Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept
Abstract
:1. Introduction
2. Methods
2.1. Systematic Review Protocols
2.2. Search Strategy and Eligibility Criteria
2.3. Data Screening
- Only those articles reporting vector-borne and zoonotic viral diseases within Africa.
- The abstract of the stored articles were first read through to find out their relevance to be included, and, if necessary, the introduction and/or results and discussion sections of each article were thoroughly investigated to ensure that the articles met the inclusion criteria.
2.4. Statistical Analysis
3. Results and Discussion
3.1. East Africa
3.1.1. Comoros Island
3.1.2. Djibouti
3.1.3. Eritrea
3.1.4. Ethiopia
3.1.5. Kenya
3.1.6. La Reunion
3.1.7. Madagascar
3.1.8. Malawi
3.1.9. Mauritius
3.1.10. Mozambique
3.1.11. Seychelles
3.1.12. Somalia
3.1.13. Tanzania
3.1.14. Uganda
3.1.15. Zambia
3.1.16. Zimbabwe
3.2. West Africa
3.2.1. Benin
3.2.2. Burkina Faso
3.2.3. Cape Verde
3.2.4. Cote d’ Ivoire
3.2.5. Gambia
3.2.6. Ghana
3.2.7. Guinea
3.2.8. Guinea-Bissau
3.2.9. Liberia
3.2.10. Mali
3.2.11. Mauritania
3.2.12. Niger
3.2.13. Nigeria
3.2.14. Senegal
3.2.15. Sierra Leone
3.2.16. Togo
3.3. Central Africa
3.3.1. Angola
3.3.2. Burundi
3.3.3. Cameroon
3.3.4. Central African Republic
3.3.5. Chad
3.3.6. Democratic Republic of Congo
3.3.7. Equatorial Guinea
3.3.8. Gabon
3.3.9. Republic of Congo
3.3.10. Rwanda
3.3.11. Sao Tome and Principe
3.3.12. South Sudan
3.4. North Africa
3.4.1. Algeria
3.4.2. Egypt
3.4.3. Libya
3.4.4. Morocco
3.4.5. Sudan
3.4.6. Tunisia
3.5. Southern Africa
3.5.1. Botswana
3.5.2. Lesotho
3.5.3. Namibia
3.5.4. South Africa
3.5.5. Kingdom of Eswatini
3.6. Summary
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ndembi, N.; Habakkuk, Y.; Takehisa, J.; Takemura, T.; Kobayashi, E.; Ngansop, C.; Songok, E.; Miura, T.; Ido, E.; Hayami, M.; et al. HIV type 1 infection in Pygmy hunter gatherers is from contact with Bantu rather than from nonhuman primates. AIDS Res. Hum. Retrovir. 2003, 19, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Lahm, S.A.; Kombila, M.; Swanepoel, R.; Barnes, R.F. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994–2003. Trans. R. Soc. Trop Med. Hyg. 2007, 101, 64–78. [Google Scholar] [CrossRef]
- World Health Organization. Ebola Virus Disease- Democratic Republic of Congo, External Situation Report 82; WHO Regional Centre for Africa: Pretoria, South Africa, 2020; pp. 1–9. [Google Scholar]
- Okareh, O.T.; Morakinyo, O.M. Monkeypox in Nigeria: A case report of re-emerged disease outbreak. J. Microbiol. Exp. 2018, 6, 89–91. [Google Scholar]
- Abolnik, C.; Olivier, A.J.; Grewar, J.; Gers, S.; Romito, M. Molecular analysis of the 2011 HPAI H5N2 outbreak in ostriches, South Africa. Avian Dis. 2012, 56, 865–879. [Google Scholar] [CrossRef]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O. Molecular detection of influenza A(H1N1)pdm09 viruses with M genes from human pandemic strains among Nigerian pigs, 2013–2015: Implications and associated risk factors. Epidemiol. Infect. 2017, 145, 3345–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriamandimby, S.F.; Marianneau, P.; Rafisandratantsoa, J.T.; Rollin, P.E.; Heraud, J.M.; Tordo, N.; Reynes, J.M. Crimean-Congo hemorrhagic fever serosurvey in at-risk professionals, Madagascar, 2008 and 2009. J. Clin. Virol. 2011, 52, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Benjelloun, A.; El Harrak, M.; Belkadi, B. West Nile Disease Epidemiology in North-West Africa: Bibliographical Review. Transbound Emerg. Dis. 2016, 63, e153–e159. [Google Scholar] [CrossRef]
- Budasha, N.H.; Gonzalez, J.P.; Sebhatu, T.T.; Arnold, E. Rift Valley fever seroprevalence and abortion frequency among livestock of Kisoro district, South Western Uganda (2016): A prerequisite for zoonotic infection. BMC Vet. Res. 2018, 14, 271. [Google Scholar] [CrossRef]
- David, D.; Hughes, G.J.; Yakobson, B.A.; Davidson, I.; Un, H.; Aylan, O.; Kuzmin, I.V.; Rupprecht, C.E. Identification of novel canine rabies virus clades in the Middle East and North Africa. J. Gen. Virol. 2007, 88, 967–980. [Google Scholar] [CrossRef] [Green Version]
- Krauss, S.; Webster, R.G. Avian Influenza Virus Surveillance and Wild Birds: Past and Present. Avian Dis. 2010, 54, 394–398. [Google Scholar] [CrossRef]
- Clark, L.; Hall, J. Avian influenza in wild birds: Status as reservoirs, and risks to humans and agriculture. Ornithol. Monogr. 2006, 60, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Allwright, D.M.; Burger, W.P.; Geyer, A.; Terblanche, A.W. Isolation of an influenza A virus from ostriches (Struthio camelus). Avian Pathol. 1993, 22, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Asante, I.A.; Bertram, S.; Awuni, J.; Commey, A.N.; Aniwa, B.; Ampofo, W.K.; Gabriel, G. Highly Pathogenic Avian Influenza A(H5N1) Virus among Poultry, Ghana, 2015. Emerg. Infect. Dis. 2016, 22, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, M.F.; Olinger, C.M.; Owoade, A.A.; Tarnagda, Z.; Tahita, M.C.; Sow, A.; De Landtsheer, S.; Ammerlaan, W.; Ouedraogo, J.B.; Osterhaus, A.D.; et al. Molecular and antigenic evolution and geographical spread of H5N1 highly pathogenic avian influenza viruses in western Africa. J. Gen. Virol. 2007, 88, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; Treurnicht, F.K.; Buys, A.; Tempia, S.; Samudzi, R.; McAnerney, J.; Jacobs, C.A.; Thomas, J.; Blumberg, L. Risk of Human Infections with Highly Pathogenic H5N2 and Low Pathogenic H7N1 Avian Influenza Strains During Outbreaks in Ostriches in South Africa. J. Infect. Dis. 2017, 216, S512–S519. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Skidmore, A.K.; Wang, T.; de Boer, W.F.; Debba, P.; Toxopeus, A.G.; Li, L.; Prins, H.H. Spatio-temporal dynamics of global H5N1 outbreaks match bird migration patterns. Geospat Health 2009, 4, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Gschweng, M.; Kalko, E.K.; Querner, U.; Fiedler, W.; Berthold, P. All across Africa: Highly individual migration routes of Eleonora’s falcon. Proc. Biol. Sci. 2008, 275, 2887–2896. [Google Scholar] [CrossRef] [Green Version]
- Ofula, V.O.; Franklin, A.B.; Root, J.J.; Sullivan, H.J.; Gichuki, P.; Makio, A.; Bulimo, W.; Abong’o, B.O.; Muchai, M.; Schnabel, D. Detection of Avian Influenza Viruses in Wild Waterbirds in the Rift Valley of Kenya Using Fecal Sampling. Vector-Borne Zoonot 2013, 13, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, J.; Qiao, C.; Yang, H.; Zhang, Y.; Xin, X.; Chen, H. Co-circulation of pandemic 2009 H1N1, classical swine H1N1 and avian-like swine H1N1 influenza viruses in pigs in China. Infect. Genet. Evol. 2013, 13, 331–338. [Google Scholar] [CrossRef]
- Brown, I.H. The epidemiology and evolution of influenza viruses in pigs. Vet. Microbiol. 2000, 74, 29–46. [Google Scholar] [CrossRef]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog 2013, 9, e1003176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meseko, C.A.; Odaibo, G.N.; Olaleye, D.O. Detection and isolation of 2009 pandemic influenza A/H1N1 virus in commercial piggery, Lagos Nigeria. Vet. Microbiol. 2014, 168, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Gramer, M.R.; Vincent, A.L.; Holmes, E.C. Global transmission of influenza viruses from humans to swine. J. Gen. Virol. 2012, 93, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Awada, L.; Brown, I.; Chen, H.; Claes, F.; Dauphin, G.; Donis, R.; Culhane, M.; Hamilton, K.; Lewis, N.; et al. Review of influenza A virus in swine worldwide: A call for increased surveillance and research. Zoonoses Public Health 2014, 61, 4–17. [Google Scholar] [CrossRef]
- Webster, R.G.; Shortridge, K.F.; Kawaoka, Y. Influenza: Interspecies transmission and emergence of new pandemics. FEMS Immunol. Med. Microbiol. 1997, 18, 275–279. [Google Scholar] [CrossRef]
- Webster, R.G. Influenza virus: Transmission between species and relevance to emergence of the next human pandemic. Arch. Virol. Suppl. 1997, 13, 105–113. [Google Scholar]
- Wentworth, D.E.; McGregor, M.W.; Macklin, M.D.; Neumann, V.; Hinshaw, V.S. Transmission of swine influenza virus to humans after exposure to experimentally infected pigs. J. Infect. Dis. 1997, 175, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Munyua, P.; Onyango, C.; Mwasi, L.; Waiboci, L.W.; Arunga, G.; Fields, B.; Mott, J.A.; Cardona, C.J.; Kitala, P.; Nyaga, P.N.; et al. Identification and characterization of influenza A viruses in selected domestic animals in Kenya, 2010–2012. PLoS ONE 2018, 13, e0192721. [Google Scholar] [CrossRef]
- Meseko, C.A.; Globig, A.; Ijomanta, J.; Joannis, T.; Nwosuh, C.; Shamaki, D.; Harder, T.; Hoffman, D.; Pohlmann, A.; Beer, M.; et al. Evidence of exposure of domestic pigs to Highly Pathogenic Avian Influenza H5N1 in Nigeria. Sci. Rep. 2018, 8, 5900. [Google Scholar] [CrossRef]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O. Detection of pandemic strain of influenza virus (A/H1N1/pdm09) in pigs, West Africa: Implications and considerations for prevention of future influenza pandemics at the source. Infect. Ecol. Epidemiol. 2015, 5, 30227. [Google Scholar] [CrossRef]
- Njabo, K.Y.; Fuller, T.L.; Chasar, A.; Pollinger, J.P.; Cattoli, G.; Terregino, C.; Monne, I.; Reynes, J.M.; Njouom, R.; Smith, T.B. Pandemic A/H1N1/2009 influenza virus in swine, Cameroon, 2010. Vet. Microbiol. 2012, 156, 189–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducatez, M.F.; Awoume, F.; Webby, R.J. Influenza A(H1N1)pdm09 virus in pigs, Togo, 2013. Vet. Microbiol. 2015, 177, 201–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, M.R.; Kandeil, A.; El-Shesheny, R.; Shehata, M.M.; McKenzie, P.P.; Webby, R.J.; Ali, M.A.; Kayali, G. Evidence of infection with avian, human, and swine influenza viruses in pigs in Cairo, Egypt. Arch. Virol. 2018, 163, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, E.K.; Awoyelu, E.H.; Oloke, J.K. Yellow fever, Dengue fever and West Nile viruses co-circulation in Ogbomoso. BioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- McVey, D.S.; Wilson, W.C.; Gay, C.G. West Nile virus. Rev. Sci Tech. 2015, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect. Dis 2002, 8, 392–397. [Google Scholar] [CrossRef]
- Kenawy, M.A.; Abdel-Hamid, Y.M.; Beier, J.C. Rift Valley Fever in Egypt and other African countries: Historical review, recent outbreaks and possibility of disease occurrence in Egypt. Acta Trop 2018, 181, 40–49. [Google Scholar] [CrossRef]
- Gear, J.H.; Thomson, P.D.; Hopp, M.; Andronikou, S.; Cohn, R.J.; Ledger, J.; Berkowitz, F.E. Congo-Crimean haemorrhagic fever in South Africa. Report of a fatal case in the Transvaal. S. Afr. Med. J. 1982, 62, 576–580. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, W49–W65. [Google Scholar] [CrossRef]
- Melade, J.; McCulloch, S.; Ramasindrazana, B.; Lagadec, E.; Turpin, M.; Pascalis, H.; Goodman, S.M.; Markotter, W.; Dellagi, K. Serological Evidence of Lyssaviruses among Bats on Southwestern Indian Ocean Islands. PLoS ONE 2016, 11, e0160553. [Google Scholar] [CrossRef] [Green Version]
- Cosby, M.T.; Pimentel, G.; Nevin, R.L.; Fouad Ahmed, S.; Klena, J.D.; Amir, E.; Younan, M.; Browning, R.; Sebeny, P.J. Outbreak of H3N2 influenza at a US military base in Djibouti during the H1N1 pandemic of 2009. PLoS ONE 2013, 8, e82089. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.; Ball, J.D.; Rojas, D.P.; Berhane, A.; Ghebrat, Y.; Mebrahtu, G.; Gebresellasie, A.; Zehaie, A.; Mufunda, J.; Liseth, O.; et al. Dengue fever outbreaks in Eritrea, 2005–2015: A case for strengthening surveillance, control and reporting. Glob. Health Res. Policy 2016, 1, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Correction: Estimating the global burden of endemic canine rabies. PLoS Negl. Trop Dis 2015, 9, e0003786. [Google Scholar] [CrossRef] [PubMed]
- Jemberu, W.T.; Molla, W.; Almaw, G.; Alemu, S. Incidence of rabies in humans and domestic animals and people’s awareness in North Gondar Zone, Ethiopia. PLoS Negl. Trop Dis. 2013, 7, e2216. [Google Scholar] [CrossRef] [Green Version]
