Bovine Papillomavirus Type 2 Infection Associated with Papillomatosis of the Amniotic Membrane in Water Buffaloes (Bubalus bubalis)
Abstract
1. Introduction
2. Results
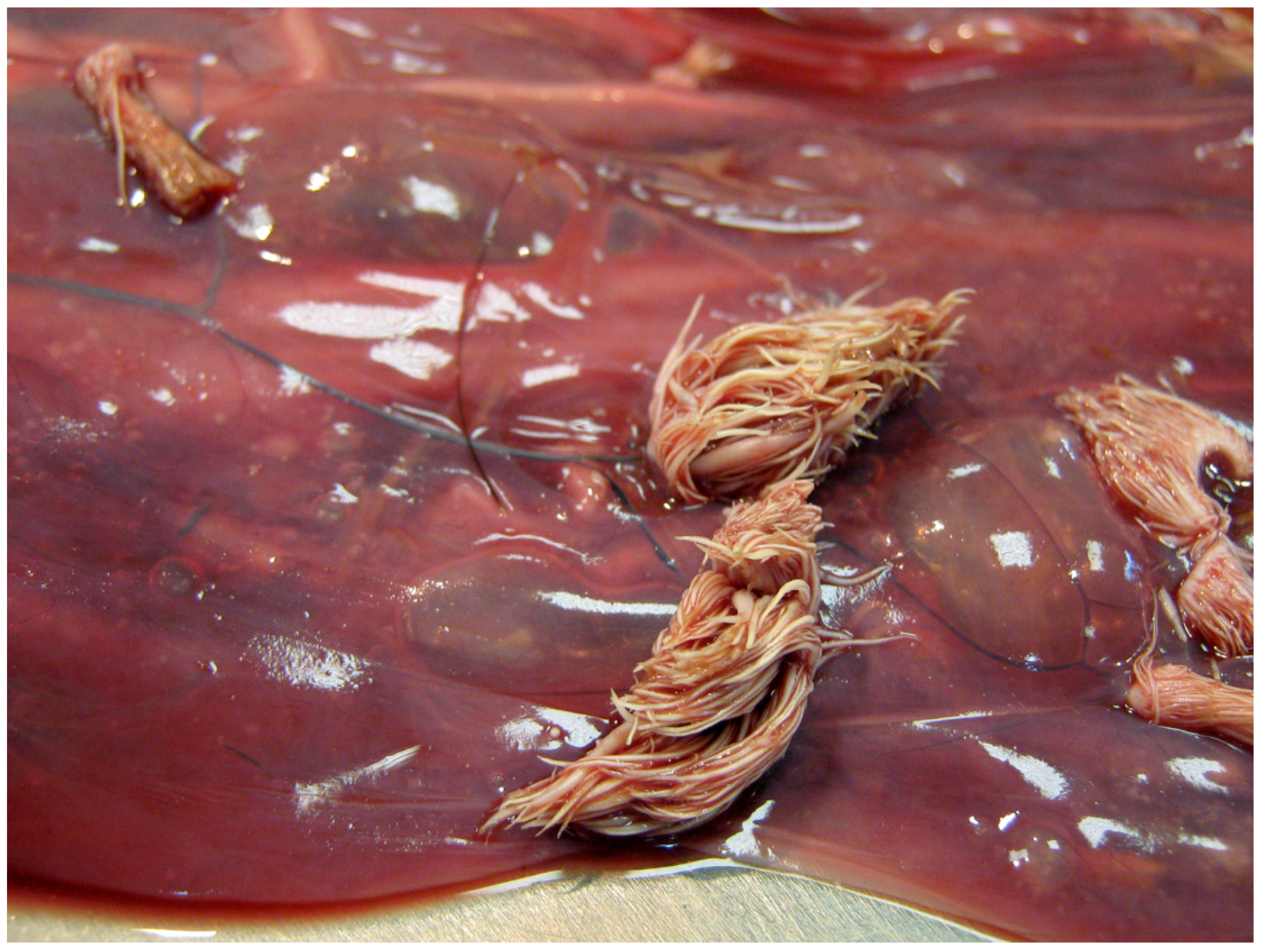
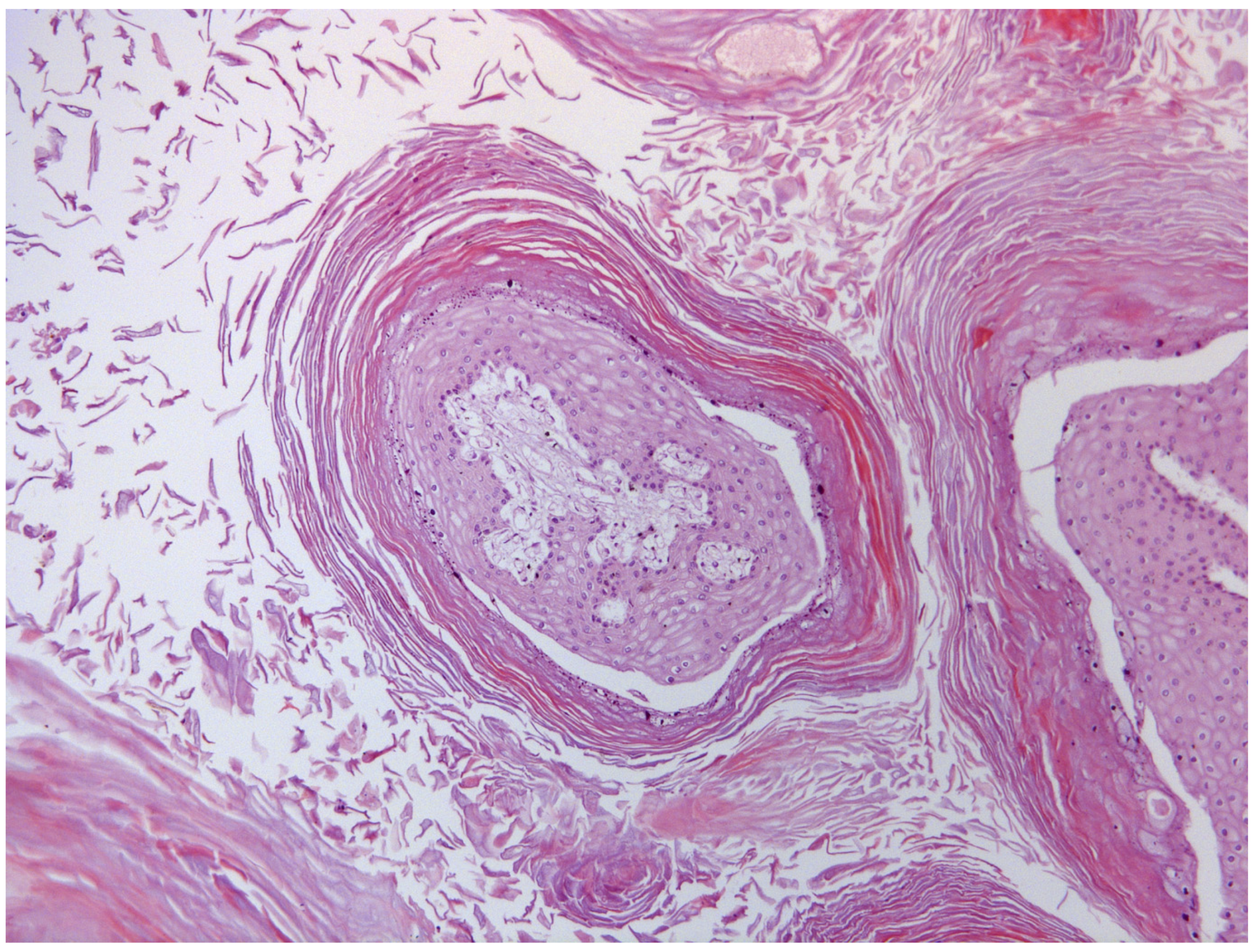
2.1. Gross and Microscopic Morphology
2.2. Virological Findings
2.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Animal and Tissue Samples
4.2. Immunohistochemistry
4.3. DNA Extraction and PCR Amplification
4.4. RNA Extraction and Reverse Transcription (RT)-PCR
4.5. Sequence Analysis
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomavirus; World Health Organization: Lyon, France, 2007; Volume 90, p. 47. [Google Scholar]
- Daudt, C.; da Silva, F.R.; Streck, A.F.; Weber, M.N.; Mayer, F.Q.; Cibulski, S.P.; Canal, C.W. How many papillomavirus species can go undetected in papilloma lesions? Sci. Rep. 2018, 6, 36480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crespo, S.E.I.; Lunardi, M.; Otonel, R.A.A.; Headley, S.A.; Alfieri, A.F.; Alfieri, A.A. Genetic characterization of a putative new type of bovine papillomavirus in the Xipapillomavirus 1 species in a Brazilian dairy herd. Virus Genes 2019, 55, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Papillomavirus Episteme (PaVE). 2017. Available online: http://pave.niaid.nih.gov (accessed on 1 March 2020).
