Recent Advances in Diagnostic Approaches for Epstein–Barr Virus
Abstract
:1. Introduction
2. EBV-Associated Diseases
3. Diagnoses of EBV-Associated Diseases
3.1. EBER-ISH
3.2. EBV Serology
3.2.1. Heterophile Antibody Test
3.2.2. Specific EBV Antibodies Tests
IFA and EIA
Western Blot
3.2.3. Avidity Testing
3.3. Molecular Assays
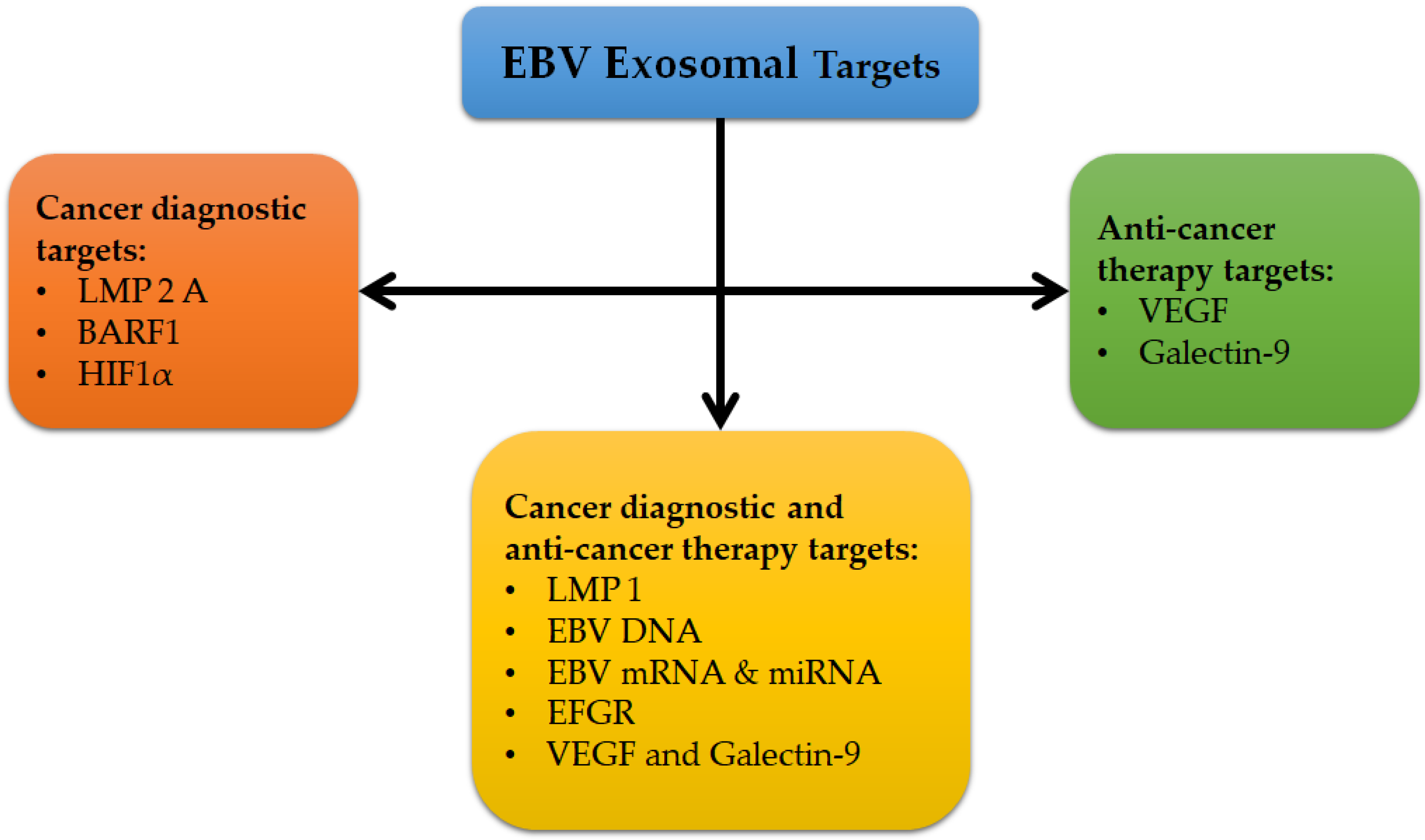
3.4. Exosomes as Promising Biomarkers
4. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prabhu, S.R.; Wilson, D.F. Evidence of Epstein-Barr Virus Association with Head and Neck Cancers: A Review. J. Can. Dent. Assoc. 2016, 82, g2. [Google Scholar] [PubMed]
- Thompson, M.P.; Kurzrock, R. Epstein-Barr virus and cancer. Clin. Cancer Res. 2004, 10, 803–821. [Google Scholar] [CrossRef] [Green Version]
- Bolis, V.; Karadedos, C.; Chiotis, I.; Chaliasos, N.; Tsabouri, S. Atypical manifestations of Epstein-Barr virus in children: A diagnostic challenge. J. Pediatr. (Rio J) 2016, 92, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulley, M.L. Molecular diagnosis of Epstein-Barr virus-related diseases. J. Mol. Diagn. 2001, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stanfield, B.A.; Luftig, M.A. Recent advances in understanding Epstein-Barr virus. F1000Res 2017, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Hjalgrim, H.; Friborg, J.; Melbye, M. The epidemiology of EBV and its association with malignant disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Thorley-Lawson, D.A. EBV Persistence—Introducing the Virus. Curr. Top Microbiol. Immunol. 2015, 390, 151–209. [Google Scholar] [CrossRef] [Green Version]
- Young, L.S.; Arrand, J.R.; Murray, P.G. EBV gene expression and regulation. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- De Paschale, M.; Clerici, P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar] [CrossRef]
- Young, L.S.; Dawson, C.W.; Eliopoulos, A.G. The expression and function of Epstein-Barr virus encoded latent genes. Mol. Pathol. 2000, 53, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Ungerleider, N.; Concha, M.; Lin, Z.; Roberts, C.; Wang, X.; Cao, S.; Baddoo, M.; Moss, W.N.; Yu, Y.; Seddon, M.; et al. The Epstein Barr virus circRNAome. PLoS Pathog. 2018, 14, e1007206. [Google Scholar] [CrossRef]
- Murray, P.G.; Swinnen, L.J.; Flavell, J.R.; Ragni, M.V.; Baumforth, K.R.; Toomey, S.M.; Filipovich, A.H.; Lowe, D.; Schnell, C.S.; Johl, J.; et al. Frequent expression of the tumor necrosis factor receptor-associated factor 1 in latent membrane protein 1-positive posttransplant lymphoproliferative disease and HIV-associated lymphomas. Hum. Pathol. 2001, 32, 963–969. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; EAR, E.N.S.; Boer, J.C.; Ferji, K.; Six, J.L.; Chen, X.; Elkord, E.; Plebanski, M.; Mohamud, R. Synergistic Effects of Nanomedicine Targeting TNFR2 and DNA Demethylation Inhibitor-An Opportunity for Cancer Treatment. Cells 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Al-Hatamleh, M.A.; Ahmad, S.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. A Perspective Review on the Role of Nanomedicine in the Modulation of TNF-TNFR2 Axis in Breast Cancer Immunotherapy. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, N.A.; Jain, V.; Wang, Y.; Maness, N.J.; Blair, R.V.; Alvarez, X.; Midkiff, C.; Kolson, D.; Bai, S.; Roberts, C.; et al. Comparative Analysis of Gammaherpesvirus Circular RNA Repertoires: Conserved and Unique Viral Circular RNAs. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Elgui de Oliveira, D.; Muller-Coan, B.G.; Pagano, J.S. Viral Carcinogenesis Beyond Malignant Transformation: EBV in the Progression of Human Cancers. Trends Microbiol. 2016, 24, 649–664. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.S.; Kieff, E. Epstein-Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandraud, N.; Meynard, J.B.; Auger, I.; Sovran, H.; Mugnier, B.; Reviron, D.; Roudier, J.; Roudier, C. Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: Accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 2003, 48, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Takei, M. Epstein-Barr virus and autoimmune diseases. Clin. Exp. Neuroimmunol. 2015, 6, 38–48. [Google Scholar] [CrossRef]
- Draborg, A.; Izarzugaza, J.M.; Houen, G. How compelling are the data for Epstein–Barr virus being a trigger for systemic lupus and other autoimmune diseases? Curr. Opin. Rheum. 2016, 28, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Niedobitek, G.; Meru, N.; Delecluse, H.J. Epstein-Barr virus infection and human malignancies. Int. J. Exp. Pathol. 2001, 82, 149–170. [Google Scholar] [CrossRef]
- Pender, M.P. Epstein-Barr virus and autoimmunity. In Infection and Autoimmunity; Elsvier: Amsterdam, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Zhang, K.; Wakefield, E.; Marsh, R. Lymphoproliferative Disease, X-Linked. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Gulley, M.L.; Tang, W. Laboratory assays for Epstein-Barr virus-related disease. J. Mol. Diagn. 2008, 10, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.S.; Kim, W.H. Epstein-Barr virus in human malignancy: A special reference to Epstein-Barr virus associated gastric carcinoma. Cancer Res. Treat. 2005, 37, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans R Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Huppmann, A.R.; Nicolae, A.; Slack, G.W.; Pittaluga, S.; Davies-Hill, T.; Ferry, J.A.; Harris, N.L.; Jaffe, E.S.; Hasserjian, R.P. EBV may be expressed in the LP cells of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) in both children and adults. Am. J. Surg. Pathol. 2014, 38, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Marshall-Andon, T.; Heinz, P. How to use… the Monospot and other heterophile antibody tests. Arc. Disease Childhood-Educ. Pract. 2017, 102, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Gloghini, A. Epstein Barr Virus-Associated Hodgkin Lymphoma. Cancers 2018, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Fanaian, N.K.; Cohen, C.; Waldrop, S.; Wang, J.; Shehata, B.M. Epstein-Barr virus (EBV)-encoded RNA: Automated in-situ hybridization (ISH) compared with manual ISH and immunohistochemistry for detection of EBV in pediatric lymphoproliferative disorders. Pediatr. Dev. Pathol. 2009, 12, 195–199. [Google Scholar] [CrossRef]
- Gulley, M.L.; Glaser, S.L.; Craig, F.E.; Borowitz, M.; Mann, R.B.; Shema, S.J.; Ambinder, R.F. Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting Epstein-Barr virus in Hodgkin lymphoma. Am. J. Clin. Pathol. 2002, 117, 259–267. [Google Scholar] [CrossRef]
- Nakatsuka, S.-I.; Homma, K.; Aozasa, K. When to use in situ hybridization for the detection of Epstein-Barr virus: A review of Epstein-Barr virus-associated lymphomas. J. Hematopathol. 2015, 8, 61–70. [Google Scholar] [CrossRef]