- Paulos, A.; Eshetu, Y.; Bethelhem, N.; Abebe, B.; Badeg, Z. A study on the prevalence of animal rabies in Addis Ababa during 1999–2002. Ethiop. Vet. J. 2002, 7, 69–77. [Google Scholar]
- Reusken, C.B.; Messadi, L.; Feyisa, A.; Ularamu, H.; Godeke, G.J.; Danmarwa, A.; Dawo, F.; Jemli, M.; Melaku, S.; Shamaki, D.; et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014, 20, 1370–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisay, Z.; Djikeng, A.; Berhe, N.; Belay, G.; Abegaz, W.E.; Wang, Q.H.; Saif, L.J. First detection and molecular characterization of sapoviruses and noroviruses with zoonotic potential in swine in Ethiopia. Arch. Virol. 2016, 161, 2739–2747. [Google Scholar] [CrossRef]
- Salem, E.; Cook, E.A.J.; Lbacha, H.A.; Oliva, J.; Awoume, F.; Aplogan, G.L.; Hymann, E.C.; Muloi, D.; Deem, S.L.; Alali, S.; et al. Serologic Evidence for Influenza C and D Virus among Ruminants and Camelids, Africa, 1991–2015. Emerg Infect. Dis. 2017, 23, 1556–1559. [Google Scholar] [CrossRef] [Green Version]
- Shieh, W.J.; Paddock, C.D.; Lederman, E.; Rao, C.Y.; Gould, L.H.; Mohamed, M.; Mosha, F.; Mghamba, J.; Bloland, P.; Njenga, M.K.; et al. Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am. J. Trop Med. Hyg. 2010, 83, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Amimo, J.O.; El Zowalaty, M.E.; Githae, D.; Wamalwa, M.; Djikeng, A.; Nasrallah, G.K. Metagenomic analysis demonstrates the diversity of the fecal virome in asymptomatic pigs in East Africa. Arch. Virol. 2016, 161, 887–897. [Google Scholar] [CrossRef]
- Caini, S.; Spreeuwenberg, P.; Kusznierz, G.F.; Rudi, J.M.; Owen, R.; Pennington, K.; Wangchuk, S.; Gyeltshen, S.; Ferreira de Almeida, W.A.; Pessanha Henriques, C.M.; et al. Distribution of influenza virus types by age using case-based global surveillance data from twenty-nine countries, 1999–2014. BMC Infect. Dis. 2018, 18, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apopo, A.A.; Kariithi, H.M.; Ateya, L.O.; Binepal, Y.S.; Sirya, J.H.; Dulu, T.D.; Welch, C.N.; Hernandez, S.M.; Afonso, C.L. A retrospective study of Newcastle disease in Kenya. Trop. Anim. Health Prod. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ommeh, S.; Zhang, W.; Zohaib, A.; Chen, J.; Zhang, H.; Hu, B.; Ge, X.Y.; Yang, X.L.; Masika, M.; Obanda, V.; et al. Genetic Evidence of Middle East Respiratory Syndrome Coronavirus (MERS-Cov) and Widespread Seroprevalence among Camels in Kenya. Virol. Sin. 2018, 33, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raharinosy, V.; Olive, M.M.; Andriamiarimanana, F.M.; Andriamandimby, S.F.; Ravalohery, J.P.; Andriamamonjy, S.; Filippone, C.; Rakoto, D.A.D.; Telfer, S.; Heraud, J.M. Geographical distribution and relative risk of Anjozorobe virus (Thailand orthohantavirus) infection in black rats (Rattus rattus) in Madagascar. Virol. J. 2018, 15, 83. [Google Scholar] [CrossRef] [Green Version]
- Schwartz-Cornil, I.; Mertens, P.P.; Contreras, V.; Hemati, B.; Pascale, F.; Breard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue virus: Virology, pathogenesis and immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef] [Green Version]
- Andriamandimby, S.F.; Viarouge, C.; Ravalohery, J.P.; Reynes, J.M.; Sailleau, C.; Tantely, M.L.; Elissa, N.; Cardinale, E.; Sall, A.A.; Zientara, S.; et al. Detection in and circulation of Bluetongue virus among domestic ruminants in Madagascar. Vet. Microbiol. 2015, 176, 268–273. [Google Scholar] [CrossRef]
- Chevalier, V.; Rakotondrafara, T.; Jourdan, M.; Heraud, J.M.; Andriamanivo, H.R.; Durand, B.; Ravaomanana, J.; Rollin, P.E.; Rakotondravao, R. An unexpected recurrent transmission of Rift Valley fever virus in cattle in a temperate and mountainous area of Madagascar. PLoS Negl. Trop Dis. 2011, 5, e1423. [Google Scholar] [CrossRef]
- Temmam, S.; Besnard, L.; Andriamandimby, S.F.; Foray, C.; Rasamoelina-Andriamanivo, H.; Heraud, J.M.; Cardinale, E.; Dellagi, K.; Pavio, N.; Pascalis, H.; et al. High prevalence of hepatitis E in humans and pigs and evidence of genotype-3 virus in swine, Madagascar. Am. J. Trop Med. Hyg. 2013, 88, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Peel, A.J.; Sargan, D.R.; Baker, K.S.; Hayman, D.T.S.; Barr, J.A.; Crameri, G.; Suu-Ire, R.; Broder, C.C.; Lembo, T.; Wang, L.F.; et al. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 2013, 4, 2770. [Google Scholar] [CrossRef] [Green Version]
- Haresnape, J.M.; Lungu, S.A.; Mamu, F.D. A four-year survey of African swine fever in Malawi. J. Hyg. (Lond.) 1985, 95, 309–323. [Google Scholar] [CrossRef] [Green Version]
- Haresnape, J.M.; Wilkinson, P.J.; Mellor, P.S. Isolation of African swine fever virus from ticks of the Ornithodoros moubata complex (Ixodoidea: Argasidae) collected within the African swine fever enzootic area of Malawi. Epidemiol. Infect. 1988, 101, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudo, E.S.; Lesko, B.; Vene, S.; Lagerqvist, N.; Candido, S.I.; Razao de Deus, N.; Pinto, F.D.; Pinto, G.; Monteiro, V.; Evaristo, V.L.; et al. Seroepidemiologic Screening for Zoonotic Viral Infections, Maputo, Mozambique. Emerg. Infect. Dis. 2016, 22, 915–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tivane, A.; Daniels, R.; Nguenha, N.; Machalele, L.; Nacoto, A.; Pale, M.; Mateonane, E.; Mavale, S.; Chilundo, J.; Muteto, D.; et al. Antigenic and genetic characterization of influenza viruses isolated in Mozambique during the 2015 season. PLoS ONE 2018, 13, e0201248. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, A.; Aceti, A.; Paparo, B.S.; Pennica, A.; Ilardi, I.; Bile, K.; Mohamud, O.M. Hepatitis B virus circulation in three different villages of Somalia. Trans. R. Soc. Trop Med. Hyg. 1985, 79, 162–164. [Google Scholar] [CrossRef]
- Watts, D.M.; Corwin, A.L.; Omar, M.A.; Hyams, K.C. Low risk of sexual transmission of hepatitis C virus in Somalia. Trans. R. Soc. Trop Med. Hyg. 1994, 88, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.S.; Todd, S.; Marsh, G.A.; Crameri, G.; Barr, J.; Kamins, A.O.; Peel, A.J.; Yu, M.; Hayman, D.T.; Nadjm, B.; et al. Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol. 2013, 87, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Cleaveland, S.; Appel, M.G.; Chalmers, W.S.; Chillingworth, C.; Kaare, M.; Dye, C. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Vet. Microbiol. 2000, 72, 217–227. [Google Scholar] [CrossRef]
- Roelke-Parker, M.E.; Munson, L.; Packer, C.; Kock, R.; Cleaveland, S.; Carpenter, M.; O’Brien, S.J.; Pospischil, A.; Hofmann-Lehmann, R.; Lutz, H.; et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 1996, 379, 441–445. [Google Scholar] [CrossRef]
- Mtui-Malamsha, N.; Sallu, R.; Mahiti, G.R.; Mohamed, H.; OleNeselle, M.; Rubegwa, B.; Swai, E.S.; Makungu, S.; Otieno, E.G.; Lupindu, A.M.; et al. Ecological and Epidemiological Findings Associated with Zoonotic Rabies Outbreaks and Control in Moshi, Tanzania, 2017–2018. Int. J. Environ. Res. Public Health 2019, 16, 2816. [Google Scholar] [CrossRef] [Green Version]
- Leroy, E.M.; Epelboin, A.; Mondonge, V.; Pourrut, X.; Gonzalez, J.P.; Muyembe-Tamfum, J.J.; Formenty, P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009, 9, 723–728. [Google Scholar] [CrossRef]
- Palmenberg, A.C. Rhinovirus C, Asthma, and Cell Surface Expression of Virus Receptor CDHR3. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, E.J.; Basnet, S.; Wrangham, R.W.; Muller, M.N.; Otali, E.; Hyeroba, D.; Grindle, K.A.; Pappas, T.E.; Thompson, M.E.; Machanda, Z.; et al. Lethal Respiratory Disease Associated with Human Rhinovirus C in Wild Chimpanzees, Uganda, 2013. Emerg. Infect. Dis. 2018, 24, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edridge, A.W.D.; Deijs, M.; Namazzi, R.; Cristella, C.; Jebbink, M.F.; Maurer, I.; Kootstra, N.A.; Buluma, L.R.; van Woensel, J.B.M.; de Jong, M.D.; et al. Novel Orthobunyavirus Identified in the Cerebrospinal Fluid of a Ugandan Child with Severe Encephalopathy. Clin. Infect. Dis. 2019, 68, 139–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Go, Y.Y.; Balasuriya, U.B.; Lee, C.K. Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014, 3, 58–77. [Google Scholar] [CrossRef] [Green Version]
- Byarugaba, D.K.; Erima, B.; Millard, M.; Kibuuka, H.; Lkwago, L.; Bwogi, J.; Mimbe, D.; Kiconco, J.B.; Tugume, T.; Mworozi, E.A.; et al. Whole-genome analysis of influenza A(H1N1)pdm09 viruses isolated in Uganda from 2009 to 2011. Influenza Other Respir. Viruses 2016, 10, 486–492. [Google Scholar] [CrossRef]
- Ndumu, D.; Zecchin, B.; Fusaro, A.; Arinaitwe, E.; Erechu, R.; Kidega, E.; Kayiwa, J.; Muwanga, E.; Kirumira, M.; Kirembe, G.; et al. Highly pathogenic avian influenza H5N8 Clade 2.3.4.4B virus in Uganda, 2017. Infect. Genet Evol. 2018, 66, 269–271. [Google Scholar] [CrossRef]
- Simulundu, E.; Chambaro, H.M.; Sinkala, Y.; Kajihara, M.; Ogawa, H.; Mori, A.; Ndebe, J.; Dautu, G.; Mataa, L.; Lubaba, C.H.; et al. Co-circulation of multiple genotypes of African swine fever viruses among domestic pigs in Zambia (2013–2015). Transbound Emerg Dis. 2018, 65, 114–122. [Google Scholar] [CrossRef]
- Simulundu, E.; Sinkala, Y.; Chambaro, H.M.; Chinyemba, A.; Banda, F.; Mooya, L.E.; Ndebe, J.; Chitanga, S.; Makungu, C.; Munthali, G.; et al. Genetic characterisation of African swine fever virus from 2017 outbreaks in Zambia: Identification of p72 genotype II variants in domestic pigs. Onderstepoort J. Vet. Res. 2018, 85, e1–e5. [Google Scholar] [CrossRef] [Green Version]
- Bailey, A.L.; Lauck, M.; Ghai, R.R.; Nelson, C.W.; Heimbruch, K.; Hughes, A.L.; Goldberg, T.L.; Kuhn, J.H.; Jasinska, A.J.; Freimer, N.B.; et al. Arteriviruses, Pegiviruses, and Lentiviruses Are Common among Wild African Monkeys. J. Virol. 2016, 90, 6724–6737. [Google Scholar] [CrossRef] [Green Version]
- Orba, Y.; Hang’ombe, B.M.; Mweene, A.S.; Wada, Y.; Anindita, P.D.; Phongphaew, W.; Qiu, Y.; Kajihara, M.; Mori-Kajihara, A.; Eto, Y.; et al. First isolation of West Nile virus in Zambia from mosquitoes. Transbound Emerg Dis. 2018, 65, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Mweene-Ndumba, I.; Siziya, S.; Monze, M.; Mazaba, M.L.; Masaninga, F.; Songolo, P.; Mwaba, P.; Babaniyi, O.A. Seroprevalence of West Nile Virus specific IgG and IgM antibodies in North-Western and Western provinces of Zambia. Afr. Health Sci. 2015, 15, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kapiya, J.; Nalubamba, K.S.; Kaimoyo, E.; Changula, K.; Chidumayo, N.; Saasa, N.; Simuunza, M.C.; Takada, A.; Mweene, A.S.; Chitanga, S.; et al. First genetic detection and characterization of canine parvovirus from diarrheic dogs in Zambia. Arch. Virol. 2019, 164, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Muleya, W.; Chambaro, H.M.; Sasaki, M.; Gwenhure, L.F.; Mwenechanya, R.; Kajihara, M.; Saasa, N.; Mupila, Z.; Mori-Kajihara, A.; Qiu, Y.; et al. Genetic diversity of rabies virus in different host species and geographic regions of Zambia and Zimbabwe. Virus Genes 2019. [Google Scholar] [CrossRef]
- Simulundu, E.; Mweene, A.S.; Tomabechi, D.; Hang’ombe, B.M.; Ishii, A.; Suzuki, Y.; Nakamura, I.; Sawa, H.; Sugimoto, C.; Ito, K.; et al. Characterization of H3N6 avian influenza virus isolated from a wild white pelican in Zambia. Arch. Virol. 2009, 154, 1517–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simulundu, E.; Ishii, A.; Igarashi, M.; Mweene, A.S.; Suzuki, Y.; Hang’ombe, B.M.; Namangala, B.; Moonga, L.; Manzoor, R.; Ito, K.; et al. Characterization of influenza A viruses isolated from wild waterfowl in Zambia. J. Gen. Virol. 2011, 92, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Wastika, C.E.; Sasaki, M.; Yoshii, K.; Anindita, P.D.; Hang’ombe, B.M.; Mweene, A.S.; Kobayashi, S.; Kariwa, H.; Carr, M.J.; Hall, W.W.; et al. Serological evidence of Zika virus infection in non-human primates in Zambia. Arch. Virol. 2019, 164, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Chiweshe, N.; Mundava, J.; Abolnik, C.; Capobianco Dondona, A.; Scacchia, M.; Gaidet, N. Avian Viral Pathogens in Swallows, Zimbabwe: Infectious Diseases in Hirundinidae: A Risk to Swallow? Ecohealth 2017, 14, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coetzer, A.; Gwenhure, L.; Makaya, P.; Markotter, W.; Nel, L. Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010–2017) in Harare, Zimbabwe. PLoS ONE 2019, 14, e0210018. [Google Scholar] [CrossRef]