- Campo, M.S.; Jarrett, W.F.H.; Barron, R.J.; O’Neil, B.W.; Smith, K.T. Association of bovine papillomavirus type 2 and bracken fern with bladder cancer in cattle. Cancer Res. 1992, 52, 6898–6904. [Google Scholar] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Roperto, S.; Munday, J.S.; Corrado, F.; Goria, M.; Roperto, F. Detection of bovine papillomavirus type 14 DNA sequences in urinary bladder tumors of cattle. Vet. Microbiol. 2016, 190, 1–4. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Leonardi, L.; Martano, M.; Corrado, F.; Riccardi, M.G.; Roperto, F. Bovine papillomavirus type 13 expression in the urothelial bladder tumours of cattle. Transbound. Emerg. Dis. 2016, 63, 628–634. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Ozkul, A.; Sepici-Dincel, A.; Maiolino, P.; Borzacchiello, G.; Esposito, I.; Riccardi, M.G.; Roperto, F. Bovine papillomavirus type 2 infects the urinary bladder of water buffalo (Bubalus bubalis) and plays a crucial role in bubaline urothelial carcinogenesis. J. Gen. Virol. 2013, 94, 403–408. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Corrado, F.; De Falco, F.; Munday, J.S.; Roperto, F. Oral fibropapillomatosis and cutaneous pseudocarcinomatous hyperplasia of the muzzle in newborn lambs associated with bovine Deltapapillomavirus: A novel trans-species infection. Sci. Rep. 2018, 8, 13310. [Google Scholar] [CrossRef]
- Lancaster, W.D.; Theilen, G.H.; Olson, C. Hybridization of bovine papilloma virus type 1 and type 2 DNA to DNA from virus-induced hamster tumors and naturally occurring equine tumors. Intervirology 1979, 11, 227–233. [Google Scholar] [CrossRef]
- Lunardi, M.; de Alcântar, B.K.; Ortonel, R.A.; Rodrigues, W.B.; Alfieri, A.F.; Alfieri, A.A. Bovine papillomavirus type 13 DNA in equine sarcoids. J. Clin. Microbiol. 2013, 51, 2167–2171. [Google Scholar] [CrossRef]
- Orbell, G.M.; Young, S.; Munday, J.S. Cutaneous sarcoids in captive African lions associated with feline sarcoid-associated papillomavirus infection. Vet. Pathol. 2011, 48, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Thomson, N.; Dunowska, M.; Knight, C.G.; Laurie, R.E.; Hills, S. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet. Microbiol. 2015, 177, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kainzbauer, C.; Rushton, J.; Tober, R.; Scase, T.; Nell, B.; Sykora, S.; Brandt, S. Bovine papillomavirus type 1 and Equus caballus papillomavirus 2 in equine squamous cell carcinoma of the head and neck in a Connemara mare. Equine Vet. J. 2012, 44, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Epperson, E.D.; Castleman, W.L. Bovine papillomavirus DNA and S100 profiles in sarcoids and other cutaneous spindle cell tumors in horses. Vet. Pathol. 2017, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; van Dyk, E.; Nel, P.J.; Lane, E.; Van Wilpe, E.; Bengis, R.G.; de Klerk-Lorist, L.M.; van Heerden, J. Pathology and immunohistochemistry of papillomavirus-associated cutaneous lesions in Cape mountain zebra, giraffe, sable antelope and African buffalo in South Africa. J. S. Afr. Vet. Assoc. 2011, 82, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Russo, V.; Corrado, F.; Munday, J.S.; De Falco, F.; Roperto, F. Detection of bovine Deltapapillomavirus DNA in peripheral blood of healthy sheep (Ovis aries). Transbound. Emerg. Dis. 2018, 65, 758–764. [Google Scholar] [CrossRef]
- Russo, V.; Roperto, F.; Esposito, I.; Ceccarelli, D.M.; Zizzo, N.; Leonardi, L.; Capparelli, R.; Borzacchiello, G.; Roperto, S. ERas protein is overexpressed and binds to the activated platelet-derived growth factor β receptor in bovine urothelial cells associated with papillomavirus infection. Vet. J. 2016, 212, 44–47. [Google Scholar] [CrossRef]
- Savini, F.; Dal Molin, E.; Gallina, L.; Casà, G.; Scagliarini, A. Papillomavirus in healthy skin and mucosa of wild ruminants in the Italian Alps. J. Wildl. Dis. 2016, 52, 82–87. [Google Scholar] [CrossRef]