- Skalsky, R.L.; Cullen, B.R. EBV Noncoding RNAs. Curr. Top Microbiol. Immunol. 2015, 391, 181–217. [Google Scholar] [CrossRef] [Green Version]
- Iwakiri, D. Epstein-Barr Virus-Encoded RNAs: Key Molecules in Viral Pathogenesis. Cancers 2014, 6, 1615–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, G.; Coates, P.J.; Kangro, H.O.; Slavin, G. Epstein Barr virus (EBV) encoded small RNAs: Targets for detection by in situ hybridisation with oligonucleotide probes. J. Clin. Pathol. 1992, 45, 616–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez-Calvillo, G.; Martin, S.; Hamm, C.; Sztuba-Solinska, J. The Structure-To-Function Relationships of Gammaherpesvirus-Encoded Long Non-Coding RNAs and Their Contributions to Viral Pathogenesis. Noncoding RNA 2018, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Pathmanathan, R.; Prasad, U.; Sadler, R.; Flynn, K.; Raab-Traub, N. Clonal proliferations of cells infected with Epstein–Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Eng. J. Med. 1995, 333, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.; Tanner, M. Chromogenic in situ hybridization in tumor pathology. In Molecular Diagnosis of Cancer; Roulston, J.E., Bartlett, J.M.S., Eds.; Springer Humana Press: Totowa, NJ, USA, 2004; pp. 133–144. [Google Scholar]
- Gartner, B.C.; Kortmann, K.; Schafer, M.; Mueller-Lantzsch, N.; Sester, U.; Kaul, H.; Pees, H. No correlation in Epstein-Barr virus reactivation between serological parameters and viral load. J. Clin. Microbiol. 2000, 38, 2458. [Google Scholar] [CrossRef]
- Callan, M.F.; Tan, L.; Annels, N.; Ogg, G.S.; Wilson, J.D.; O’Callaghan, C.A.; Steven, N.; McMichael, A.J.; Rickinson, A.B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J. Exp. Med. 1998, 187, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Ito, Y.; Suzuki, R.; Nishiyama, Y. Measuring Epstein-Barr virus (EBV) load: The significance and application for each EBV-associated disease. Rev. Med. Virol. 2008, 18, 305–319. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein-Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front. Oncol. 2018, 8, 211. [Google Scholar] [CrossRef]
- Coghill, A.E.; Hildesheim, A. Epstein-Barr virus antibodies and the risk of associated malignancies: Review of the literature. Am. J. Epidemiol. 2014, 180, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Kostadinova, T.; Ivanova, L.; Hristov, I.; Todorova, T.; Stoykova, Z.; Tsaneva, D. The role of anti-EBNA1 IgG determination in EBV diagnostics. J. IMAB–Annual Proc. Sci. Papers 2018, 24, 2181–2185. [Google Scholar] [CrossRef] [Green Version]
- Henle, W.; Henle, G. Epstein-Barr virus-specific serology in immunologically compromised individuals. Cancer Res. 1981, 41, 4222–4225. [Google Scholar] [PubMed]
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H., Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 2011, 24, 193–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, R.D. Routine Epstein-Barr virus diagnostics from the laboratory perspective: Still challenging after 35 years. J. Clin. Microbiol. 2004, 42, 3381–3387. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.J.; Pachnio, A.; Pedroza-Pacheco, I.; Leese, A.M.; Begum, J.; Long, H.M.; Croom-Carter, D.; Stacey, A.; Moss, P.A.H.; Hislop, A.D.; et al. Asymptomatic Primary Infection with Epstein-Barr Virus: Observations on Young Adult Cases. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Ramos, A.; Patel, M.; Kadakia, K.; Haque, T. Performance of the architect EBV antibody panel for determination of Epstein-Barr virus infection stage in immunocompetent adolescents and young adults with clinical suspicion of infectious mononucleosis. Clin. Vaccine Immunol. 2014, 21, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Henle, W.; Henle, G.; Andersson, J.; Ernberg, I.; Klein, G.; Horwitz, C.A.; Marklund, G.; Rymo, L.; Wellinder, C.; Straus, S.E. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc. Natl. Acad. Sci. USA 1987, 84, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Neocleous, C.; Adramerina, A.; Spanou, C.; Spyrou, G.; Mitsios, A.; Dragoumi, M.; Tzanetis, F. How accurate are diagnostic tools for Epstein-Barr virus (EBV) to establish causal association of an uncommon clinical condition with EBV? Acta Virol. 2013, 57, 283–291. [Google Scholar]
- Crowley, A.; Connell, J.; Schaffer, K.; Hall, W.; Hassan, J. Is there diagnostic value in detection of immunoglobulin g antibodies to the epstein-barr virus early antigen? Biores. Open Access 2012, 1, 291–296. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Birmann, B.M.; Chang, E.T.; Spiegelman, D.; Aster, J.C.; Zhang, S.M.; Laden, F. A prospective study of Epstein-Barr virus antibodies and risk of non-Hodgkin lymphoma. Blood 2010, 116, 3547–3553. [Google Scholar] [CrossRef] [Green Version]
- Neparidze, N.; Lacy, J. Malignancies associated with epstein-barr virus: Pathobiology, clinical features, and evolving treatments. Clin. Adv. Hematol. Oncol. 2014, 12, 358–371. [Google Scholar] [PubMed]
- Song, C.; Yang, S. A meta-analysis on the EBV DNA and VCA-IgA in diagnosis of Nasopharyngeal Carcinoma. Pak. J. Med. Sci. 2013, 29, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Klutts, J.S.; Ford, B.A.; Perez, N.R.; Gronowski, A.M. Evidence-based approach for interpretation of Epstein-Barr virus serological patterns. J. Clin. Microbiol. 2009, 47, 3204–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenson, H.B. Virologic Diagnosis, Viral Monitoring, and Treatment of Epstein-Barr Virus Infectious Mononucleosis. Curr. Infect. Dis. Rep. 2004, 6, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, B.; Sreeshyla, H.; Kishore, A.; Hegde, U.; Archana, S.; Sumana, M. Evaluation of infectious mononucleosis status among a cohort of dental students. Int. J. Adv. Med. 2016, 3, 116. [Google Scholar] [CrossRef]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H., Jr. Primary Epstein-Barr virus infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef]
- Binnicker, M.; Jespersen, D.; Harring, J.; Rollins, L.; Beito, E. Evaluation of a multiplex flow immunoassay for detection of Epstein-Barr virus-specific antibodies. Clin. Vaccine Immunol. 2008, 15, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Strašek, K.; Marin, J. Epstein-barr virus infections–avidity test for igg antibodies. Slovenian Med. J. 2001, 70. [Google Scholar]
- Niller, H.-H.; Bauer, G. Epstein-Barr virus: Clinical diagnostics. In Epstein Barr Virus; Springer Humana Press: New York, NY, USA, 2017; pp. 33–55. [Google Scholar]
- Kim, K.Y.; Le, Q.T.; Yom, S.S.; Pinsky, B.A.; Bratman, S.V.; Ng, R.H.; El Mubarak, H.S.; Chan, K.C.; Sander, M.; Conley, B.A. Current State of PCR-Based Epstein-Barr Virus DNA Testing for Nasopharyngeal Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Al Moustafa, A.E.; Ahmed, H.G.; Wulf, G.; Sultan, A.A. Editorial: EBV-Associated Carcinomas: Presence, Role, and Prevention Strategies. Front. Oncol. 2018, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- De Paschale, M.; Clerici, P. EBV infection in immunocompetent patient: Problems and solutions in serological diagnosis. Microbiol. Med. 2011, 26. [Google Scholar] [CrossRef] [Green Version]