- Couacy-Hymann, E.; Kouakou, V.A.; Aplogan, G.L.; Awoume, F.; Kouakou, C.K.; Kakpo, L.; Sharp, B.R.; McClenaghan, L.; McKenzie, P.; Webster, R.G.; et al. Surveillance for influenza viruses in poultry and swine, west Africa, 2006–2008. Emerg. Infect. Dis. 2012, 18, 1446–1452. [Google Scholar] [CrossRef]
- Boussini, H.; Lamien, C.E.; Nacoulma, O.G.; Kabore, A.; Poda, G.; Viljoen, G. Prevalence of Rift Valley fever in domestic ruminants in the central and northern regions of Burkina Faso. Rev. Sci. Tech. 2014, 33, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.M.; Wandaogo, S.C.M.; Poda, A.; Tamini, L.; Kyere, A.E.; Sagna, T.; Ouedraogo, M.S.; Pauly, M.; Hubschen, J.M.; Muller, C.P.; et al. Epidemiology and molecular characterization of influenza viruses in Burkina Faso, sub-Saharan Africa. Influenza Other Respir. Viruses 2018, 12, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zecchin, B.; Minoungou, G.; Fusaro, A.; Moctar, S.; Ouedraogo-Kabore, A.; Schivo, A.; Salviato, A.; Marciano, S.; Monne, I. Influenza A(H9N2) Virus, Burkina Faso. Emerg. Infect. Dis. 2017, 23, 2118–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dia, N.; Ndiaye, M.N.; Monteiro Mde, L.; Koivogui, L.; Bara, M.O.; Diop, O.M. A subregional analysis of epidemiologic and genetic characteristics of influenza A(H1N1)pdm09 in Africa: Senegal, Cape Verde, Mauritania, and Guinea, 2009–2010. Am. J. Trop Med. Hyg. 2013, 88, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourie, B.; Bingham, P.G.; Evans, H.H.; Foster, S.O.; Nakano, J.H.; Herrmann, K.L. Human infection with monkeypox virus: Laboratory investigation of six cases in West Africa. Bull. World Health Organ. 1972, 46, 633–639. [Google Scholar] [PubMed]
- Sklenovska, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Morozov, V.A.; Leendertz, F.H.; Junglen, S.; Boesch, C.; Pauli, G.; Ellerbrok, H. Frequent foamy virus infection in free-living chimpanzees of the Tai National Park (Cote d’Ivoire). J. Gen. Virol. 2009, 90, 500–506. [Google Scholar] [CrossRef]
- Ayouba, A.; Akoua-Koffi, C.; Calvignac-Spencer, S.; Esteban, A.; Locatelli, S.; Li, H.; Li, Y.; Hahn, B.H.; Delaporte, E.; Leendertz, F.H.; et al. Evidence for continuing cross-species transmission of SIVsmm to humans: Characterization of a new HIV-2 lineage in rural Cote d’Ivoire. AIDS 2013, 27, 2488–2491. [Google Scholar] [CrossRef] [Green Version]
- Kouakou, A.V.; Kouakou, V.; Kouakou, C.; Godji, P.; Kouassi, A.L.; Krou, H.A.; Langeois, Q.; Webby, R.J.; Ducatez, M.F.; Couacy-Hymann, E. Prevalence of Newcastle disease virus and infectious bronchitis virus in avian influenza negative birds from live bird markets and backyard and commercial farms in Ivory-Coast. Res. Vet. Sci. 2015, 102, 83–88. [Google Scholar] [CrossRef]
- Bittaye, M.; Idoko, P.; Ekele, B.A.; Obed, S.A.; Nyan, O. Hepatitis B virus sero-prevalence amongst pregnant women in the Gambia. BMC Infect Dis. 2019, 19, 259. [Google Scholar] [CrossRef]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O. Antigenic Detection of Human Strain of Influenza Virus A (H3N2) in Swine Populations at Three Locations in Nigeria and Ghana during the Dry Early Months of 2014. Zoonoses Public Health 2016, 63, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.A.; Olugasa, B.O.; Emikpe, B.O.; Folitse, R.D. Syndromic survey and molecular analysis of influenza viruses at the human-swine interface in two West African cosmopolitan cities suggest the possibility of bidirectional interspecies transmission. Zoonoses Public Health 2019, 66, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Awuni, J.A.; Bianco, A.; Dogbey, O.J.; Fusaro, A.; Yingar, D.T.; Salviato, A.; Ababio, P.T.; Milani, A.; Bonfante, F.; Monne, I. Avian influenza H9N2 subtype in Ghana: Virus characterization and evidence of co-infection. Avian Pathol. 2019, 48, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Dzotsi, E.K.; Ohene, S.A.; Asiedu-Bekoe, F.; Amankwa, J.; Sarkodie, B.; Adjabeng, M.; Thouphique, A.M.; Ofei, A.; Oduro, J.; Atitogo, D.; et al. The first cases of Lassa fever in Ghana. Ghana Med. J. 2012, 46, 166–170. [Google Scholar]
- Punguyire, D.T.; Osei-Tutu, A.; Aleser, E.V.; Letsa, T. Level and pattern of human rabies and dog bites in Techiman Municipality in the Middle Belt of Ghana: A six year retrospective records review. Pan. Afr. Med. J. 2017, 28, 281. [Google Scholar] [CrossRef]
- Bonney, J.H.K.; Hayashi, T.; Dadzie, S.; Agbosu, E.; Pratt, D.; Nyarko, S.; Asiedu-Bekoe, F.; Ido, E.; Sarkodie, B.; Ohta, N.; et al. Molecular detection of dengue virus in patients suspected of Ebola virus disease in Ghana. PLoS ONE 2018, 13, e0208907. [Google Scholar] [CrossRef]
- Ankrah, G.A.; Bonney, J.H.K.; Agbosu, E.E.; Pratt, D.; Adiku, T.K. Serological evidence of Zika virus infection in febrile patients at Greater Accra Regional Hospital, Accra Ghana. BMC Res. Notes 2019, 12, 326. [Google Scholar] [CrossRef]
- Gatherer, D. The 2014 Ebola virus disease outbreak in West Africa. J. Gen. Virol. 2014, 95, 1619–1624. [Google Scholar] [CrossRef]
- International Ebola Response, T.; Agua-Agum, J.; Ariyarajah, A.; Aylward, B.; Bawo, L.; Bilivogui, P.; Blake, I.M.; Brennan, R.J.; Cawthorne, A.; Cleary, E.; et al. Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study. PLoS Med. 2016, 13, e1002170. [Google Scholar] [CrossRef] [Green Version]
- Sissoko, D.; Keita, M.; Diallo, B.; Aliabadi, N.; Fitter, D.L.; Dahl, B.A.; Akoi Bore, J.; Raymond Koundouno, F.; Singethan, K.; Meisel, S.; et al. Ebola Virus Persistence in Breast Milk After No Reported Illness: A Likely Source of Virus Transmission From Mother to Child. Clin. Infect. Dis. 2017, 64, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Mate, S.E.; Kugelman, J.R.; Nyenswah, T.G.; Ladner, J.T.; Wiley, M.R.; Cordier-Lassalle, T.; Christie, A.; Schroth, G.P.; Gross, S.M.; Davies-Wayne, G.J.; et al. Molecular Evidence of Sexual Transmission of Ebola Virus. N. Engl. J. Med. 2015, 373, 2448–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keita, M.; Duraffour, S.; Loman, N.J.; Rambaut, A.; Diallo, B.; Magassouba, N.; Carroll, M.W.; Quick, J.; Sall, A.A.; Glynn, J.R.; et al. Unusual Ebola Virus Chain of Transmission, Conakry, Guinea, 2014–2015. Emerg. Infect. Dis. 2016, 22, 2149–2152. [Google Scholar] [CrossRef]
- Diallo, B.; Sissoko, D.; Loman, N.J.; Bah, H.A.; Bah, H.; Worrell, M.C.; Conde, L.S.; Sacko, R.; Mesfin, S.; Loua, A.; et al. Resurgence of Ebola Virus Disease in Guinea Linked to a Survivor with Virus Persistence in Seminal Fluid for More Than 500 Days. Clin. Infect. Dis. 2016, 63, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Bausch, D.G.; Demby, A.H.; Coulibaly, M.; Kanu, J.; Goba, A.; Bah, A.; Conde, N.; Wurtzel, H.L.; Cavallaro, K.F.; Lloyd, E.; et al. Lassa fever in Guinea: I. Epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis. 2001, 1, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Demby, A.H.; Inapogui, A.; Kargbo, K.; Koninga, J.; Kourouma, K.; Kanu, J.; Coulibaly, M.; Wagoner, K.D.; Ksiazek, T.G.; Peters, C.J.; et al. Lassa fever in Guinea: II. Distribution and prevalence of Lassa virus infection in small mammals. Vector Borne Zoonotic Dis. 2001, 1, 283–297. [Google Scholar] [CrossRef]
- van Tienen, C.; de Silva, T.I.; Alcantara, L.C.; Onyango, C.O.; Jarju, S.; Goncalves, N.; Vincent, T.; Aaby, P.; Whittle, H.; Schim van der Loeff, M.; et al. Molecular epidemiology of endemic human T-lymphotropic virus type 1 in a rural community in Guinea-Bissau. PLoS Negl. Trop Dis. 2012, 6, e1690. [Google Scholar] [CrossRef] [Green Version]
- Blackley, D.J.; Wiley, M.R.; Ladner, J.T.; Fallah, M.; Lo, T.; Gilbert, M.L.; Gregory, C.; D’Ambrozio, J.; Coulter, S.; Mate, S.; et al. Reduced evolutionary rate in reemerged Ebola virus transmission chains. Sci. Adv. 2016, 2, e1600378. [Google Scholar] [CrossRef] [Green Version]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of Monkeypox-West and Central Africa, 1970–2017. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 306–310. [Google Scholar] [CrossRef]
- Safronetz, D.; Sogoba, N.; Lopez, J.E.; Maiga, O.; Dahlstrom, E.; Zivcec, M.; Feldmann, F.; Haddock, E.; Fischer, R.J.; Anderson, J.M.; et al. Geographic distribution and genetic characterization of Lassa virus in sub-Saharan Mali. PLoS Negl. Trop Dis. 2013, 7, e2582. [Google Scholar] [CrossRef] [Green Version]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Koita, O.A.; Sangare, L.; Poudiougou, B.; Aboubacar, B.; Samake, Y.; Coulibaly, T.; Pronyk, P.; Salez, N.; Kieffer, A.; Ninove, L.; et al. A seroepidemiological study of pandemic A/H1N1(2009) influenza in a rural population of Mali. Clin. Microbiol. Infect. 2012, 18, 976–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bob, N.S.; Ba, H.; Fall, G.; Ishagh, E.; Diallo, M.Y.; Sow, A.; Sembene, P.M.; Faye, O.; El Kouri, B.; Sidi, M.L.; et al. Detection of the Northeastern African Rift Valley Fever Virus Lineage During the 2015 Outbreak in Mauritania. Open Forum Infect Dis. 2017, 4, ofx087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagare, A.; Fall, G.; Ibrahim, A.; Ousmane, S.; Sadio, B.; Abdoulaye, M.; Alhassane, A.; Mahaman, A.E.; Issaka, B.; Sidikou, F.; et al. First occurrence of Rift Valley fever outbreak in Niger, 2016. Vet. Med. Sci. 2019, 5, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoye, J.; Eze, D.; Krueger, W.S.; Heil, G.L.; Friary, J.A.; Gray, G.C. Serologic evidence of avian influenza virus infections among Nigerian agricultural workers. J. Med. Virol. 2013, 85, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Munoz, O.; De Nardi, M.; van der Meulen, K.; van Reeth, K.; Koopmans, M.; Harris, K.; von Dobschuetz, S.; Freidl, G.; Meijer, A.; Breed, A.; et al. Genetic Adaptation of Influenza A Viruses in Domestic Animals and Their Potential Role in Interspecies Transmission: A Literature Review. Ecohealth 2016, 13, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.A.; Adeniji, J.A. Prevalence of antibodies to influenza viruses among handlers of live pigs at three locations in Ibadan, Nigeria. Vet. Ital. 2010, 46, 147–153. [Google Scholar] [PubMed]
- Awosanya, E.J.; Ogundipe, G.; Babalobi, O.; Omilabu, S. Prevalence and correlates of influenza-A in piggery workers and pigs in two communities in Lagos, Nigeria. Pan. Afr. Med. J. 2013, 16, 102. [Google Scholar] [CrossRef]
- Snoeck, C.J.; Abiola, O.J.; Sausy, A.; Okwen, M.P.; Olubayo, A.G.; Owoade, A.A.; Muller, C.P. Serological evidence of pandemic (H1N1) 2009 virus in pigs, West and Central Africa. Vet. Microbiol. 2015, 176, 165–171. [Google Scholar] [CrossRef]
- Oluwayelu, D.O.; Bankole, O.; Ajagbe, O.; Adebiyi, A.I.; Abiola, J.O.; Otuh, P.; Omobowale, O.T. Serological survey for emerging canine H3N8 and H3N2 influenza viruses in pet and village dogs in Nigeria. Afr. J. Med. Med. Sci. 2014, 43, 111–115. [Google Scholar]
- Faye, O.; Pratt, C.B.; Faye, M.; Fall, G.; Chitty, J.A.; Diagne, M.M.; Wiley, M.R.; Yinka-Ogunleye, A.F.; Aruna, S.; Etebu, E.N.; et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis. 2018, 18, 246. [Google Scholar] [CrossRef] [Green Version]
- Kabuga, A.I.; El Zowalaty, M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 2019, 91, 533–540. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Bulletin on Outbreaks and Other Emergencies. In Week 26 2018. 2018. Available online: https://apps.who.int/iris/handle/10665/272981 (accessed on 13 April 2020).
