathogens. 2020; 9(3):227. https://doi.org/10.3390/pathogens9030227
Chicago/Turabian StyleHe, Mingming, Mohammad Shah Jahan, Yu Wang, Jin Sun, Sheng Shu, and Shirong Guo. 2020. "Compost Amendments Based on Vinegar Residue Promote Tomato Growth and Suppress Bacterial Wilt Caused by Ralstonia Solanacearum" Pathogens 9, no. 3: 227. https://doi.org/10.3390/pathogens9030227
APA StyleHe, M., Shah Jahan, M., Wang, Y., Sun, J., Shu, S., & Guo, S. (2020). Compost Amendments Based on Vinegar Residue Promote Tomato Growth and Suppress Bacterial Wilt Caused by Ralstonia Solanacearum. Pathogens, 9(3), 227. https://doi.org/10.3390/pathogens9030227