- Gulley, M.L.; Tang, W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin. Microbiol. Rev. 2010, 23, 350–366. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.Y.; Zhang, Y.; Li, Y.H.; Gao, H.Y.; Feng, H.X.; Wu, Q.L.; Cui, N.J.; Cheng, G.; Hu, B.; Hu, L.F.; et al. Comparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. 2004, 24, 4059–4066. [Google Scholar]
- Teow, S.-Y.; Peh, S.-C. Exosomes as the promising biomarker for Epstein-Barr virus (EBV)-associated cancers. In Novel Implications of Exosomes in Diagnosis And Treatment of Cancer And Infectious Diseases; Wang, J., Ed.; Books on Demand: Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Zhao, M.; Nanbo, A.; Sun, L.; Lin, Z. Extracellular Vesicles in Epstein-Barr Virus’ Life Cycle and Pathogenesis. Microorganisms 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teow, S.Y.; Liew, K.; Khoo, A.S.; Peh, S.C. Pathogenic Role of Exosomes in Epstein-Barr Virus (EBV)-Associated Cancers. Int. J. Biol. Sci. 2017, 13, 1276–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Infected Cells | |||||
|---|---|---|---|---|---|
| Native B-cells | Germinal Center B-cells | Peripheral Memory B-cells | Dividing Peripheral Memory B-cells | Plasma Cells | |
| Transcription program | Latency III | Latency II | Latency 0 | Latency I | Lytic |
| Viral proteins | All EBNAs, EBERs, LMP-1, LMP-2A and LMP-2B | EBNA-1, EBERs, LMP-1 and LMP-2A | EBERs | EBNA-1 and EBERs. | All lytic genes |
| Function of viral proteins | Activate B-cell | Differentiate activated B-cell into memory B-cell | Allow for lifetime persistence | Allow for the virus in latency-programmed cell to divide | Assist viral replication in plasma cells |
| Associated malignancies | IM and post-transplant lymphoproliferative disorder | Nasal NK cell lymphoma, Hodgkin’s lymphoma, chronic active EBV infection, NPC and peripheral NK/T cell lymphoma | Healthy carrier | Burkitt lymphoma and gastric carcinoma | IM and NPC |
| Specimens for measuring viral load | Plasma or serum, MNCs and WBC | Plasma or serum, MNCs (for chronic active EBV infection), tissue biopsy | Plasma or serum, WBC | Plasma or serum | Plasma or serum |
| Tumor | Subtypes | Association with EBV (% cases) | References |
|---|---|---|---|
| Autoimmune disease | Multiple sclerosis | 99 | [23] |
| Systemic lupus erythematous | 99 | [23] | |
| Rheumatoid arthritis | 88 | [23] | |
| Sjogren’s syndrome | 57 | [20] | |
| XLP | XLP1 and XLP2 | 65 | [24] |
| Benign reactive infection | Infectious mononucleosis | >99 | [25] |
| Oral hairy leukoplakia | >95 | [25] | |
| Chronic active EBV infection | 100 | [25] | |
| Nasopharyngeal carcinoma | Non-keratinizing | 100 | [26] |
| Keratinizing | 30–100 | [26] | |
| Gastric carcinoma | UCNT | 100 | [26] |
| Adenocarcinoma | 5–15 | [26] | |
| Non-Hodgkin’s Lymphoma and Related Neoplasms | |||
| Burkitt lymphoma | Endemic | 100 | [27] |
| Sporadic | 10–80 | [27] | |
| AIDS-associated | 30–40 | [27] | |
| B-lymphoproliferative disease | Post-transplant | >90 | [27] |
| HIV-related | >90 | [27] | |
| Diffuse large B cell lymphoma | NOS | 10 | [27] |
| PAL | 100 | [27] | |
| HIV-related | 20–60 | [27] | |