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Antia, R.E.; Adekola, A.A.; Jubril, A.J.; Ohore, O.G.; Emikpe, B.O. Hepatitis E Virus infection seroprevalence and the associated risk factors in animals raised in Ibadan, Nigeria. J. Immunoass. Immunochem. 2018, 39, 509–520. [Google Scholar] [CrossRef]
- Ogunro, B.N.; Olugasa, B.O.; Verschoor, E.J.; Olarinmoye, A.O.; Theyse, I.; Niphuis, H. Serological Detection of Ebola Virus Exposures in Native Non-human Primates of Southern Nigeria. J. Epidemiol. Glob. Health 2018, 8, 162–170. [Google Scholar] [CrossRef]
- Welch, C.N.; Shittu, I.; Abolnik, C.; Solomon, P.; Dimitrov, K.M.; Taylor, T.L.; Williams-Coplin, D.; Goraichuk, I.V.; Meseko, C.A.; Ibu, J.O.; et al. Genomic comparison of Newcastle disease viruses isolated in Nigeria between 2002 and 2015 reveals circulation of highly diverse genotypes and spillover into wild birds. Arch. Virol. 2019, 164, 2031–2047. [Google Scholar] [CrossRef]
- Shittu, I.; Sharma, P.; Joannis, T.M.; Volkening, J.D.; Odaibo, G.N.; Olaleye, D.O.; Williams-Coplin, D.; Solomon, P.; Abolnik, C.; Miller, P.J.; et al. Complete Genome Sequence of a Genotype XVII Newcastle Disease Virus, Isolated from an Apparently Healthy Domestic Duck in Nigeria. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Traore-Lamizana, M.; Zeller, H.G.; Mondo, M.; Hervy, J.P.; Adam, F.; Digoutte, J.P. Isolations of West Nile and Bagaza viruses from mosquitoes (Diptera: Culicidae) in central Senegal (Ferlo). J. Med. Entomol. 1994, 31, 934–938. [Google Scholar] [CrossRef]
- Chevalier, V.; Lancelot, R.; Diaite, A.; Mondet, B.; Sall, B.; De Lamballerie, X. Serological assessment of West Nile fever virus activity in the pastoral system of Ferlo, Senegal. Ann. N. Y. Acad. Sci. 2006, 1081, 216–225. [Google Scholar] [CrossRef]
- Chevalier, V.; Reynaud, P.; Lefrancois, T.; Durand, B.; Baillon, F.; Balanca, G.; Gaidet, N.; Mondet, B.; Lancelot, R. Predicting West Nile virus seroprevalence in wild birds in Senegal. Vector Borne Zoonotic Dis. 2009, 9, 589–596. [Google Scholar] [CrossRef]
- Seck, M.C.; Badiane, A.S.; Thwing, J.; Moss, D.; Fall, F.B.; Gomis, J.F.; Deme, A.B.; Diongue, K.; Sy, M.; Mbaye, A.; et al. Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists. Pathogens 2019, 8, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sow, A.; Faye, O.; Faye, O.; Diallo, D.; Sadio, B.D.; Weaver, S.C.; Diallo, M.; Sall, A.A. Rift Valley fever in Kedougou, southeastern Senegal, 2012. Emerg. Infect. Dis. 2014, 20, 504–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, A.; Watson, S.J.; Asogun, D.; Tobin, E.A.; Lu, J.; Phan, M.V.T.; Jah, U.; Wadoum, R.E.G.; Meredith, L.; Thorne, L.; et al. Rapid outbreak sequencing of Ebola virus in Sierra Leone identifies transmission chains linked to sporadic cases. Virus Evol. 2016, 2, vew016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpren, C.; Sloan, M.; Boegler, K.A.; Martin, D.W.; Ervin, E.; Washburn, F.; Rickert, R.; Singh, T.; Redd, J.T.; Interagency Investigation, T. Notes from The Field: Ebola Virus Disease Cluster-Northern Sierra Leone, January 2016. MMWR Morb. Mortal. Wkly Rep. 2016, 65, 681–682. [Google Scholar] [CrossRef] [Green Version]
- Subissi, L.; Keita, M.; Mesfin, S.; Rezza, G.; Diallo, B.; Van Gucht, S.; Musa, E.O.; Yoti, Z.; Keita, S.; Djingarey, M.H.; et al. Ebola Virus Transmission Caused by Persistently Infected Survivors of the 2014–2016 Outbreak in West Africa. J. Infect. Dis. 2018, 218, S287–S291. [Google Scholar] [CrossRef]
- Park, D.J.; Dudas, G.; Wohl, S.; Goba, A.; Whitmer, S.L.; Andersen, K.G.; Sealfon, R.S.; Ladner, J.T.; Kugelman, J.R.; Matranga, C.B.; et al. Ebola Virus Epidemiology, Transmission, and Evolution during Seven Months in Sierra Leone. Cell 2015, 161, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.C.; Neto de Vasconcelos, J.; Granja, B.G.; Theze, J.; Jandondo, D.; Neto, Z.; Mirandela, M.; Sebastiao, C.D.S.; Candido, A.L.M.; Clemente, C.; et al. Early Genomic Detection of Cosmopolitan Genotype of Dengue Virus Serotype 2, Angola, 2018. Emerg. Infect. Dis. 2019, 25, 784–787. [Google Scholar] [CrossRef]
- Ntagirabiri, R.; Poveda, J.D.; Mumana, A.; Ndayishimiye, H. Genotypes and subtypes of hepatitis C virus in Burundi: A particularity in sub-Saharan Africa. Pan. Afr. Med. J. 2014, 19, 69. [Google Scholar] [CrossRef]
- Roy, S.; Vandenberghe, L.H.; Kryazhimskiy, S.; Grant, R.; Calcedo, R.; Yuan, X.; Keough, M.; Sandhu, A.; Wang, Q.; Medina-Jaszek, C.A.; et al. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog. 2009, 5, e1000503. [Google Scholar] [CrossRef]
- Larison, B.; Njabo, K.Y.; Chasar, A.; Fuller, T.; Harrigan, R.J.; Smith, T.B. Spillover of pH1N1 to swine in Cameroon: An investigation of risk factors. BMC Vet. Res. 2014, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Wade, A.; Jumbo, S.D.; Zecchin, B.; Fusaro, A.; Taiga, T.; Bianco, A.; Rodrigue, P.N.; Salomoni, A.; Kameni, J.M.F.; Zamperin, G.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus, Cameroon, 2017. Emerg. Infect. Dis. 2018, 24, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Sadeuh-Mba, S.A.; Yonga Wansi, G.M.; Demanou, M.; Gessain, A.; Njouom, R. Serological evidence of Rift VAlley fever Phlebovirus and Crimean-Congo hemorrhagic fever orthonairovirus infections among pygmies in the east region of Cameroon. Virol. J. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvala, H.; Sharp, C.P.; Ngole, E.M.; Delaporte, E.; Peeters, M.; Simmonds, P. Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: Relationship with human and simian enteroviruses. J. Virol. 2011, 85, 4480–4486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernet, O.; Schneider, B.S.; Beaty, S.M.; LeBreton, M.; Yun, T.E.; Park, A.; Zachariah, T.T.; Bowden, T.A.; Hitchens, P.; Ramirez, C.M.; et al. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 2014, 5, 5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modiyinji, A.F.; Atsama, M.A.; Monamele, G.C.; Nola, M.; Njouom, R. High seroprevalence of hepatitis E among pigs suggests an animal reservoir in Cameroon. J. Infect. Dev. Ctries. 2018, 12, 676–679. [Google Scholar] [CrossRef]
- Switzer, W.M.; Garcia, A.D.; Yang, C.; Wright, A.; Kalish, M.L.; Folks, T.M.; Heneine, W. Coinfection with HIV-1 and simian foamy virus in West Central Africans. J. Infect. Dis. 2008, 197, 1389–1393. [Google Scholar] [CrossRef] [Green Version]
- LeBreton, M.; Yang, O.; Tamoufe, U.; Mpoudi-Ngole, E.; Torimiro, J.N.; Djoko, C.F.; Carr, J.K.; Tassy Prosser, A.; Rimoin, A.W.; Birx, D.L.; et al. Exposure to wild primates among HIV-infected persons. Emerg. Infect. Dis. 2007, 13, 1579–1582. [Google Scholar] [CrossRef]
- Zheng, H.; Wolfe, N.D.; Sintasath, D.M.; Tamoufe, U.; Lebreton, M.; Djoko, C.F.; Diffo Jle, D.; Pike, B.L.; Heneine, W.; Switzer, W.M. Emergence of a novel and highly divergent HTLV-3 in a primate hunter in Cameroon. Virology 2010, 401, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Prosser, A.T.; Lebreton, M.; Mpoudi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. USA 2005, 102, 7994–7999. [Google Scholar] [CrossRef] [Green Version]
- Calattini, S.; Betsem, E.; Bassot, S.; Chevalier, S.A.; Tortevoye, P.; Njouom, R.; Mahieux, R.; Froment, A.; Gessain, A. Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology 2011, 410, 48–55. [Google Scholar] [CrossRef]
- Calattini, S.; Betsem, E.B.; Froment, A.; Mauclere, P.; Tortevoye, P.; Schmitt, C.; Njouom, R.; Saib, A.; Gessain, A. Simian foamy virus transmission from apes to humans, rural Cameroon. Emerg. Infect. Dis. 2007, 13, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Betsem, E.; Rua, R.; Tortevoye, P.; Froment, A.; Gessain, A. Frequent and recent human acquisition of simian foamy viruses through apes’ bites in central Africa. PLoS Pathog. 2011, 7, e1002306. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, N.D.; Switzer, W.M.; Carr, J.K.; Bhullar, V.B.; Shanmugam, V.; Tamoufe, U.; Prosser, A.T.; Torimiro, J.N.; Wright, A.; Mpoudi-Ngole, E.; et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 2004, 363, 932–937. [Google Scholar] [CrossRef]
- Courgnaud, V.; Van Dooren, S.; Liegeois, F.; Pourrut, X.; Abela, B.; Loul, S.; Mpoudi-Ngole, E.; Vandamme, A.; Delaporte, E.; Peeters, M. Simian T-cell leukemia virus (STLV) infection in wild primate populations in Cameroon: Evidence for dual STLV type 1 and type 3 infection in agile mangabeys (Cercocebus agilis). J. Virol. 2004, 78, 4700–4709. [Google Scholar] [CrossRef] [Green Version]
- Adlhoch, C.; Kaiser, M.; Kingsley, M.T.; Schwarz, N.G.; Ulrich, M.; de Paula, V.S.; Ehlers, J.; Lowa, A.; Daniel, A.M.; Poppert, S.; et al. Porcine hokovirus in domestic pigs, Cameroon. Emerg. Infect. Dis. 2013, 19, 2060–2062. [Google Scholar] [CrossRef]
- Monamele, C.G.; Karlsson, E.A.; Vernet, M.A.; Wade, A.; Okomo, M.A.; Abah, A.S.A.; Yann, S.; Etoundi, G.A.M.; Mohamadou, N.R.; Feussom, J.M.; et al. Evidence of exposure and human seroconversion during an outbreak of avian influenza A(H5N1) among poultry in Cameroon. Emerg. Microbes. Infect. 2019, 8, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Besombes, C.; Gonofio, E.; Konamna, X.; Selekon, B.; Grant, R.; Gessain, A.; Berthet, N.; Manuguerra, J.C.; Fontanet, A.; Nakoune, E. Intrafamily Transmission of Monkeypox Virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019, 25, 1602–1604. [Google Scholar] [CrossRef]
- Abakar, M.F.; Nare, N.B.; Schelling, E.; Hattendorf, J.; Alfaroukh, I.O.; Zinsstag, J. Seroprevalence of Rift Valley fever, Q fever, and brucellosis in ruminants on the southeastern shore of Lake Chad. Vector Borne Zoonotic Dis. 2014, 14, 757–762. [Google Scholar] [CrossRef]
- Twabela, A.T.; Tshilenge, G.M.; Sakoda, Y.; Okamatsu, M.; Bushu, E.; Kone, P.; Wiersma, L.; Zamperin, G.; Drago, A.; Zecchin, B.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus, Democratic Republic of the Congo, 2017. Emerg. Infect. Dis. 2018, 24, 1371–1374. [Google Scholar] [CrossRef] [Green Version]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Learned, L.A.; Reynolds, M.G.; Wassa, D.W.; Li, Y.; Olson, V.A.; Karem, K.; Stempora, L.L.; Braden, Z.H.; Kline, R.; Likos, A.; et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop Med. Hyg. 2005, 73, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessain, A.; Rua, R.; Betsem, E.; Turpin, J.; Mahieux, R. HTLV-3/4 and simian foamy retroviruses in humans: Discovery, epidemiology, cross-species transmission and molecular virology. Virology 2013, 435, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, V.; Leupold, J.; Clotten, J.; Urbanyi, E.; Herchenroder, O.; Spatz, W.; Volk, B.; Bohm, N.; Toniolo, A.; Neumann-Haefelin, D.; et al. Sites of simian foamy virus persistence in naturally infected African green monkeys: Latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology 1999, 257, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Linial, M.L. Foamy virus infection in primates. J. Med. Primatol. 2006, 35, 225–235. [Google Scholar] [CrossRef]