- Pangty, K.; Singh, S.; Goswami, R.; Saikumar, G.; Somvanshi, R. Detection of BPV-1 and -2 and quantification of BPV-1 by real-time PCR in cutaneous warts in cattle and buffaloes. Transbound. Emerg. Dis. 2010, 57, 185–196. [Google Scholar] [CrossRef]
- Silvestre, O.; Borzacchiello, G.; Nava, D.; Iovane, G.; Russo, V.; Vecchio, D.; D’Ausilio, F.; Gault, E.A.; Campo, S.; Paciello, O. Bovine papillomavirus type 1 DNA and E5 oncoprotein expression in water buffalo fibropapillomas. Vet. Pathol. 2009, 46, 636–641. [Google Scholar] [CrossRef]
- Somvanshi, R. Papillomatosis in buffaloes: A less-known disease. Transbound. Emerg. Dis. 2011, 58, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.J.; Murphy, E.C. Rare bovine placental tumour–A case report. Vet. Rec. 1965, 77, 1234–1235. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, C.A.; Bicknell, E.J.; Robl, M.G. Hemangiomas of a bovine fetus with a chorioangioma of the placenta. Vet. Pathol. 1973, 10, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Miliaras, D.; Anagnostou, E.; Papoulidis, I.; Miliara, X. Non-trophoblastic tumor of the placenta with combined histologic features of chorangioma and leiomyoma. Placenta 2011, 32, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.; Douroudis, K.; Westgren, M.; Papadogiannakis, N. Association of chorangiomas to hypoxia-related placental changes in singleton and multiple pregnancy placentas. Placenta 2016, 39, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; Foster, R.A. Female Genital System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 3, pp. 359–464. [Google Scholar]
- Karabadzhak, A.G.; Petti, L.M.; Barrera, F.N.; Edwards, A.P.B.; Moya-Rodriguez, A.; Polikanov, Y.S.; Freites, J.A.; Tobias, D.J.; Engelman, D.M.; DiMaio, D. Two transmembrane dimers of the bovine papillomavirus E5 oncoprotein clamp the PDGF β receptor in an active dimeric conformation. Proc. Natl. Acad. Sci. USA 2017, 114, E7262–E7271. [Google Scholar] [CrossRef]
- Gaultier, F.; Ejeil, A.L.; Lepelletier, F.; Gogly, B.; Cherifi, H.; Bayet, K.; Dridi, S.M. Clinical and histopathological features of a lingual mucosal horn: First time described clinical case series. Dent. Oral Craniofac. Res. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Naaktgeboren, C.; Zwillenberg, H.H. Research on the tumors of the amnion and umbilical cord in whales and hoofed animals, with special regard to European domestic cattle. Acta Morphol. Neerl. Scand. 1961, 4, 31–60. [Google Scholar]
- Roperto, S.; Russo, V.; De Falco, F.; Taulescu, M.; Roperto, F. Congenital papillomavirus infection in cattle: Evidence for transplacental transmission. Vet. Microbiol. 2019, 230, 95–100. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Russo, V.; Gentile, F.; Roperto, F.; Venuti, A.; Nitsch, L.; Campo, M.S.; Roperto, S. Bovine papillomavirus E5 oncoprotein binds to the activated form of the platelet-derived growth factor β receptor in naturally occurring bovine urinary bladder tumours. Oncogene 2006, 25, 1251–1260. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Ozkul, A.; Corteggio, A.; Sepici-Dincel, A.; Catoi, C.; Esposito, I.; Riccardi, M.C.; Urraro, C.; Lucà, R.; et al. Productive infection of bovine papillomavirus type 2 in the urothelial cells of naturally occurring urinary bladder tumors in cattle and water buffaloes. PLoS ONE 2013, 8, e62227. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Kaufmann, P.; Baergen, R.N. Pathology of the Human Placenta, 5th ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Da Silva, V.M.F.; Carter, A.M.; Ambrosio, C.E.; Carvalho, A.F.; Bonatelli, M.; Lima, M.C.; Miglino, M.A. Placentation in dolphins from the Amazon River basin: The Boto, Inia geoffrensis, and the Tucuxi, Sotalia fluviatilis. Reprod. Biol. Endocrinol. 2007, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.A.; Seal, U.S.; Erickson, A.W. Ultrastructure of the amnion and amniotic plaques of the white-tailed deer. Am. J. Anat. 1970, 127, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.; Phaneuf, J.B.; Cécyre, A.; Larouche, Y. Métaplasie et kératinisation des enveloppes foetales. Can. Vet. J. 1974, 15, 354–356. [Google Scholar]