| Rare immunocompromised B lymphomas | Plasmablastic lymphoma | 75–90 | [27] |
| Primary effusion lymphoma | 75–90 | [27] | |
| T/NK lymphoproliferative disease | CAEBV | 100 | [27] |
| Extra-nodal T/NK lymphoma | 100 | [27] | |
| Aggressive NK lymphoma | 100 | [27] | |
| Hodgkin’s Lymphoma | |||
| NLPHL | - | <4 (usually absent) | [28] |
| Classical Hodgkin’s lymphoma | All subtypes | 40 | [29] |
| Nodular sclerosis | 10–40 (variably present) | [27,30] | |
| Mixed cellularity | 70–80 (usually present) | [27,30] | |
| Lymphocyte depleted | 10–50 (variably present) | [27,30] | |
| Lymphocyte rich | 30–60 (variably present) | [27,30] | |
| HIV-related | >90 | [27,30] | |
| Method | Advantages | Disadvantages |
|---|---|---|
| Molecular methods (PCR and other nucleic amplification methods) | (1) Ability to differentiate between healthy carriers and patients with EBV-related disease based on viral load (low or high) (2) Low risk of contamination and reduced labor costs and turnaround time in qPCR (3) Allow for quantitative EBV DNA detection to monitor disease status. (4) Rapid (within 1 to 2 days) (5) More reliable than serological methods in terms of evaluating EBV status in immunocompromised patients (6) For early intervention, it is useful in screening high-risk populations and in monitoring EBV reactivation (7) Sensitive and specific across a wide dynamic range | (1) Could generate false-positive results due to improper blood sample storage and false-negative results due to the presence of nucleases (2) Lack of standardization (3) Expensive (4) Require special equipment |
| ISH | (1) Ability to identify EBV DNA or EBER transcripts in EBV–associated tumors. (2) Highly reliable confirmatory test for EBV (gold standard for EBV diagnosis) | (1) Only applicable to cells (2) Requires special skills (3) Could get counterproductive due to the histological interference between non-Hodgkin’s and Hodgkin’s lymphoma (4) EBER is downregulated in oral hairy leukoplakia |
| Heterophile antibody test | (1) Can measure heterophile antibodies released against serum viral proteins (2) Can differentiate between late primary infection and reactivation (3) Cost effective and easy to perform | (1) Less sensitive and less specific (especially in children) (2) Possibility of false-positive result in some cases of autoimmune disease (3) Possibility of false negative is high in young children |
| IFA (immunofluorescence assay) | (1) Gold standard reference method (2) Highly specific (3) Allows for the staging of EBV infections | (1) A high degree of variability (2) Lacks standardization (3) Equivocal diagnosis of acute EBV infection |
| EIAs and ELISA | (1) Rapid method (2) More sensitive than the IFA (3) Suitable for automation (4) Inexpensive (5) Less hands-on time | (1) Less specific (2) Difficulty in the staging of EBV infection (single patient’s serum) (3) Lack of standardization (4) Equivocal diagnosis of acute EBV infection |
| CLIA (chemiluminescence immunoassay) | Sensitive and specific in distinguishing primary infection (transient) from past infection | Requires further validation |
| Immunoblotting analysis | (1) Highly specific (2) Confirmatory method (3) Possibility of detecting the stage of EBV infection from serum (4) Detection of EBV-specific antibodies against several antigens | (1) Lack of the standardization of buffer conditions, the combination of recombinant antigens and the lysates from cell lines (2) Expensive |