- Murray, S.M.; Picker, L.J.; Axthelm, M.K.; Hudkins, K.; Alpers, C.E.; Linial, M.L. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J. Virol 2008, 82, 5981–5985. [Google Scholar] [CrossRef] [Green Version]
- Switzer, W.M.; Tang, S.; Ahuka-Mundeke, S.; Shankar, A.; Hanson, D.L.; Zheng, H.; Ayouba, A.; Wolfe, N.D.; LeBreton, M.; Djoko, C.F.; et al. Novel simian foamy virus infections from multiple monkey species in women from the Democratic Republic of Congo. Retrovirology 2012, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Halawi, A.D.; Saasa, N.; Pongombo, B.L.; Kajihara, M.; Chambaro, H.M.; Hity, M.; Sawa, H.; Takada, A.; Mweene, A.S.; Nsembo, L.L.; et al. Seroprevalence of Rift Valley fever in cattle of smallholder farmers in Kwilu Province in the Democratic Republic of Congo. Trop Anim. Health Prod. 2019. [Google Scholar] [CrossRef]
- Xie, D.D.; Li, J.; Chen, J.T.; Eyi, U.M.; Matesa, R.A.; Obono, M.M.; Ehapo, C.S.; Yang, L.Y.; Yang, H.; Yang, H.T.; et al. Seroprevalence of Human Immunodeficiency Virus, Hepatitis B Virus, Hepatitis C Virus, and Treponema pallidum Infections among Blood Donors on Bioko Island, Equatorial Guinea. PLoS ONE 2015, 10, e0139947. [Google Scholar] [CrossRef] [Green Version]
- Paupy, C.; Kassa Kassa, F.; Caron, M.; Nkoghe, D.; Leroy, E.M. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012, 12, 167–169. [Google Scholar] [CrossRef]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med. Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Esposito, J.J.; Gras, F.; Kolakowski, T.; Fatras, M.; Muller, G. First appearance of monkey pox in human beings in Gabon. Med. Trop (Mars) 1991, 51, 53–57. [Google Scholar] [PubMed]
- Mouinga-Ondeme, A.; Caron, M.; Nkoghe, D.; Telfer, P.; Marx, P.; Saib, A.; Leroy, E.; Gonzalez, J.P.; Gessain, A.; Kazanji, M. Cross-species transmission of simian foamy virus to humans in rural Gabon, Central Africa. J. Virol. 2012, 86, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouinga-Ondeme, A.; Betsem, E.; Caron, M.; Makuwa, M.; Salle, B.; Renault, N.; Saib, A.; Telfer, P.; Marx, P.; Gessain, A.; et al. Two distinct variants of simian foamy virus in naturally infected mandrills (Mandrillus sphinx) and cross-species transmission to humans. Retrovirology 2010, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Mouinga-Ondeme, A.; Kazanji, M. Simian foamy virus in non-human primates and cross-species transmission to humans in Gabon: An emerging zoonotic disease in central Africa? Viruses 2013, 5, 1536–1552. [Google Scholar] [CrossRef] [Green Version]
- Liegeois, F.; Boue, V.; Mouacha, F.; Butel, C.; Ondo, B.M.; Pourrut, X.; Leroy, E.; Peeters, M.; Rouet, F. New STLV-3 strains and a divergent SIVmus strain identified in non-human primate bushmeat in Gabon. Retrovirology 2012, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Nurtop, E.; Moyen, N.; Dzia-Lepfoundzou, A.; Dimi, Y.; Ninove, L.; Drexler, J.F.; Gallian, P.; de Lamballerie, X.; Priet, S. A Report of Zika Virus Seroprevalence in Republic of the Congo. Vector Borne Zoonotic Dis. 2019. [Google Scholar] [CrossRef]
- Umuhoza, T.; Berkvens, D.; Gafarasi, I.; Rukelibuga, J.; Mushonga, B.; Biryomumaisho, S. Seroprevalence of Rift Valley fever in cattle along the Akagera-Nyabarongo rivers, Rwanda. J. S. Afr. Vet. Assoc. 2017, 88, e1–e5. [Google Scholar] [CrossRef] [Green Version]
- Wane, J.; Nyatanyi, T.; Nkunda, R.; Rukelibuga, J.; Ahmed, Z.; Biedron, C.; Kabeja, A.; Muhimpundu, M.A.; Kabanda, A.; Antara, S.; et al. 2009 pandemic influenza A (H1N1) virus outbreak and response–Rwanda, October, 2009–May, 2010. PLoS ONE 2012, 7, e31572. [Google Scholar] [CrossRef] [Green Version]
- Yen, T.Y.; Trovoada dos Santos Mde, J.; Tseng, L.F.; Chang, S.F.; Cheng, C.F.; Carvalho, A.V.; Shu, P.Y.; Lien, J.C.; Tsai, K.H. Seroprevalence of antibodies against dengue virus among pregnant women in the Democratic Republic of Sao Tome and Principe. Acta Trop 2016, 155, 58–62. [Google Scholar] [CrossRef]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.C.; Davidson, W.B.; Karem, K.L.; et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, T.; Darnell, D.; Gradi, E.A.; Benali, Y.; Kara, R.; Guetarni, D.; Rubrum, A.; Seiler, P.J.; Crumpton, J.C.; Webby, R.J.; et al. A(H9N2) influenza viruses associated with chicken mortality in outbreaks in Algeria 2017. Influenza Other Respir Viruses 2019. [Google Scholar] [CrossRef]
- Kautman, M.; Tiar, G.; Papa, A.; Siroky, P. AP92-like Crimean-Congo Hemorrhagic Fever Virus in Hyalomma aegyptium Ticks, Algeria. Emerg. Infect. Dis. 2016, 22, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Laabassi, F.; Lecouturier, F.; Amelot, G.; Gaudaire, D.; Mamache, B.; Laugier, C.; Legrand, L.; Zientara, S.; Hans, A. Epidemiology and Genetic Characterization of H3N8 Equine Influenza Virus Responsible for Clinical Disease in Algeria in 2011. Transbound Emerg. Dis. 2015, 62, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, A.; Manoncourt, S.; Abd el Kareem, E.; Mohamed Ahmed, A.N.; El-Refaie, S.; Essmat, H.; Tjaden, J.; de Mattos, C.C.; Earhart, K.C.; Marfin, A.A.; et al. Zoonotic transmission of avian influenza virus (H5N1), Egypt, 2006–2009. Emerg. Infect. Dis. 2010, 16, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.D.; Ahmed, L.S.; Gamal-Eldein, M.A.; Fouda, M.K.; Khalil, F.; Yingst, S.L.; Parker, M.A.; Montevillel, M.R. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg. Infect. Dis. 2007, 13, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Younan, M.; Poh, M.K.; Elassal, E.; Davis, T.; Rivailler, P.; Balish, A.L.; Simpson, N.; Jones, J.; Deyde, V.; Loughlin, R.; et al. Microevolution of highly pathogenic avian influenza A(H5N1) viruses isolated from humans, Egypt, 2007–2011. Emerg. Infect. Dis. 2013, 19, 43–50. [Google Scholar] [CrossRef]
- Bahgat, M.M.; Kutkat, M.A.; Nasraa, M.H.; Mostafa, A.; Webby, R.; Bahgat, I.M.; Ali, M.A. Characterization of an avian influenza virus H5N1 Egyptian isolate. J. Virol Methods 2009, 159, 244–250. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [Green Version]
- Arafa, A.S.; Yamada, S.; Imai, M.; Watanabe, T.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Kiso, M.; Sakai-Tagawa, Y.; Ito, M.; Imamura, T.; et al. Risk assessment of recent Egyptian H5N1 influenza viruses. Sci. Rep. 2016, 6, 38388. [Google Scholar] [CrossRef]
- Helmy, Y.A.; El-Adawy, H.; Abdelwhab, E.M. A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt. Pathogens 2017, 6, 33. [Google Scholar] [CrossRef]
- Imam, I.Z.; El-Karamany, R.; Darwish, M.A. An epidemic of Rift Valley fever in Egypt. 2. Isolation of the virus from animals. Bull. World Health Organ. 1979, 57, 441–443. [Google Scholar] [PubMed]
- Samy, A.M.; Peterson, A.T.; Hall, M. Phylogeography of Rift Valley Fever Virus in Africa and the Arabian Peninsula. PLoS Negl. Trop Dis. 2017, 11, e0005226. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.M.; Hoogstraal, H.; Moussa, M.I. An epizootic of Rift Valley fever in Egypt in 1977. Vet. Rec. 1979, 105, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Yu, H.; Yu, X.J. Evidence for zoonotic origins of Middle East respiratory syndrome coronavirus. J. Gen. Virol. 2016, 97, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Shehata, M.M.; Gomaa, M.R.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; El-Taweel, A.N.; Atea, M.; Hassan, N.; Bagato, O.; et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes Infect. 2017, 6, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, R.A.; Wang, P.; Gomaa, M.R.; El-Shesheny, R.; Kandeil, A.; Bagato, O.; Siu, L.Y.; Shehata, M.M.; Kayed, A.S.; Moatasim, Y.; et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill 2013, 18, 20574. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Imam, I.Z.; Omar, F.M.; Hoogstraal, H. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta Virol. 1978, 22, 77. [Google Scholar]
- Horton, K.C.; Wasfy, M.; Samaha, H.; Abdel-Rahman, B.; Safwat, S.; Abdel Fadeel, M.; Mohareb, E.; Dueger, E. Serosurvey for zoonotic viral and bacterial pathogens among slaughtered livestock in Egypt. Vector Borne Zoonotic Dis. 2014, 14, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Weidmann, M.; Avsic-Zupanc, T.; Bino, S.; Bouloy, M.; Burt, F.; Chinikar, S.; Christova, I.; Dedushaj, I.; El-Sanousi, A.; Elaldi, N.; et al. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2016, 97, 2799–2808. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.; Mohareb, E.; Salman, D.; Saad, M.; Salama, S.; Fayez, C.; Hanafi, H.; Medhat, I.; Labib, E.; Rakha, M.; et al. Studies on West Nile virus infection in Egypt. J Infect Public Health 2010, 3, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Kamal, S.A. Observations on Rift Valley fever virus and vaccines in Egypt. Virol. J. 2011, 8, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, R.R.; el-Sharkawy, M.S.; Cope, S.E.; Botros, B.A.; Oun, S.; Morrill, J.C.; Shope, R.E.; Hibbs, R.G.; Darwish, M.A.; Imam, I.Z. Recurrence of Rift Valley fever in Egypt. Lancet 1993, 342, 1149–1150. [Google Scholar] [CrossRef]
- Imam, I.Z.; Darwish, M.A.; El-Karamany, R. An epidemic of Rift Valley fever in Egypt. 1. Diagnosis of Rift Valley fever in man. Bull. World Health Organ. 1979, 57, 437–439. [Google Scholar] [PubMed]
- Abdel-Moneim, A.S.; Afifi, M.A.; El-Kady, M.F. Isolation and mutation trend analysis of influenza A virus subtype H9N2 in Egypt. Virol. J. 2012, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anis, A.; AboElkhair, M.; Ibrahim, M. Characterization of highly pathogenic avian influenza H5N8 virus from Egyptian domestic waterfowl in 2017. Avian Pathol. 2018, 47, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, E.M.; Watanabe, Y.; Daidoji, T.; Arai, Y.; Ikuta, K.; Ibrahim, M.S.; Nakaya, T. Genetic characterization of highly pathogenic avian influenza H5N1 viruses isolated from naturally infected pigeons in Egypt. Virus Genes 2016, 52, 867–871. [Google Scholar] [CrossRef]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Selim, U.; Nasef, S.; Aggor, M.G.; Abdelwhab, E.M.; et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Kandeil, A.; Kayed, A.S.; Elabd, M.A.; Zaki, S.A.; Abu Zeid, D.; El Rifay, A.S.; Mousa, A.A.; Farag, M.M.; McKenzie, P.P.; et al. Serological Evidence of Human Infection with Avian Influenza A H7virus in Egyptian Poultry Growers. PLoS ONE 2016, 11, e0155294. [Google Scholar] [CrossRef] [Green Version]