- Pourlis, A.F.; Christodoulopoulos, G.; Magras, I.N. The amniotic plaques in sheep of the Karagouniko breed. Res. Vet. Sci. 2008, 85, 201–203. [Google Scholar] [CrossRef]
- Turner, C.B.; Garlick, P.R. Structure of amniotic papillae in sheep. J. Anat. 1978, 126, 385–392. [Google Scholar]
- Roperto, S.; Borzacchiello, G.; Esposito, I.; Riccardi, M.G.; Urraro, C.; Lucà, R.; Corteggio, A.; Tatè, R.; Cermola, M.; Paciello, O.; et al. Productive infection of bovine papillomavirus type 2 in the placenta of pregnant cows affected with urinary bladder tumors. PLoS ONE 2012, 27, e33569. [Google Scholar] [CrossRef]
- Savini, F.; Gallina, L.; Mazza, F.; Mariella, J.; Castagnetti, C.; Scagliarini, A. Molecular detection of bovine papillomavirus DNA in the placenta and blood of healthy mares and respective foals. Vet. Sci. 2019, 6, 14. [Google Scholar] [CrossRef]
- Bjarnegård, M.; Enge, M.; Norlin, J.; Gustafsdottir, S.; Fredriksson, S.; Abramsson, A.; Takemoto, M.; Gustafsson, E.; Fässler, R.; Betsholtz, C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 2004, 131, 1847–1857. [Google Scholar] [CrossRef]
- Magnusson, P.U.; Looman, C.; Åhgren, A.; Wu, Y.; Claesson-Welsh, L.; Heuchel, R.L. Platelet-derived growth factor receptor-β constitutive activity promotes angiogenesis In Vivo and In Vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2142–2149. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Donders, G.; Verstraetem, L.; Vanden Broeck, D.; Beert, J.; Salembier, G.; Bosmans, E.; DhontT, N.; Van Der Auwera, I.; Vandenborne, K.; et al. Time has come to include Human Papillomavirus (HPV) testing in sperm donors bank. Facts Views Vis. Obgyn 2018, 10, 201–205. [Google Scholar] [PubMed]
- Moragianni, D.; Dryllis, G.; Andromidas, P.; Kapeta-Korkouli, R.; Kouskouni, E.; Pessach, I.; Papalexis, P.; Kodonaki, A.; Athanasiou, N.; Pouliakis, A.; et al. Genital tract infection and associated factors affect reproductive outcome in fertile females and females undergoing In Vitro fertilization. Biomed. Rep. 2019, 10, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Beert, J.; Bosmans, E.; Salember, G. Human papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis. Obgyn 2016, 8, 211–222. [Google Scholar] [PubMed]
- Yoo, H.S. Infectious causes of reproductive disorders in cattle. J. Reprod. Dev. 2010, 56, S53–S60. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, V.; Roperto, F.; De Biase, D.; Cerino, P.; Urraro, C.; Munday, J.S.; Roperto, S. Bovine Papillomavirus Type 2 Infection Associated with Papillomatosis of the Amniotic Membrane in Water Buffaloes (Bubalus bubalis). Pathogens 2020, 9, 262. https://doi.org/10.3390/pathogens9040262
Russo V, Roperto F, De Biase D, Cerino P, Urraro C, Munday JS, Roperto S. Bovine Papillomavirus Type 2 Infection Associated with Papillomatosis of the Amniotic Membrane in Water Buffaloes (Bubalus bubalis). Pathogens. 2020; 9(4):262. https://doi.org/10.3390/pathogens9040262
Chicago/Turabian StyleRusso, Valeria, Franco Roperto, Davide De Biase, Pellegrino Cerino, Chiara Urraro, John S. Munday, and Sante Roperto. 2020. "Bovine Papillomavirus Type 2 Infection Associated with Papillomatosis of the Amniotic Membrane in Water Buffaloes (Bubalus bubalis)" Pathogens 9, no. 4: 262. https://doi.org/10.3390/pathogens9040262
APA StyleRusso, V., Roperto, F., De Biase, D., Cerino, P., Urraro, C., Munday, J. S., & Roperto, S. (2020). Bovine Papillomavirus Type 2 Infection Associated with Papillomatosis of the Amniotic Membrane in Water Buffaloes (Bubalus bubalis). Pathogens, 9(4), 262. https://doi.org/10.3390/pathogens9040262