| Immunoglobulin G (IgG) avidity testing | (1) Confirmatory test for intermediate results (2) Specifies the period of primary infection (3) Distinguishes active from past infections | (1) Depends on the individual maturation rates of antibodies (2) Not useful in newborns (due to maternal antibodies) |
| Viral cell culture | A precise and semi-quantitative method | (1) Expensive and time consuming (4–8 weeks) (2) Performed only in special laboratories (3) Requires trained personnel |
| Country | Sample Type | Sample Size | Seroprevalence (%) | Diagnostic Assay Used | Year |
|---|---|---|---|---|---|
| USA | Whole blood | 143 | 42 (29.3) | qPCR | 2012 |
| Whole blood | 92 | 75 (82) | In-house qPCR | 2012 | |
| Plasma | 116 | 15 (13) | - | - | |
| PMNCs | 64 | 56 (88) | - | - | |
| Oral wash: cell pellet | 143 | 66 (46) | - | - | |
| Supernatant | 61 (42.6) | - | - | ||
| Whole blood | 19 | 5 (26) | qPCR | 2016 | |
| Whole blood | 66 | 42 (64) | qPCR | 2013 | |
| Whole blood | 86 | 7 (8) | qPCR | 2016 | |
| Colombia | Saliva | 17 | 9 (52.9) | In-house qPCR | 2016 |
| Brazil | Saliva | 100 | 60 (60) | Nested PCR | 2018 |
| Saliva and fresh tissue | 17 each | 64.7 | Nested PCR | 2016 | |
| samples | 35.3 | ||||
| Scraping samples of the tongue lateral border | 53 | 53 (100) | Nested PCR | 2008 | |
| Australia | Tissue | 55 | 55 (100) | DNA sequence analysis | 2012 |
| CzechRepublic | Whole blood | 29 | 19 (66) | qPCR | 2011 |
| Plasma | 29 | 22 (76) | |||
| Poland | Fresh frozen tumor tissue oropharyngeal cancer | 78 | 40 (51.3) | Nested PCR | 2016 |
| Saliva | 40 healthy | 8 (20) | - | - | |
| Saliva | 56 | 22 (39.3) | Nested PCR | 2004 | |
| Sweden | Cervical secretion | 305 | 32 (10.5) | qPCR | - |
| Germany | Saliva | 47 | 14 (30) | PCR | 2017 |
| Serbia | Tissue | 80 | 37 (46.6) | Nested PCR | 2016 |
| Qatar | PMNCs | 673 | 354 (52.6) | qPCR | 2013 |
| China | PMNCs | 859 | 206 (24) | PCR-RFLP | 2017 |
| Plasma | 1318 | 69 (5.2) | qPCR | 2013 | |
| Saliva | 20 | 20 (100) | qPCR | 2015 | |
| Paraffin-embedded tissues | 209 | 146 (69.9) | qPCR | 2014 | |
| India | Serum | 40 | 37 (92.5) | Standard PCDE and PCR | 2016 |
| Egypt | Paraffin-embedded samples of breast tissue | 84 | 32 (38) | Nested PCR | 2017 |
| Eritrea | Formalin-fixed paraffin-embedded breast cancer tissue | 144 | 40 (27.77) | PCR | 2017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abusalah, M.A.H.; Gan, S.H.; Al-Hatamleh, M.A.I.; Irekeola, A.A.; Shueb, R.H.; Yean Yean, C. Recent Advances in Diagnostic Approaches for Epstein–Barr Virus. Pathogens 2020, 9, 226. https://doi.org/10.3390/pathogens9030226
Abusalah MAH, Gan SH, Al-Hatamleh MAI, Irekeola AA, Shueb RH, Yean Yean C. Recent Advances in Diagnostic Approaches for Epstein–Barr Virus. Pathogens. 2020; 9(3):226. https://doi.org/10.3390/pathogens9030226
Chicago/Turabian StyleAbusalah, Mai Abdel Haleem, Siew Hua Gan, Mohammad A. I. Al-Hatamleh, Ahmad Adebayo Irekeola, Rafidah Hanim Shueb, and Chan Yean Yean. 2020. "Recent Advances in Diagnostic Approaches for Epstein–Barr Virus" Pathogens 9, no. 3: 226. https://doi.org/10.3390/pathogens9030226
APA StyleAbusalah, M. A. H., Gan, S. H., Al-Hatamleh, M. A. I., Irekeola, A. A., Shueb, R. H., & Yean Yean, C. (2020). Recent Advances in Diagnostic Approaches for Epstein–Barr Virus. Pathogens, 9(3), 226. https://doi.org/10.3390/pathogens9030226