- Selim, A.A.; Erfan, A.M.; Hagag, N.; Zanaty, A.; Samir, A.H.; Samy, M.; Abdelhalim, A.; Arafa, A.A.; Soliman, M.A.; Shaheen, M.; et al. Highly Pathogenic Avian Influenza Virus (H5N8) Clade 2.3.4.4 Infection in Migratory Birds, Egypt. Emerg. Infect. Dis. 2017, 23, 1048–1051. [Google Scholar] [CrossRef] [Green Version]
- Kammon, A.; Heidari, A.; Dayhum, A.; Eldaghayes, I.; Sharif, M.; Monne, I.; Cattoli, G.; Asheg, A.; Farhat, M.; Kraim, E. Characterization of Avian Influenza and Newcastle Disease Viruses from Poultry in Libya. Avian Dis. 2015, 59, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lahlou Amine, I.; Bajjou, T.; El Rhaffouli, H.; Laraqui, A.; Hilali, F.; Menouar, K.; Ennibi, K.; Boudlal, M.; Bouaiti, E.A.; Sbai, K.; et al. Pandemic influenza A(H1N1)2009 in Morocco: Experience of the Mohammed V Military Teaching Hospital, Rabat, 12 June to 24 December 2009. Euro. Surveill 2011, 16, 6479–6488. [Google Scholar]
- Boukharta, M.; Azlmat, S.; Elharrak, M.; Ennaji, M.M. Multiple alignment comparison of the non-structural genes of three strains of equine influenza viruses (H3N8) isolated in Morocco. BMC Res. Notes 2015, 8, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Houadfi, M.; Fellahi, S.; Nassik, S.; Guerin, J.L.; Ducatez, M.F. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virol. J. 2016, 13, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, L.H.; Osman, M.S.; Farnon, E.C.; Griffith, K.S.; Godsey, M.S.; Karch, S.; Mulenda, B.; El Kholy, A.; Grandesso, F.; de Radigues, X.; et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans. R. Soc. Trop Med. Hyg. 2008, 102, 1247–1254. [Google Scholar] [CrossRef]
- Malik, A.; Earhart, K.; Mohareb, E.; Saad, M.; Saeed, M.; Ageep, A.; Soliman, A. Dengue hemorrhagic fever outbreak in children in Port Sudan. J. Infect. Public Health 2011, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Himatt, S.; Osman, K.E.; Okoued, S.I.; Seidahmed, O.E.; Beatty, M.E.; Soghaier, M.A.; Elmusharaf, K. Sero-prevalence of dengue infections in the Kassala state in the eastern part of the Sudan in 2011. J. Infect. Public Health 2015, 8, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Soghaier, M.A.; Hagar, A.; Abbas, M.A.; Elmangory, M.M.; Eltahir, K.M.; Sall, A.A. Yellow Fever outbreak in Darfur, Sudan in October 2012; the initial outbreak investigation report. J. Infect. Public Health 2013, 6, 370–376. [Google Scholar] [CrossRef] [Green Version]
- El Moussi, A.; Ben Hadj Kacem, M.A.; Ledesma, J.; Pozo, F.; Teresa Cuevas, M.; Casas, I.; Slim, A. Genetic diversity of influenza B virus in 2009–2010 and 2010–2011 in Tunisia. Med. Mal. Infect. 2013, 43, 337–344. [Google Scholar] [CrossRef]
- Wasfi, F.; Dowall, S.; Ghabbari, T.; Bosworth, A.; Chakroun, M.; Varghese, A.; Tiouiri, H.; Ben Jemaa, M.; Znazen, A.; Hewson, R.; et al. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite 2016, 23, 10. [Google Scholar] [CrossRef]
- Jori, F.; Alexander, K.A.; Mokopasetso, M.; Munstermann, S.; Moagabo, K.; Paweska, J.T. Serological Evidence of Rift Valley Fever Virus Circulation in Domestic Cattle and African Buffalo in Northern Botswana (2010–2011). Front Vet. Sci. 2015, 2, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coetzer, A.; Coertse, J.; Makalo, M.J.; Molomo, M.; Markotter, W.; Nel, L.H. Epidemiology of Rabies in Lesotho: The Importance of Routine Surveillance and Virus Characterization. Trop Med. Infect Dis. 2017, 2, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellan, S.E.; Cizauskas, C.A.; Miyen, J.; Ebersohn, K.; Kusters, M.; Prager, K.C.; Van Vuuren, M.; Sabeta, C.; Getz, W.M. Black-backed jackal exposure to rabies virus, canine distemper virus, and Bacillus anthracis in Etosha National Park, Namibia. J. Wildl. Dis. 2012, 48, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umberto, M.; Aikukutu, G.; Roux, J.P.; Kemper, J.; Ntahonshikira, C.; Marruchella, G.; Khaiseb, S.; Cattoli, G.; Dundon, W.G. Avian Influenza H5N8 Outbreak in African Penguins (Spheniscus demersus), Namibia, 2019. J. Wildl. Dis. 2019, 56, 214–218. [Google Scholar]
- Monaco, F.; Pinoni, C.; Cosseddu, G.M.; Khaiseb, S.; Calistri, P.; Molini, U.; Bishi, A.; Conte, A.; Scacchia, M.; Lelli, R. Rift Valley fever in Namibia, 2010. Emerg. Infect. Dis. 2013, 19, 2025–2027. [Google Scholar] [CrossRef]
- Thalwitzer, S.; Wachter, B.; Robert, N.; Wibbelt, G.; Muller, T.; Lonzer, J.; Meli, M.L.; Bay, G.; Hofer, H.; Lutz, H. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian Farmland. Clin. Vaccine Immunol. 2010, 17, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.B. The isolation and classification of Tern virus: Influenza A-Tern South Africa–1961. J. Hyg. (Lond.) 1966, 64, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.N.; Sinclair, M.; Ganzevoort, B. Risk factors for seropositivity to H5 avian influenza virus in ostrich farms in the Western Cape Province, South Africa. Prev. Vet. Med. 2008, 86, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Helden, L.S.; Sinclair, M.; Koen, P.; Grewar, J.D. Description of an outbreak of highly pathogenic avian influenza in domestic ostriches (Struthio camelus) in South Africa in 2011. Prev. Vet. Med. 2016, 128, 6–11. [Google Scholar] [CrossRef]
- Abolnik, C.; Pieterse, R.; Peyrot, B.M.; Choma, P.; Phiri, T.P.; Ebersohn, K.; Heerden, C.J.V.; Vorster, A.A.; Zel, G.V.; Geertsma, P.J.; et al. The Incursion and Spread of Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 within South Africa. Avian Dis. 2019, 63, 149–156. [Google Scholar] [CrossRef]
- Abolnik, C.; Fehrsen, J.; Olivier, A.; van Wyngaardt, W.; Fosgate, G.; Ellis, C. Serological investigation of highly pathogenic avian influenza H5N2 in ostriches (Struthio camelus). Avian Pathol. 2013, 42, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C.; Olivier, A.; Reynolds, C.; Henry, D.; Cumming, G.; Rauff, D.; Romito, M.; Petty, D.; Falch, C. Susceptibility and Status of Avian Influenza in Ostriches. Avian Dis. 2016, 60, 286–295. [Google Scholar] [CrossRef]
- Abolnik, C.; Bisschop, S.P.; Gerdes, G.H.; Olivier, A.J.; Horner, R.F. Phylogenetic analysis of low-pathogenicity avian influenza H6N2 viruses from chicken outbreaks (2001–2005) suggest that they are reassortants of historic ostrich low-pathogenicity avian influenza H9N2 and H6N8 viruses. Avian Dis. 2007, 51, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poen, M.J.; Fouchier, R.A.; Webby, R.J.; Webster, R.G.; El Zowalaty, M.E. Evidence of the Presence of Low Pathogenic Avian Influenza A Viruses in Wild Waterfowl in 2018 in South Africa. Pathogens 2019, 8, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abolnik, C.; Strydom, C. Complete Genome Sequence of a Class I Avian Orthoavulavirus 1 Isolated from Commercial Ostriches. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, B.N.; Timothy, G.A.; Cohen, C.; Tempia, S.; Huma, M.; Blumberg, L.; Naidoo, D.; Cengimbo, A.; Schoub, B.D. Introduction of 2009 pandemic influenza A virus subtype H1N1 into South Africa: Clinical presentation, epidemiology, and transmissibility of the first 100 cases. J. Infect. Dis. 2012, 206 (Suppl. S1), S148–S153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.L.; Hellferscee, O.; Pretorius, M.; Treurnicht, F.; Walaza, S.; Madhi, S.; Groome, M.; Dawood, H.; Variava, E.; Kahn, K.; et al. Epidemiology of influenza virus types and subtypes in South Africa, 2009–2012. Emerg. Infect. Dis. 2014, 20, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Drewe, J.A.; O’Riain, M.J.; Beamish, E.; Currie, H.; Parsons, S. Survey of infections transmissible between baboons and humans, Cape Town, South Africa. Emerg. Infect. Dis. 2012, 18, 298–301. [Google Scholar] [CrossRef]
- Dickens, C.; Kew, M.C.; Purcell, R.H.; Kramvis, A. Occult hepatitis B virus infection in chacma baboons, South Africa. Emerg. Infect. Dis. 2013, 19, 598–605. [Google Scholar] [CrossRef]
- Mathengtheng, L.; Burt, F.J. Use of envelope domain III protein for detection and differentiation of flaviviruses in the Free State Province, South Africa. Vector Borne Zoonotic Dis. 2014, 14, 261–271. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.G.; Ubico, S.R.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. Curr. Top. Microbiol. Immunol. 2002, 267, 271–308. [Google Scholar] [PubMed]
- Venter, M.; Swanepoel, R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector Borne Zoonotic Dis. 2010, 10, 659–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, M.; Pretorius, M.; Fuller, J.A.; Botha, E.; Rakgotho, M.; Stivaktas, V.; Weyer, C.; Romito, M.; Williams, J. West Nile Virus Lineage 2 in Horses and Other Animals with Neurologic Disease, South Africa, 2008–2015. Emerg. Infect. Dis. 2017, 23, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Hollidge, B.S.; Gonzalez-Scarano, F.; Soldan, S.S. Arboviral encephalitides: Transmission, emergence, and pathogenesis. J. Neuroimmune Pharmacol. 2010, 5, 428–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eeden, C.; Williams, J.H.; Gerdes, T.G.; van Wilpe, E.; Viljoen, A.; Swanepoel, R.; Venter, M. Shuni virus as cause of neurologic disease in horses. Emerg Infect. Dis. 2012, 18, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, H.; Strickland-Cholmley, M.; Jackson, A.L. Sindbis virus infection in man. Report of a case with recovery of virus from skin lesions. S. Afr. Med. J. 1963, 37, 547–552. [Google Scholar]
- Storm, N.; Weyer, J.; Markotter, W.; Kemp, A.; Leman, P.A.; Dermaux-Msimang, V.; Nel, L.H.; Paweska, J.T. Human cases of Sindbis fever in South Africa, 2006–2010. Epidemiol. Infect. 2014, 142, 234–238. [Google Scholar] [CrossRef]
- Weyer, J.; Thomas, J.; Leman, P.A.; Grobbelaar, A.A.; Kemp, A.; Paweska, J.T. Human cases of Wesselsbron disease, South Africa 2010–2011. Vector Borne Zoonotic Dis. 2013, 13, 330–336. [Google Scholar] [CrossRef]
- Weiss, K.E.; Haig, D.A.; Alexander, R.A. Wesselsbron virus—A virus not previously described associated with abortion in domestic animals. Onderstepoort J. Vet. Res. 1956, 27, 183–195. [Google Scholar]
- van Niekerk, S.; Human, S.; Williams, J.; van Wilpe, E.; Pretorius, M.; Swanepoel, R.; Venter, M. Sindbis and Middelburg Old World Alphaviruses Associated with Neurologic Disease in Horses, South Africa. Emerg. Infect. Dis. 2015, 21, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Karabatsos, N. Arbovirus Serogroups: Definition and Geographic Distribution; CRC Press: Baca Raton, FL, USA, 1988. [Google Scholar]
- Vawda, S.; Goedhals, D.; Bester, P.A.; Burt, F. Seroepidemiologic Survey of Crimean-Congo Hemorrhagic Fever Virus in Selected Risk Groups, South Africa. Emerg. Infect. Dis. 2018, 24, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; Sewlall, N.H.; Ksiazek, T.G.; Blumberg, L.H.; Hale, M.J.; Lipkin, W.I.; Weyer, J.; Nichol, S.T.; Rollin, P.E.; McMullan, L.K.; et al. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg. Infect. Dis. 2009, 15, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Paweska, J.T.; McMullan, L.K.; Hutchison, S.K.; Street, C.; Palacios, G.; Khristova, M.L.; Weyer, J.; Swanepoel, R.; Egholm, M.; et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009, 5, e1000455. [Google Scholar] [CrossRef] [Green Version]
- Paweska, J.T.; Jansen van Vuren, P.; Kemp, A.; Storm, N.; Grobbelaar, A.A.; Wiley, M.R.; Palacios, G.; Markotter, W. Marburg Virus Infection in Egyptian Rousette Bats, South Africa, 2013–2014(1). Emerg. Infect. Dis. 2018, 24, 1134–1137. [Google Scholar] [CrossRef]
- Geldenhuys, M.; Weyer, J.; Nel, L.H.; Markotter, W. Coronaviruses in South African bats. Vector Borne Zoonotic Dis. 2013, 13, 516–519. [Google Scholar] [CrossRef]
- von Teichman, B.F.; de Koker, W.C.; Bosch, S.J.; Bishop, G.C.; Meredith, C.D.; Bingham, J. Mokola virus infection: Description of recent South African cases and a review of the virus epidemiology. J. S. Afr. Vet. Assoc. 1998, 69, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Shope, R.E.; Murphy, F.A.; Harrison, A.K.; Causey, O.R.; Kemp, G.E.; Simpson, D.I.; Moore, D.L. Two African viruses serologically and morphologically related to rabies virus. J. Virol. 1970, 6, 690–692. [Google Scholar] [CrossRef] [Green Version]
- Kemp, G.E.; Causey, O.R.; Moore, D.L.; Odelola, A.; Fabiyi, A. Mokola virus. Further studies on IbAn 27377, a new rabies-related etiologic agent of zoonosis in nigeria. Am. J. Trop Med. Hyg. 1972, 21, 356–359. [Google Scholar] [CrossRef]
- Mkhize, G.C.; Ngoepe, E.C.; Du Plessis, B.J.; Reininghaus, B.; Sabeta, C.T. Re-emergence of dog rabies in Mpumalanga province, South Africa. Vector. Borne Zoonotic Dis. 2010, 10, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.C.; Durrhein, D.N.; Kloeck, P.E.; Godlonton, J.D.; Bingham, J.; Speare, R. Rabies-Guide for the Medical, Veterinary and Allied Professions; Blumberg, L., Weyer, J., Pienaar, H., Markotter, W., Eds.; Department of Agriculture, Forestey and Fishries Pretoria: Gauteng, South Africa, 2010; pp. 2–16.
- Grover, M.; Bessell, P.R.; Conan, A.; Polak, P.; Sabeta, C.T.; Reininghaus, B.; Knobel, D.L. Spatiotemporal epidemiology of rabies at an interface between domestic dogs and wildlife in South Africa. Sci. Rep. 2018, 8, 10864. [Google Scholar] [CrossRef] [PubMed]
- Gummow, B. A survey of zoonotic diseases contracted by South African veterinarians. J. S Afr Vet. Assoc 2003, 74, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Mundel, B.; Gear, J. Rift Valley fever; I. The occurrence of human cases in Johannesburg. S. Afr. Med. J. 1951, 25, 797–800. [Google Scholar]
- Gear, J.; De Meillon, B.; Le Roux, A.F.; Kofsky, R.; Innes, R.R.; Steyn, J.J.; Oliff, W.D.; Schulz, K.H. Rift Valley fever in South Africa; a study of the 1953 outbreak in the Orange Free State, with special reference to the vectors and possible reservoir hosts. S. Afr. Med. J. 1955, 29, 514–518. [Google Scholar]
- Jansen van Vuren, P.; Kgaladi, J.; Patharoo, V.; Ohaebosim, P.; Msimang, V.; Nyokong, B.; Paweska, J.T. Human Cases of Rift Valley Fever in South Africa, 2018. Vector Borne Zoonotic Dis. 2018. [Google Scholar] [CrossRef] [Green Version]
- Archer, B.N.; Thomas, J.; Weyer, J.; Cengimbo, A.; Landoh, D.E.; Jacobs, C.; Ntuli, S.; Modise, M.; Mathonsi, M.; Mashishi, M.S.; et al. Epidemiologic Investigations into Outbreaks of Rift Valley Fever in Humans, South Africa, 2008–2011. Emerg. Infect. Dis. 2013, 19. [Google Scholar] [CrossRef]
- Fasina, F.O.; Mokoele, J.M.; Spencer, B.T.; Van Leengoed, L.A.; Bevis, Y.; Booysen, I. Spatio-temporal patterns and movement analysis of pigs from smallholder farms and implications for African swine fever spread, Limpopo province, South Africa. Onderstepoort J. Vet. Res. 2015, 82, 795. [Google Scholar] [CrossRef] [Green Version]
- van den Bergh, C.; Venter, E.H.; Swanepoel, R.; Thompson, P.N. High seroconversion rate to Rift Valley fever virus in cattle and goats in far northern KwaZulu-Natal, South Africa, in the absence of reported outbreaks. PLoS Negl. Trop Dis. 2019, 13, e0007296. [Google Scholar] [CrossRef]
- Drew, T.W.; Grierson, S.S.; King, D.P.; Hicks, D.; Done, S.; Neser, J.A.; Evans, D.P.; Grimbeek, P.; Banks, M. Genetic similarity between porcine circovirus type 2 isolated from the first reported case of PMWS in South Africa and North American isolates. Vet. Rec. 2004, 155, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, K.O.; Iweriebor, B.C.; Obi, L.C.; Okoh, A.I. Molecular detection of Porcine circovirus type 2 in swine herds of Eastern Cape Province South Africa. BMC Microbiol. 2017, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afolabi, K.O.; Iweriebor, B.C.; Obi, L.C.; Okoh, A.I. Prevalence of porcine parvoviruses in some South African swine herds with background of porcine circovirus type 2 infection. Acta Trop 2019, 190, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pini, A. Porcine parvovirus in pig herds in Southern Africa. J. S. Afr. Vet. Assoc. 1975, 46, 241–244. [Google Scholar] [PubMed]
- Korsman, S.; Hardie, D.; Kaba, M. Hepatitis E virus in patients with acute hepatitis in Cape Town, South Africa, 2011. S. Afr. Med. J. 2019, 109, 582–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyaga, M.M.; Peenze, I.; Potgieter, C.A.; Seheri, L.M.; Page, N.A.; Yinda, C.K.; Steele, A.D.; Matthijnssens, J.; Mphahlele, M.J. Complete genome analyses of the first porcine rotavirus group H identified from a South African pig does not provide evidence for recent interspecies transmission events. Infect. Genet. Evol 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Prozesky, L.; Theodoridis, A. Diarrhoea in pigs induced by rotavirus. Onderstepoort J. Vet. Res. 1977, 44, 275–277. [Google Scholar]
- Pfitzer, S.; Verwoerd, D.J.; Gerdes, G.H.; Labuschagne, A.E.; Erasmus, A.; Manvell, R.J.; Grund, C. Newcastle disease and avian influenza A virus in wild waterfowl in South Africa. Avian Dis. 2000, 44, 655–660. [Google Scholar] [CrossRef]
- Scagliarini, A.; Piovesana, S.; Turrini, F.; Savini, F.; Sithole, F.; McCrindle, C.M. Orf in South Africa: Endemic but neglected. Onderstepoort J. Vet. Res. 2012, 79, E1–E8. [Google Scholar] [CrossRef] [Green Version]
- Famoroti, T.; Sibanda, W.; Ndung’u, T. Prevalence and seasonality of common viral respiratory pathogens, including Cytomegalovirus in children, between 0–5 years of age in KwaZulu-Natal, an HIV endemic province in South Africa. BMC Pediatr. 2018, 18, 240. [Google Scholar] [CrossRef]
- Moyes, J.; Walaza, S.; Pretorius, M.; Groome, M.; von Gottberg, A.; Wolter, N.; Haffejee, S.; Variava, E.; Cohen, A.L.; Tempia, S.; et al. Respiratory syncytial virus in adults with severe acute respiratory illness in a high HIV prevalence setting. J. Infect. 2017, 75, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Spengane, Z.; Korsman, S.; Mkentane, K.; Davids, L.M.; Zemanay, W.; Africa, M.; Mbhele, S.; Nicol, M.; Gumedze, F.; Ngwanya, D.; et al. Blood and virus detection on barber clippers. S. Afr. Med. J. 2018, 108, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, C.D.; Prossouw, A.P.; Koch, H. An unusual case of human rabies thought to be of chiropteran origin. S Afr Med. J. 1971, 45, 767–769. [Google Scholar]
- Justman, J.; Reed, J.B.; Bicego, G.; Donnell, D.; Li, K.; Bock, N.; Koler, A.; Philip, N.M.; Mlambo, C.K.; Parekh, B.S.; et al. Swaziland HIV Incidence Measurement Survey (SHIMS): A prospective national cohort study. Lancet HIV 2017, 4, e83–e92. [Google Scholar] [CrossRef] [Green Version]
- Potter, C.W. A history of influenza. J. Appl Microbiol 2001, 91, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, D.E.; Black, S.; Rappuoli, R. Emerging infectious diseases: A proactive approach. Proc. Natl. Acad. Sci. USA 2017, 114, 4055–4059. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, S.M.; Gorham, K.; Lee, B.Y. The cost of an Ebola case. Pathog Glob. Health 2015, 109, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Dimitri, N. The Economics of Epidemic Diseases. PLoS ONE 2015, 10, e0137964. [Google Scholar] [CrossRef] [Green Version]
- Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Kelley, P.W.; Myers, M.F.; Peters, C.J. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 1999, 285, 397–400. [Google Scholar] [CrossRef]
- Zell, R. Global climate change and the emergence/re-emergence of infectious diseases. Int. J. Med. Microbiol. 2004, 293 (Suppl. S37), 16–26. [Google Scholar] [CrossRef]
- de Vries, R.D.; Ludlow, M.; Verburgh, R.J.; van Amerongen, G.; Yuksel, S.; Nguyen, D.T.; McQuaid, S.; Osterhaus, A.D.; Duprex, W.P.; de Swart, R.L. Measles vaccination of nonhuman primates provides partial protection against infection with canine distemper virus. J. Virol. 2014, 88, 4423–33305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, N.; Nakatsu, Y.; Kubota, T.; Sekizuka, T.; Seki, F.; Sakai, K.; Kuroda, M.; Yamaguchi, R.; Takeda, M. The V protein of canine distemper virus is required for virus replication in human epithelial cells. PLoS ONE 2013, 8, e82343306. [Google Scholar]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebov, J.; Grieger, K.; Womack, D.; Zaccaro, D.; Whitehead, N.; Kowalcyk, B.; MacDonald, P.D.M. A framework for One Health research. One Health 2017, 3, 44–50. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [Green Version]
- El Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A previously unknown SARS-CoV-2 virus of pandemic potential infecting humans–Call for a One Health approach. One Health 2020. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus COVID-19 Global Cases by Johns Hopkins, The Center for Systems Science and Engineering (CSSE). 2020. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 15 April 2020).
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-51; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Morse, S.S. Global infectious disease surveillance and health intelligence. Health Aff. (Millwood) 2007, 26, 1069–1077. [Google Scholar] [CrossRef]


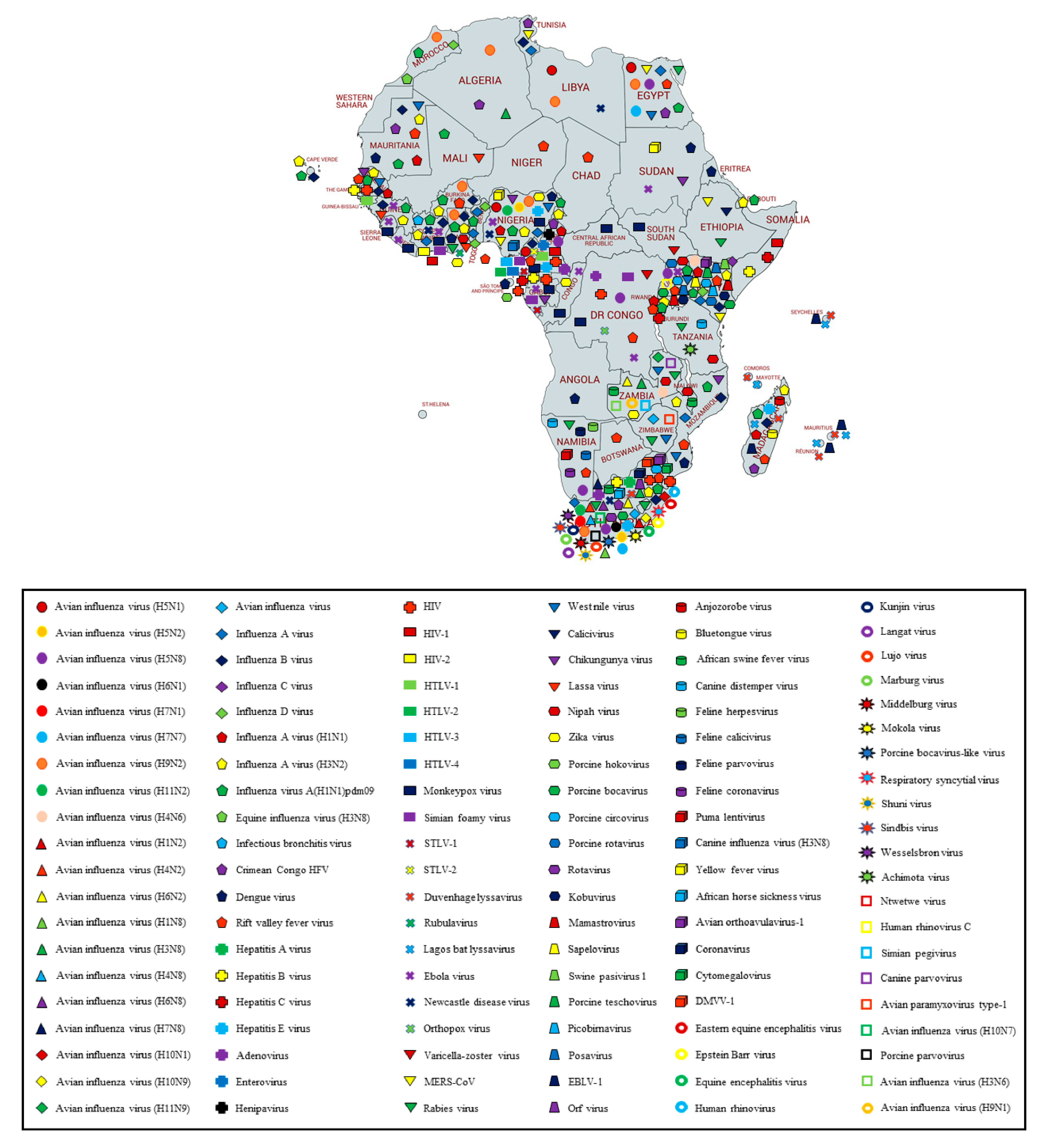
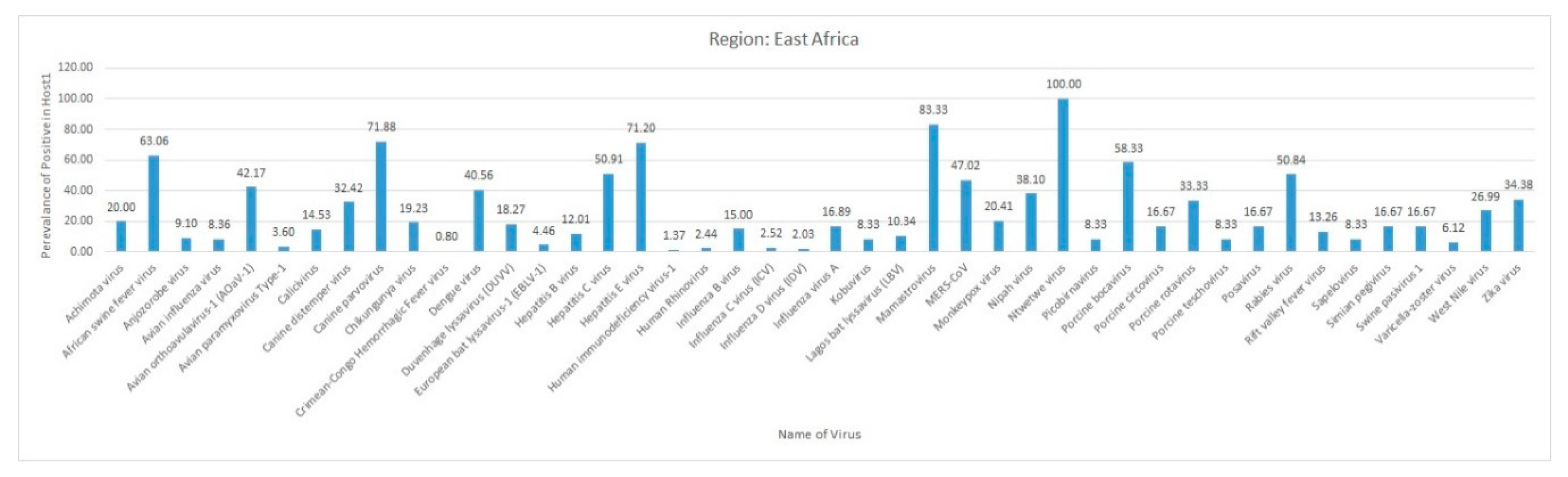










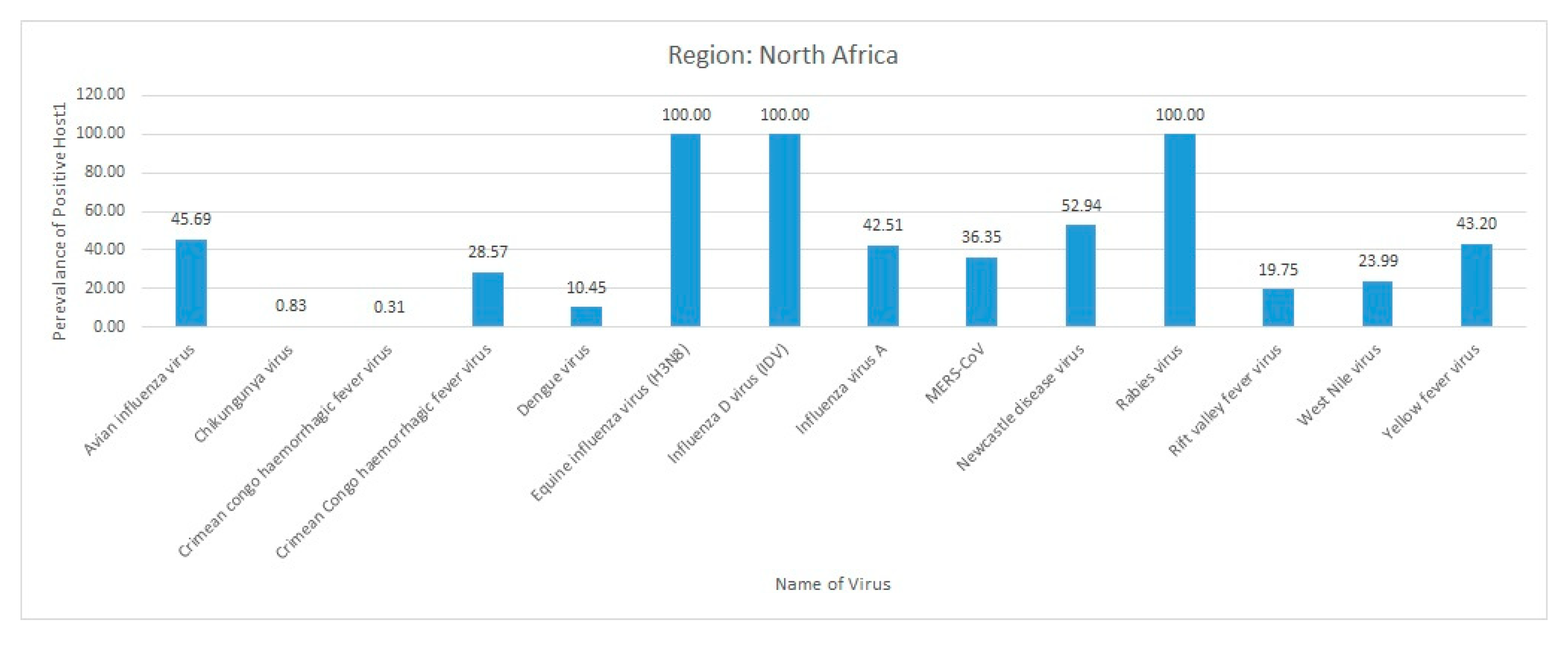










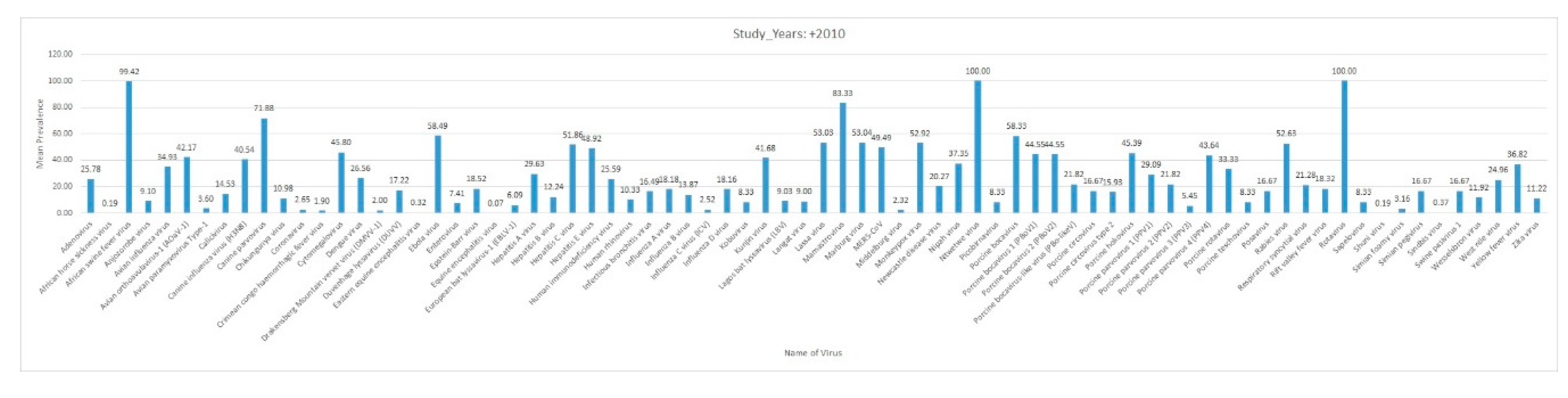
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, R.P.; Dessie, Z.G.; Noreddin, A.; El Zowalaty, M.E. Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept. Pathogens 2020, 9, 301. https://doi.org/10.3390/pathogens9040301
Chauhan RP, Dessie ZG, Noreddin A, El Zowalaty ME. Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept. Pathogens. 2020; 9(4):301. https://doi.org/10.3390/pathogens9040301
Chicago/Turabian StyleChauhan, Ravendra P., Zelalem G. Dessie, Ayman Noreddin, and Mohamed E. El Zowalaty. 2020. "Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept" Pathogens 9, no. 4: 301. https://doi.org/10.3390/pathogens9040301
APA StyleChauhan, R. P., Dessie, Z. G., Noreddin, A., & El Zowalaty, M. E. (2020). Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept. Pathogens, 9(4), 301. https://doi.org/10.3390/pathogens9040301






